A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Fiber Type and Subcellular-Specific Analysis of Lipid Droplet Content in Skeletal Muscle
In This Article
Summary
Increasing evidence indicates that excessive infiltration of lipids inside skeletal muscle results in lipotoxicity and diabetes. Here, we present a complete protocol, including tissue processing, staining with Bodipy, image acquisition, and analysis, to quantify the size, density, and subcellular distribution of lipid droplets in a fiber-type specific manner.
Abstract
Skeletal muscle lipid infiltration, known as myosteatosis, increases with obesity and ageing. Myosteatosis has also recently been discovered as a negative prognostic factor for several other disorders such as cardiovascular disease and cancer. Excessive lipid infiltration decreases muscle mass and strength. It also results in lipotoxicity and insulin resistance depending on total intramyocellular lipid content, lipid droplet (LD) morphology, and subcellular distribution. Fiber type (oxidative vs glycolytic) is also important, since oxidative fibers have a greater capacity to utilize lipids. Because of their crucial implications in pathophysiology, in-depth studies on LD dynamics and function in a fiber type-specific manner are warranted.
Herein, a complete protocol is presented for the quantification of intramyocellular lipid content and analysis of LD morphology and subcellular distribution in a fiber type-specific manner. To this end, serial muscle cryosections were stained with the fluorescent dye Bodipy and antibodies against myosin heavy chain isoforms. This protocol enables the simultaneous processing of different muscles, saving time and avoiding possible artifacts and, thanks to a personalized macro created in Fiji, the automatization of LD analysis is also possible.
Introduction
Skeletal muscle lipid infiltration, known as myosteatosis, increases with obesity and ageing. Myosteatosis is negatively correlated with muscle mass and strength and with insulin sensitivity1. Moreover, recent studies indicate that the degree of myosteatosis could be used as a prognostic factor for other conditions such as cardiovascular disease2, non-alcoholic fatty liver disease3, or cancer4. Lipids can accumulate in skeletal muscle between muscle fibers as extramyocellular lipids or within the fibers, as intramyocellular lipids (IMCLs). IMCLs are predominantly stored as triglycerides in lipid droplets (LDs) that are used as metabolic fuel during physical exercise5,6. However, when lipid supply exceeds demand, or when mitochondria become dysfunctional, IMCLs will be implicated in muscle insulin resistance, as seen in metabolically unhealthy, obese individuals and in type 2 diabetes patients7. Intriguingly, endurance athletes have similar, if not higher, levels of IMCLs to those found in obese patients with type 2 diabetes mellitus, while maintaining high insulin sensitivity. This phenomenon is described as the "athlete's paradox"8,9, and is explained by a more nuanced appraisal of muscle LDs, related to their size, density, localization, dynamics, and lipid species composition.
First, LD size is inversely correlated to insulin sensitivity and physical fitness10,11. In fact, smaller LDs exhibit a relatively greater surface area for lipase action and, thus, potentially have a greater capacity to mobilize lipids12. Second, LD density (number/surface) plays a controversial role in insulin action8,10; yet, it seems to be increased in athletes. Third, the subcellular localization of LDs is important, since LDs located just below the surface membrane (subsarcolemmal or peripheral) exert a more deleterious effect on insulin sensitivity than central ones8,9,13. The latter provide fuel to central mitochondria, which have a greater respiratory activity and are more specialized to meet the high energy demand required for contraction14. By contrast, peripheral LDs supply subsarcolemmal mitochondria, which are involved in membrane-related processes8. Finally, beyond triglycerides, specific complex lipids within the muscle may be more deleterious than others. For instance, diacylglycerol, long-chain acyl-CoA, and ceramides may accumulate in muscle when the triglyceride turnover rate is low, thereby impairing insulin signaling9,15. Returning to the "athlete's paradox," endurance athletes have a high number of smaller central LDs with elevated turnover rates in type I (oxidative) fibers, while obese and diabetic patients have larger peripheral LDs with low turnover rates in type II (glycolytic) fibers8,15,16. In addition to their role in energy storage and release, LDs via derived fatty acids (FA) and a coat protein (perilipin 5) could also function as critical players involved in the transcriptional regulation of FA oxidation and mitochondrial biogenesis8. Because of their crucial implications in physiology and pathophysiology, in-depth studies on LDs dynamics and functions are warranted.
Although there are several techniques to study IMCLs, they are not all suitable to accurately quantify LD size, density, and distribution in a fiber-specific manner. For example, the assessment of IMCLs by magnetic resonance spectroscopy, while being non-invasive, offers a level of resolution that is not sufficient to study the size and precise location of LDs within the fiber, and it is not fiber-type specific17,18. Likewise, biochemical techniques performed on whole-muscle homogenates19 cannot assess the location and size of lipids. Consequently, the most adequate method to analyze LD morphology and location is quantitative transmission electronic microscopy13, but this technique is expensive and time-consuming. Therefore, confocal fluorescence imaging on preparations with dyes such as Oil Red O (ORO)20,21, monodansylpentane (MDH)22, or Bodipy23,24,25, has emerged as the best tool for these studies.
Here, a complete protocol is described, including tissue sampling and processing, Bodipy staining, and confocal image acquisition and analysis to quantify LD size, number, and localization in mouse muscle cryosections. Since IMCLs are not evenly distributed among oxidative and glycolytic fibers, and each fiber type regulates LD dynamics differently, the study of IMCLs must be fiber-type specific16,25,26,27. Therefore, this protocol uses immunofluorescence on serial sections to identify myosin heavy chain (MyHC) isoform(s) expressed by each fiber. Another advantage of this protocol is the simultaneous processing of a glycolytic (extensor digitorum longus, EDL) and an oxidative (soleus) muscle placed side-by-side before freezing (Figure 1). This simultaneous processing not only saves time but also avoids variability due to separate processing of the samples.
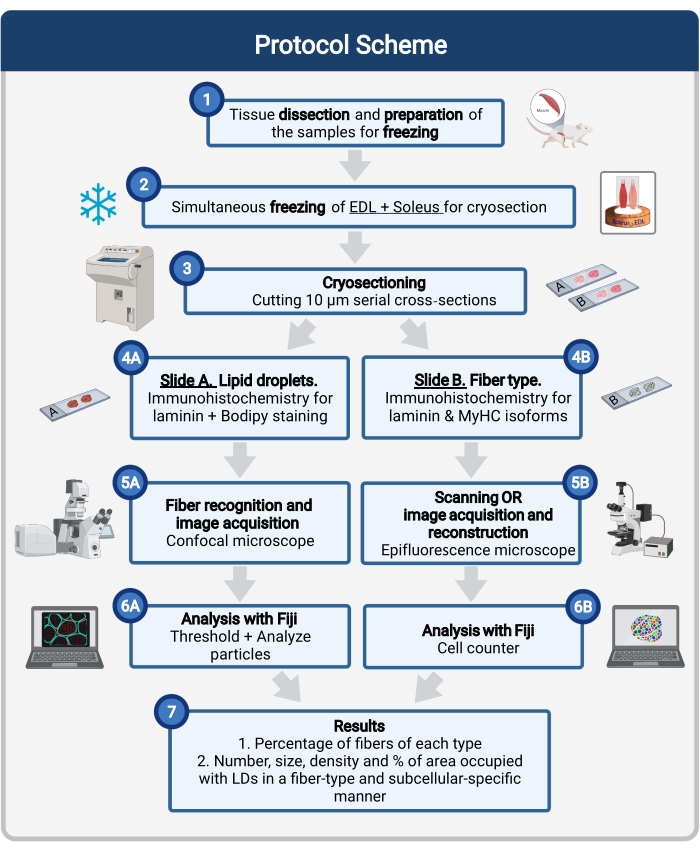
Figure 1: Schematic overview of the procedure. After muscle dissection (1), similar-size selected muscles are prepared and frozen together (2). Serial transverse sections of 10 µm are obtained using a cryostat and directly mounted on adhesion slides (3). From two serial slides, the first (4A) is immunolabeled for laminin and stained with Bodipy to recognize LDs and the second (4B) is immunostained with antibodies against MyHCs for the recognition of muscle fiber types. Images are acquired using a confocal microscope for Bodipy (5A) and an epifluorescence microscope for muscle fiber types (5B). Images are analyzed in Fiji by applying a threshold and quantifying particles (6A) to obtain the number, average size, density, and percentage of the total area occupied by LDs (7) or counting cells (6B) to obtain the percentage of fibers of each type in the section (7). Abbreviations: LDs = lipid droplets; EDL = extensor digitorum longus; MyHCs = myosin heavy chain isoforms. Please click here to view a larger version of this figure.
Protocol
All procedures conducted on mice were approved by the Ethical Committee for Animal Experimentation from the Medical Sector at Université Catholique de Louvain (2019/UCL/MD/013).
1. Dissection and preparation of the samples for freezing
- Label a 3 mm thick piece of cork for each pair of muscles.
- Through a small incision made with a blade on the center of the cork, insert perpendicularly a rectangular piece of rigid plastic (0.5 cm W, 1 cm H) that will serve as support (Figure 2B).
NOTE: The size of the rectangular plastic piece will depend on the size of the muscle. Here, the described dimensions are adapted to the size of the soleus (~9 mg, 1 cm L, 2-3 mm W) and EDL (~5 mg, 1 cm L, 2-3 mm W) of a 3-month-old C57BL/6J male mouse. - At the time of dissection, remove the soleus and EDL of the mouse hind limb. To prevent the samples from drying out during dissection, place them on a compress lightly moistened with saline solution in a Petri dish placed on ice.
NOTE: See Wang et al.28 for explanations on how to dissect these two limb muscles. - Place a small drop of Optimal Cutting Temperature compound (OCT) at the cork/plastic junction, avoiding air bubbles.
- Eliminate excess moisture from the samples by gently drying them with a paper towel (Figure 2A) and place both muscles on the plastic perpendicular to the cork (Figure 2C).
- Check the orientation of the muscle myofibers under a stereo microscope (Figure 2D).
NOTE: It is important not to cover the muscle with OCT, since its insulating effect would prevent rapid freezing and will produce freezing artifacts.
2. Freezing skeletal muscle samples for cryosectioning
CAUTION: Freezing of the muscle must be done under a chemical hood, wearing appropriate personal protective equipment (see the Table of Materials).
- Use a stainless-steel tumbler (~8 cm H, 6 cm Ø) with two side straps attached to it at least 25 cm long (Figure 2F), and fill the tumbler up to 2/3 of its capacity with isopentane.
- Grabbing the tumbler by the straps, immerse it gently in a polystyrene box filled with liquid nitrogen so that the level of nitrogen outside the container is above the level of isopentane inside (Figure 2F,G).
CAUTION: When the tumbler comes into contact with the nitrogen, the thermal shock may cause swirling. Ensure that the tumbler is sufficiently immersed but avoid the entry of nitrogen as this would cause isopentane precipitation. If this happens, let the isopentane cool down, fill the tumbler with new isopentane and start over. - When the inside of the tumbler is completely covered with a white solid layer of isopentane, take it out of the liquid nitrogen box (Figure 2H).
NOTE: The melting temperature of isopentane being -159 °C, the edges of the tumbler will turn white when it is cold enough. - Gently stir the solid isopentane pieces into the remaining liquid isopentane with forceps until the entire volume becomes liquid again.
- Re-immerse the tumbler in the liquid nitrogen until isopentane forms white pebbles all over the bottom and edges of the tumbler (Figure 2G,H).
NOTE: This second cooling step ensures the appropriate freezing temperature of the isopentane. - Remove the tumbler from the liquid nitrogen and quickly dip the muscles in the bottom of the tumbler, holding the cork with rat tooth tweezers. Swirl the cork for 15 s in the isopentane and store it at -80 °C until processing (Figure 2I).
NOTE: For short-term storage, the samples can be maintained in a -20 °C freezer. The complete protocol for rapid freezing of skeletal muscle has already been published elsewhere. For detailed references and troubleshooting, see: Meng et al.29, Kumar et al.30, and Leiva-Cepas et al.31.
3. Cryosectioning
- Bring the samples into the chamber of a cryostat previously cooled to -20 °C and the blade temperature set to -25 °C.
NOTE: Transport samples from the -20 °C/-80 °C freezer to the cryostat in a polystyrene box filled with dry ice and allow the samples to equilibrate for at least 20-30 min to the chamber temperature before cutting. - Remove the plastic from the cork with fine tweezers and place the cryostat specimen disc on the quick freeze plate to cool. Once the plate has reached -50 °C, place some OCT on the disc and quickly place the cork on top of the disk, pressing firmly. Wait until the OCT solidifies and the cork is well fixed on the disc.
- Place the disc on the object head in the desired cutting orientation (Figure 2J,K) and trim the muscle block beyond at least 1/3 of the length of the muscles and until both cross sections of the muscles are visible.
- Set the cutting thickness to 10 µm and place one section on an adhesion slide to check the correct orientation of the fibers on a brightfield microscope.
NOTE: Checking the transverse orientation of the fibers is critical. If the fibers are not adequately oriented, adjust the angle of the object head, cut another section and check again. - Place two serial cross-sections on two prelabeled adhesion slides: one slide to determine fiber types, the other to quantify lipid content (Figure 2L).
NOTE: Additional serial cross sections can be obtained and kept at -80 °C for other histological studies. However, to avoid altering the lipid content and intracellular morphology24, it is essential to process the first two slides immediately after cutting to prevent air-drying. Freezing and thawing the slides for LD quantification would have the same effect and is thus highly inadvisable.
4. Fiber typing and Bodipy staining
- Immunohistochemical detection of muscle fiber type
NOTE: For the following protocol, a total solution volume of 250 µL is sufficient to cover the entire muscle section surrounded by a circle drawn with a hydrophobic pen the approximate size of a 1 cent coin.- Surround the sections with an outline drawn with a hydrophobic pen and rinse with ice-cold 0.1 M phosphate-buffered saline (PBS) for 1 min at room temperature (RT). Place the slide in a humid chamber and block for 90 min at 37 °C in blocking solution (10% normal goat serum (NGS) and 1:30 mouse on mouse (MOM) blocking reagent in PBS).
- Remove the blocking solution and incubate the slides for 90 min at 37 °C with the solution containing the primary antibodies (5% NGS, 1:30 MOM blocking reagent, the mouse primary antibodies to recognize type I (IgG2b, at 1:10), type IIa (IgG1, at 1:10), and type IIx (IgM, at 1:5) fibers and a rat anti-laminin (α2 chain, 1:1,000) in PBS.
- Wash the slides with PBS 3 x 5 min at RT.
- Incubate the slides in the dark for 1 h at RT with the solution containing the secondary antibodies (goat anti-IgG2b AF405 (1:500), goat anti-IgG1 AF488 (1:500), goat anti-IgM AF568 (1:1,000), and goat anti-laminin AF647 (1:500) in PBS).
CAUTION: For the rest of the protocol, make sure to keep the slides away from light to preserve the fluorescence. - Wash again 3 x 5 min in PBS, rinse in double-distilled H2O, remove excess water, and mount with an antifade reagent.
NOTE: Store the slides at 4 °C, protected from light to preserve the fluorescence until image acquisition. Since the slides are not fixed, it is recommended to use freshly made solutions for each experiment, autoclave the PBS, and acquire the images as soon as possible to avoid contamination of the sections.
- Staining of LDs with Bodipy
NOTE: Similar to step 4.1., a total solution volume of 250 µL is sufficient to cover the entire muscle section surrounded by a circle drawn with a hydrophobic pen the approximate size of a 1 cent coin.- Surround the sections with an outline drawn by a hydrophobic pen and rinse with ice-cold 0.1 M PBS for 10 min at RT. Use ice-cold PBS for all the rinses and washes.
- Fix with cold 4% paraformaldehyde (PFA) without methanol for 10 min at RT. After a first quick rinse, wash the slides with PBS 3 x 5 min at RT.
CAUTION: Perform this step under a chemical hood. - Place the slide in a humid chamber and block for 1 h at RT with 5% NGS in PBS.
- Incubate the slides for 90 min at 37 °C with the solution of the primary antibody (2% NGS and rat anti-laminin (α2 chain, 1:1,000) in PBS). Wash the slides with PBS 3 x 5 min at RT.
CAUTION: For the rest of the protocol, make sure to keep the slides away from light to preserve the fluorescence. - Incubate for 1 h at RT with the secondary antibody solution containing goat anti-rat-AF647 antibody (1:500) in PBS. Wash the slides with PBS 3 x 5 min at RT.
- Incubate for 20 min at RT with a solution of 4',6-diamidino-2-phenylindole (DAPI, 0.5 µg/mL) and BODIPY (1 µg/mL) in PBS.
NOTE: To prepare BODIPY stock solution, dissolve it in DMSO at a concentration of 1 mg/mL. Different Bodipy formulations are commercially available for LD staining. Depending on the choice made, the staining method is the same (same steps, concentration, and incubation time); however, the acquisition method will be slightly different. - After a first quick rinse, wash the slides with PBS 3 x 5 min at RT, rinse in double-distilled H2O, remove excess water, and mount with an antifade reagent.
NOTE: Store the slides at 4 °C, protected from light, to preserve the fluorescence until image acquisition.
5. Acquisition of images
NOTE: Once the staining protocols have been completed, it is important to proceed immediately to image acquisition (within the following 24 h), not only to avoid contamination but also to preserve the morphology, size, and number of LDs.
- Acquisition of images to assess the fiber-types of each muscle sampled
NOTE: This step could be achieved with a whole-slide fluorescence scanning microscope or with a conventional epifluorescence microscope. With the latter, manual or automated stitching of the images must be done to reconstruct the section.- For fiber-type recognition, use an epifluorescence microscope with a 10x/0.3 objective. Select excitation filters for DAPI (405 nm), FITC, TRITC, and Cy5 to detect type I, IIa, IIx fibers, and laminin, respectively.
NOTE: Type IIb fibers will not be immunolabeled. They will be recognized as fibers stained by laminin on the limits of the sarcolemma with a black sarcoplasm. - Adjust the appropriate exposure time for each channel.
- When using a conventional epifluorescence microscope, always acquire the images of the entire muscle following the same order to facilitate muscle reconstruction. Make sure that the fibers on the right edge of one image also appear on the left edge of the following image. The same applies for the top and the bottom parts of the images (Figure 3A).
NOTE: As a reference, for a section of the EDL or the soleus dissected from a 3-month-old mouse, an average of six and eight images, respectively, will cover the entire muscle cross-section. - After the muscle is scanned, upload the captured digital images into any image-processing software for reconstruction (stitching), based on the fiber morphology (laminin) and the histology of the muscle section, and save it as a TIFF, PNG, or JPG file with all the channels (colors) merged (Figure 3A).
- For fiber-type recognition, use an epifluorescence microscope with a 10x/0.3 objective. Select excitation filters for DAPI (405 nm), FITC, TRITC, and Cy5 to detect type I, IIa, IIx fibers, and laminin, respectively.
- Acquisition of images with laminin and Bodipy co-staining
NOTE: To recognize the fiber type and have an estimate of the number of fibers for each type captured, it is essential to have the fiber-type section already scanned and the muscles reconstructed before starting the image acquisition of Bodipy-laminin (Figure 3B).- For Bodipy image observation and acquisition, use a confocal microscope with a 40x oil immersion objective lens with a numerical aperture of 1.4.
- Use the following settings: pinhole at 1 AU, 2,048 pixel x 2,048 pixel resolution, pixel size at 0.08 µm, unidirectional mode, scan speed at 4 (~4 µs/pixel), line averaging set to 4x, and digital zoom set to 1.
- To avoid cross-talk between Bodipy-558/568 and laminin-AF647, use the sequential scan mode on the confocal software.
NOTE: When the dye chosen is Bodipy-493/503, simultaneous confocal laser scanning is possible with no crosstalk between the Bodipy channel and laminin-AF647 channel. This will speed up the acquisition of images. - Excite Bodipy-493/503 using the 488 nm laser line or argon laser line, and excite Bodipy-555/568 using the 561 nm diode laser line. Finally, detect laminin-AF647 with a 640 nm diode laser line.
CAUTION: Bear in mind that Bodipy molecules are very sensitive to photobleaching, so avoid unnecessary laser scanning. To recognize fibers, use only the laser for laminin. - Depending on the dye chosen, set the emission ranges at 570-650 nm for Bodipy-493/50324 and at 565-620 nm for Bodipy-558/568. Set the emission range for laminin at 656-700 nm.
- Set the gain and digital gain appropriately so no saturated pixels are detected on the range indicator. Correct the background signal by adjusting the offset.
NOTE: Filter selection and other above-mentioned scanning parameters must be optimized for each confocal microscope. It is important that all the above-mentioned settings are kept constant for all the images captured from samples to be compared. - To identify the type of fiber among those visualized on the confocal microscope, use a personal laptop on which the image of the section reconstructed after fiber-type immunodetection is checked (Figure 3B).
- Once a group of fibers is correctly identified, acquire the image with the Bodipy and laminin channels.
NOTE: It is recommended to note the Bodipy-laminin image name on the region of the muscle where these fibers are located on the image acquired for MyHC recognition to facilitate the later fiber-specific analysis of LDs.
6. Analysis of images
- Analysis of the fiber-types on each muscle sample
- In Fiji (or ImageJ)32, open the TIFF, PNG, or JPG file with the reconstructed muscle obtained from the merge of all the channels used to detect the fiber isoforms.
- To start the Cell Counting tool, click on Plugins | Analyze | Cell counter | Cell counter.
- On the Cell counter window, click on Actions | Initialize.
- Under Counters in the same window, select Type 1.
- On the Fiji main window, select the Wand tool.
- To quantify the number of fibers of each type, click on each fiber of the same type, so that the program records the number of fibers clicked.
- Once finished, select the next type of fiber and repeat the same steps.
- When all fibers of the image have been assigned to a given fiber type, click on Results on the Cell Counter window to display the results in a table.
- Save this table as a spreadsheet table by clicking on File | Save As on the Results window.
- Save and reload the selections on the same image at any time by clicking on Save Markers or Load Markers, respectively, on the Cell Counting window.
- Analysis of lipid droplets in a fiber-type-dependent manner
- Analyze the images of Bodipy and laminin obtained on the confocal using Fiji for the quantification of LDs.
NOTE: The authors designed a customized macro to automatize the analysis. This macro, along with a step-by-step explanation on how to use it, is available as Supplemental File 1 and Supplemental File 2, respectively. - Open each image with the aid of the Bio-Formats Importer from Fiji. Under the View Stack with option, select Hyperstack | Color mode, Default. Make sure that the Autoscale window is selected.
NOTE: The following steps will describe the protocol for the analysis of one fiber on the image, but it must be repeated as many times as the number of entire fibers appear on the image. - Use the freehand selection tool to manually select the sarcolemma of the fiber based on the laminin channel (Figure 4A) and press T on the keyboard to record the selection or Region of Interest (ROI) on the ROI window.
- Go to Fiji's main window and click on Analyze | Set Measurements, and on the pop-up window, select Area and Feret's diameter. Leave the remaining boxes unchecked and other parameters as they appear by default.
- Click on Measure on the ROI window to obtain the Area and Minimal Feret's Diameter (MF) of the fiber selected, and note them for later use.
NOTE: When analyzing LDs, it is important to have in mind that their size and density vary between the center and the periphery (subsarcolemmal region-SS) of the fiber. Therefore, the analysis must be done separately. - Calculate the value of 1/6 of the MF to delimit the central part of the fiber.
NOTE: In the macro, the default MF value is set to 6, which means that the reduction applied will be set as 1/6 of the MF. This value was chosen based on the empirical data obtained from the soleus of animals fed a high-fat diet. However, each researcher must change this number based on the empirical data and the muscle analyzed, the type of fiber, and the animal condition. - On the ROI window, click on Add to have a duplicate of the first ROI and select the second ROI that appears on the window.
- On Fiji's main window, click on Edit | Selection | Enlarge and introduce the previously calculated value (from step 6.2.6.) with a minus sign before the number and click on OK. On the ROI window, click on Add[t] (a third ROI must appear) and Delete, to delete the second ROI.
NOTE: The researcher can check the results by clicking on the Show All box on the ROI window. At this point, two ROIs must appear, one that surrounds the entire perimeter of the fiber (Figure 4B) and another one that is placed in the center (Figure 4C). - Select both ROIs on the ROI window and click on More | XOR | Add[t]. Wait for a third ROI to appear, which corresponds to the periphery of the fiber (Figure 4D).
- Save the ROIs by clicking on More | Save to save the ROIs in case a later reanalysis of the same fibers is needed.
- Select the Bodipy channel and open the Threshold tool by clicking on Image | Adjust | Threshold on Fiji's main window.
- On the Threshold pop-up window, set the values to 70/255, select Yen | B&W method, and click on Dark background | Apply.
NOTE: The values applied on the Threshold may vary depending on the conditions of the experiment and the threshold must be appropriately set to optimize the analysis. A B&W window with the Bodipy signal above the threshold limit shown in white and the background in black must appear (compare the original Bodipy image in Figure 4E with the one in Figure 4F). - Go to Fiji's main window and click on Analyze | Set Measurements and on the pop-up window, select Area, Area fraction, and Limit to threshold. Leave the remaining boxes unchecked and other parameters as they appear by default.
NOTE: If the researcher wants to analyze the "circularity" of LDs, which is an index of the spherical morphology that ranges from 1 for a perfect sphere to 0 for a line, click on the Shape descriptors box of the Set Measurements pop-up window. - Go to the ROI window and select the first ROI. On Fiji's main window, click on Analyze | Analyze particles tool.
NOTE: This tool quantifies the number, size, area covered, and percentage of the total area covered by particles on each selection and saves the results as a spreadsheet file. - Set the values from 2 to Infinity (2-Infinity) on the Analyze Particles window, check the Pixels box, maintain the default circularity values, select Summarize, and click on OK.
NOTE: To check the results on the fiber, under the Show option, select any of the available options. To have the information of each LD recognized on the selection in a table, check the Display results option on the Analyze Particles window. The results from the analysis of the total area of the fiber are averaged and summarized in a table with several columns (Count, Total Area, Average size, % Area; these correspond to the number of particles [LDs], the area occupied by these particles, their average size, and the percentage of the total area of the selection occupied by particles, respectively). To calculate the density, divide the number of particles by the total area of each selection. - To obtain the values of the center and the periphery of the fiber, repeat steps 6.2.14 and 6.2.15, selecting the second (center) and the third ROI (periphery) each time.
- Save the results by clicking on File | Save as on the Summary window.
NOTE: Include the type of fiber, the condition, and the image name on the assigned name of the results to facilitate later unification and statistical analysis of the data. To analyze the rest of the fibers in the same image, repeat steps 6.2.3 to 6.2.17. For the statistical analysis, at least 10-15 fibers of each type must be analyzed per animal.
- Analyze the images of Bodipy and laminin obtained on the confocal using Fiji for the quantification of LDs.
Results
The protocol described herein provides an efficient method to easily quantify LDs in a fiber type and subcellular-specific manner. It shows how, by freezing together two muscles of similar size, such as the EDL and the soleus, the time and resources spent on the following steps are reduced by half.
A complete protocol is provided for immunostaining, image acquisition, and analysis of the different MyHC isoforms expressed in adult mouse muscles. This protocol is based on the one first designed ...
Discussion
The protocol detailed here describes an efficient method to quantify LDs tagged with Bodipy on a fiber-type- and subcellular-specific basis. In recent years, classical lipid dyes, such as ORO or Sudan Black B, have been substituted with a new array of cell-permeable, lipophilic, fluorescent dyes that bind to neutral lipids (e.g., Bodipy). Available as different conjugates, Bodipy has been proven very effective at tagging LDs to study their morphology, dynamics, and interaction with other organelles, not only in different...
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
This work was supported by grants from the Fonds National de la Recherche Scientifique (FNRS-Crédit de Recherche J.0022.20) and the Société Francophone du Diabète (SFD-Roche Diabetes Care).C.M.S. is the recipient of a Ph.D. fellowship from the FRIA (FNRS). M.A.D.-L.d.C. received a fellowship from the Wallonie-Bruxelles International Excellence Program.
The authors thank Alice Monnier for her contribution to the development of this protocol and Caroline Bouzin for her expertise and technical help in the image acquisition process. We also thank the 2IP-IREC imaging platform for access to the cryostat and the microscopes (2IP-IREC Imaging Platform, Institute of Experimental and Clinical Research, Université Catholique de Louvain, 1200 Brussels, Belgium). Finally, the authors would like to thank Nicolas Dubuisson, Romain Versele, and Michel Abou-Samra for constructive criticism of the manuscript. Some of the figures of these article were created with BioRender.com.
Materials
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| AxioCam 506 mono 6 Mpix camera | Zeiss | ||
| AxioCam MRm 1.4MPix CCD camera | Zeiss | ||
| Chemical hood | Potteau Labo | EN-14175 | |
| Confocal microscope | Zeiss | LSM800 | |
| Cork discs (ø 20 mm, 3 mm thick) | Electron Microscopy Sciences | 63305 | |
| Cryo-Gloves | Tempshield | 16072252 | |
| Cryostat | Thermo Scientific | Microm Cryo Star HM 560 | |
| Dissecting Stereo Microscope SMZ745 | Nikon | ||
| Dry Ice | |||
| Dumont Forceps | F.S.T | 11295-10 | |
| Epifluorescence microscope | Zeiss | AxioImage-Apotome Z1 | |
| Extra Fine Bonn Scissors | F.S.T | 14084-08 | |
| FisherBrand Disposable Base Molds (0.7 x 0.7 cm) | ThermoFisher | 22-363-552 | Used to cut a piece to hold the muscle on the cork for freezing |
| Glass petri dish (H 25 mm, ø 150 mm) | BRAND Petri dish, MERK | BR455751 | Used to place the muscles on ice during dissection |
| ImmEdge Hydrophobic barrier PAP Pen | Vector Labs | H-4000 | Used to create an hidrophobic barrier around the muscle sections |
| Incubator | MMM Medcenter | Incucell 707 | |
| Microscope Cover Glasses (24x50 mm) | Assistent | 40990151 | |
| Microscope Slide Boxes | Kartell | 278 | Used as humid chambers for immunohistochemistry |
| Neck holder | Linie zwo | SB-035X-02 | Used as strap to hold the stainless steel tumbler |
| No 15 Sterile Carbon Steel Scalpel Blade | Swann-Morton | 0205 | |
| Paint brushes | Van Bleiswijck | Amazon B07W7KJQ2X | Used to handle cryosections |
| Permanent Marker Pen Black | Klinipath/VWR | 98307-R | Used to label slides |
| Pierce Fixation Forceps | F.S.T | 18155-13 | |
| Polystyrene Box | H 12 cm x L 25 cm x W 18 cm, used as a liquid nitrogen container and to transport the samples to the cryostat | ||
| Scalpel Handle, 125 mm (5"), No. 3 | Aesculap | BB073R | |
| Stainless Steel Cup 10oz | Eboxer | B07GFCBPFH | Tumbler to fill with isopentene for muscle freezing |
| Superfrost Ultra Plus slides | ThermoFisher | J1800AMNZ | |
| Surgical tweezers 1/2 teeth | Medische Vakhandel | 1303152 | Also called "Rat teeth tweezers" |
| Vannas Spring Scissors - 3 mm Cutting Edge | F.S.T | 15000-00 | |
| Weighing boats | VWR international | 611-2249 | |
| Whole-Slide Scanner for Fluorescence | Zeiss | Axio Scan.Z1 | |
| Reagents | |||
| Alexa Fluor 405 Goat Anti-Mouse IgG2b | Sigma-Aldrich | SAB4600477 | Used at a final concentration of 1:500 |
| Alexa Fluor 488 Goat Anti-Mouse IgG1 | ThermoFisher | A-21121 | Used at a final concentration of 1:500 |
| Alexa Fluor 568 Goat Anti-Mouse IgM | Abcam | ab175702 | Used at a final concentration of 1:1,000 |
| Alexa Fluor 647 goat anti rat-IgG (H+L) secondary antibody | ThermoFisher | A-21247 | Used at a final concentration of 1:500 |
| BODIPY-493/503 (4,4-difluoro-1,3,5,7,8-pentametil-4-bora-3a,4a-diaza-s-indaceno) | ThermoFisher | D3922 | Used at a final concentration of 1 µg/mL |
| BODIPY-558/568 C12 (4,4-Difluoro-5-(2-Thienyl)-4-Bora-3a,4a-Diaza-s-Indacene-3-Dodecanoic Acid) | ThermoFisher | D3835 | Used at a final concentration of 1 µg/mL |
| DAPI (4',6-diamidino-2-phenylindole) | ThermoFisher | D1306 | Used at a final concentration of 0.5 µg/mL |
| Dimethyl Sulfoxide (DMSO) | Sigma-Aldrich | D-8418 | Used to solve Bodipy for the 1 mg/mL stock solution. CAUTION: Toxic and flammable. Vapors may cause irritation. Manipulate in a fume hood. Avoid direct contact with skin. Wear rubber gloves, protective eye goggles. |
| Formaldehyde solution 4%, buffered, pH 6.9 | Sigma-Aldrich | 1004969011 | CAUTION: May cause an allergic skin reaction. Suspected of causing genetic defects. May cause cancer. Manipulate in a fume hood. Avoid direct contact with skin. Wear rubber gloves, protective eye goggles. |
| Isopentane GPR RectaPur | VWR international | 24872.298 | CAUTION: Extremely flammable liquid and vapor. May be fatal if swallowed and enters airways. May cause drowsiness or dizziness. Repeated exposure may cause skin dryness or cracking. Wear protective gloves/protective clothing/eye protection/face protection. |
| Liquid Nitrogen | CAUTION: Extremely cold. Wear gloves. Handle slowly to minimize boiling and splashing and in well ventilated areas. Use containers designed for low-temperature liquids. | ||
| Mouse on mouse Blocking Reagent | Vector Labs | MKB-2213-1 | Used at concentration of 1:30 |
| Myosin heavy chain Type I (BA-D5-s Primary Antibody) Gene: MYH7, monoclonal bovine anti mouse IgG2b | DSHB University of Iowa | BA-D5-supernatant | Used at a final concentration of 1:10 |
| Myosin heavy chain Type IIA (SC-71-s Primary Antibody) Gene: MYH2, Monoclonal bovine anti mouse IgG1 | DSHB University of Iowa | SC-71-supernatant | Used at a final concentration of 1:10 |
| Myosin heavy chain Type IIX (6H1-s Primary Antibody), Gene: MYH1, Monoclonal rabbit anti mouse IgM | Developmental Studies Hybridoma Bank, University of Iowa | 6H1-supernatant | Used at a final concentration of 1:5 |
| Normal Goat Serum (NGS) | Vector Labs | S-1000 | |
| PBS 0.1 M | Commonly used on histology laboratories | ||
| ProLong Gold Antifade Mountant | Invitrogen | P36930 | |
| Rat anti-Laminin-2 (α-2 Chain) primary antibody (monoclonal) | Sigma-Aldrich | L0663 | Used at a final concentration of 1:1,000 |
| Tissue-Tek O.C.T compound | Sakura | 4583 | |
| Software | |||
| Adobe Illustrator CC | Adobe Inc. | Used to design the figures | |
| Adobe Photoshop | Adobe Inc. | Confocal software | |
| BioRender | https://biorender.com/ | Used to design the figures | |
| Fiji/ImageJ | https://imagej.net/software/fiji/ | Used to analyse the acquired images | |
| Microsoft PowerPoint | Microsoft | Used to reconstruct the histology of the whole muscle after scanning the fiber types | |
| Zen Blue 2.6 | Zeiss | Used to reconstruct the histology of the whole muscle after scanning the fiber types |
References
- Correa-de-Araujo, R., et al. Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging. Frontiers in Physiology. 11, 963 (2020).
- Miljkovic, I., et al. Greater skeletal muscle fat infiltration is associated with higher all-cause and cardiovascular mortality in older men. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 70 (9), 1133-1140 (2015).
- Nachit, M., et al. Myosteatosis rather than sarcopenia associates with non-alcoholic steatohepatitis in non-alcoholic fatty liver disease preclinical models. Journal of Cachexia, Sarcopenia, and Muscle. 12 (1), 144-158 (2021).
- Aleixo, G. F. P., et al. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Critical Reviews in Oncology/Hematolgoy. 145, 102839 (2020).
- Gemmink, A., Schrauwen, P., Hesselink, M. K. C. Exercising your fat (metabolism) into shape: a muscle-centred view. Diabetologia. 63 (8), 1453-1463 (2020).
- van Loon, L. J. Use of intramuscular triacylglycerol as a substrate source during exercise in humans. Journal of Applied Physiology. 97 (4), 1170-1187 (2004).
- Coen, P. M., Goodpaster, B. H. Role of intramyocelluar lipids in human health. Trends in Endocrinology and Metabolism. 23 (8), 391-398 (2012).
- Seibert, J. T., Najt, C. P., Heden, T. D., Mashek, D. G., Chow, L. S. Muscle lipid droplets: cellular signaling to exercise physiology and beyond. Trends in Endocrinology and Metabolism. 31 (12), 928-938 (2020).
- Bergman, B. C., Goodpaster, B. H. Exercise and muscle lipid content, composition, and localization: influence on muscle insulin sensitivity. Diabetes. 69 (5), 848-858 (2020).
- Nielsen, J., Christensen, A. E., Nellemann, B., Christensen, B. Lipid droplet size and location in human skeletal muscle fibers are associated with insulin sensitivity. American Journal of Physiology-Endocrinology and Metabolism. 313 (6), 721-730 (2017).
- Covington, J. D., et al. Intramyocellular lipid droplet size rather than total lipid content is related to insulin sensitivity after 8 weeks of overfeeding. Obesity (Silver Spring). 25 (12), 2079-2087 (2017).
- Bosma, M. Lipid droplet dynamics in skeletal muscle). Experimental Cell Research. 340 (2), 180-186 (2016).
- Nielsen, J., et al. Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism. 298 (3), 706-713 (2010).
- Ferreira, R., et al. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics. 10 (17), 3142-3154 (2010).
- Barrett, J. S., Whytock, K. L., Strauss, J. A., Wagenmakers, A. J. M., Shepherd, S. O. High intramuscular triglyceride turnover rates and the link to insulin sensitivity: influence of obesity, type 2 diabetes and physical activity. Applied Physiology, Nutrition and Metabolism. , 1-14 (2022).
- Daemen, S., et al. Distinct lipid droplet characteristics and distribution unmask the apparent contradiction of the athlete's paradox. Molecular Metabolism. 17, 71-81 (2018).
- Bredella, M. A., Ghomi, R. H., Thomas, B. J., Miller, K. K., Torriani, M. Comparison of 3.0 T proton magnetic resonance spectroscopy short and long echo-time measures of intramyocellular lipids in obese and normal-weight women. Journal of Magnetic Resonance Imaging. 32 (2), 388-393 (2010).
- Schrauwen-Hinderling, V. B., Hesselink, M. K., Schrauwen, P., Kooi, M. E. Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring). 14 (3), 357-367 (2006).
- De Bock, K., et al. Evaluation of intramyocellular lipid breakdown during exercise by biochemical assay, NMR spectroscopy, and Oil Red O staining. American Journal of Physiology-Endocrinology and Metabolism. 293 (1), 428-434 (2007).
- Koopman, R., Schaart, G., Hesselink, M. K. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochemistry and Cell Biology. 116 (1), 63-68 (2001).
- Gueugneau, M., et al. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. Journal of Gerontology Series A: Biomedical Sciences and Medical Sciences. 70 (5), 566-576 (2015).
- Gemmink, A., et al. Super-resolution microscopy localizes perilipin 5 at lipid droplet-mitochondria interaction sites and at lipid droplets juxtaposing to perilipin 2. Biochimica et Biophysica Acta-Molecular and Cell Biology of Lipids. 1863 (11), 1423-1432 (2018).
- Spangenburg, E. E., Pratt, S. J. P., Wohlers, L. M., Lovering, R. M. Use of BODIPY (493/503) to visualize intramuscular lipid droplets in skeletal muscle. Journal of Biomedicine and Biotechnology. 598358, (2011).
- Prats, C., et al. An optimized histochemical method to assess skeletal muscle glycogen and lipid stores reveals two metabolically distinct populations of type I muscle fibers. PLoS One. 8 (10), 77774 (2013).
- Strauss, J. A., Shepherd, D. A., Macey, M., Jevons, E. F. P., Shepherd, S. O. Divergence exists in the subcellular distribution of intramuscular triglyceride in human skeletal muscle dependent on the choice of lipid dye. Histochemistry and Cell Biology. 154 (4), 369-382 (2020).
- Shepherd, S. O., et al. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. Journal of Physiology. 591 (3), 657-675 (2013).
- Whytock, K. L., et al. A 7-day high-fat, high-calorie diet induces fibre-specific increases in intramuscular triglyceride and perilipin protein expression in human skeletal muscle. Journal of Physiology. 598 (6), 1151-1167 (2020).
- Wang, C., Yue, F., Kuang, S. Muscle histology characterization using h&e staining and muscle fiber type classification using immunofluorescence staining. Bio-Protocol. 7 (10), (2017).
- Meng, H., et al. Tissue triage and freezing for models of skeletal muscle disease. Journal of Visualized Experiments: JoVE. (89), e51586 (2014).
- Kumar, A., Accorsi, A., Rhee, Y., Girgenrath, M. Do's and don'ts in the preparation of muscle cryosections for histological analysis. Journal of Visualized Experiments: JoVE. (99), e52793 (2015).
- Leiva-Cepas, F., et al. Laboratory methodology for the histological study of skeletal muscle. Archivos de Medicina del Deporte. 35 (186), 254-262 (2018).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Schiaffino, S., et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. Journal of Muscle Research & Cell Motility. 10 (3), 197-205 (1989).
- Komiya, Y., et al. Mouse soleus (slow) muscle shows greater intramyocellular lipid droplet accumulation than EDL (fast) muscle: fiber type-specific analysis. Journal of Muscle Research & Cell Motility. 38 (2), 163-173 (2017).
- Andrich, D. E., et al. Altered lipid metabolism impairs skeletal muscle force in young rats submitted to a short-term high-fat diet. Frontiers in Physiology. 9, 1327 (2018).
- Schiaffino, S. Fibre types in skeletal muscle: a personal account. Acta Physiologica. 199 (4), 451-463 (2010).
- Bloemberg, D., Quadrilatero, J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One. 7 (4), 35273 (2012).
- Gemmink, A., et al. Decoration of intramyocellular lipid droplets with PLIN5 modulates fasting-induced insulin resistance and lipotoxicity in humans. Diabetologia. 59 (5), 1040-1048 (2016).
- Askinas, C., et al. . Biophotonics Congress: Biomedical Optics Congress 2018 (Microscopy/Translational/Brain/OTS). , (2018).
- Morén, B., et al. EHD2 regulates adipocyte function and is enriched at cell surface-associated lipid droplets in primary human adipocytes. Molecular Biology of the Cell. 30 (10), 1147-1159 (2019).
- Benador, I. Y., et al. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metabolism. 27 (4), 869-885 (2018).
- de la Rosa Rodriguez, M. A., et al. Hypoxia-inducible lipid droplet-associated induces DGAT1 and promotes lipid storage in hepatocytes. Molecular Metabolism. 47, 101168 (2021).
- Jevons, E. F. P., Gejl, K. D., Strauss, J. A., Ørtenblad, N., Shepherd, S. O. Skeletal muscle lipid droplets are resynthesized before being coated with perilipin proteins following prolonged exercise in elite male triathletes. American Journal of Physiology-Endocrinology and Metabolism. 318 (3), 357-370 (2020).
- Ohsaki, Y., Maeda, T., Fujimoto, T. Fixation and permeabilization protocol is critical for the immunolabeling of lipid droplet proteins. Histochemistry and Cell Biology. 124 (5), 445-452 (2005).
- Prats, C., et al. Decrease in intramuscular lipid droplets and translocation of HSL in response to muscle contraction and epinephrine. Journal of Lipid Research. 47 (11), 2392-2399 (2006).
- Listenberger, L. L., Brown, D. A. Fluorescent detection of lipid droplets and associated proteins. Current Protocols in Cell Biology. , (2007).
- Xue, Y., Lim, S., Bråkenhielm, E., Cao, Y. Adipose angiogenesis: quantitative methods to study microvessel growth, regression and remodeling in vivo. Nature Protocols. 5 (5), 912-920 (2010).
- Muliyil, S., et al. ADAM17-triggered TNF signalling protects the ageing Drosophila retina from lipid droplet-mediated degeneration. The EMBO Journal. 39 (17), 104415 (2020).
- Yan, Q., et al. Autophagy activation contributes to lipid accumulation in tubular epithelial cells during kidney fibrosis. Cell Death Discovery. 4, 2 (2018).
- Coassin, S., et al. Investigation and functional characterization of rare genetic variants in the adipose triglyceride lipase in a large healthy working population. PLoS Genetics. 6 (12), 1001239 (2010).
- Daemen, S., van Zandvoort, M., Parekh, S. H., Hesselink, M. K. C. Microscopy tools for the investigation of intracellular lipid storage and dynamics. Molecular Metabolism. 5 (3), 153-163 (2016).
- Chen, Q., et al. Rab8a deficiency in skeletal muscle causes hyperlipidemia and hepatosteatosis by impairing muscle lipid uptake and storage. Diabetes. 66 (9), 2387-2399 (2017).
- Gemmink, A., et al. Decoration of myocellular lipid droplets with perilipins as a marker for in vivo lipid droplet dynamics: A super-resolution microscopy study in trained athletes and insulin resistant individuals. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 1866 (2), 158852 (2021).
- Bergman, B. C., Hunerdosse, D. M., Kerege, A., Playdon, M. C., Perreault, L. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia. 55 (4), 1140-1150 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved