Method Article
Isolation and Processing of Murine White Adipocytes for Transcriptome and Epigenome Analyses
In This Article
Summary
The present protocol summarizes a universal method for isolating, purifying, and upstream processing of murine white adipocytes optimized for downstream total RNA sequencing, Nuclei Extraction by SONication (NEXSON), and ChIP-seq.
Abstract
Obesity is a complex disease influenced by genetics, epigenetics, the environment, and their interactions. Mature adipocytes represent the major cell type in white adipose tissue. Understanding how adipocytes function and respond to (epi)genetic and environmental signals is essential for identifying the cause(s) of obesity. RNA and chromatin have previously been isolated from adipocytes using enzymatic digestion. In addition, protocols have been developed for nuclear isolation, where purification is achieved by fluorescence-activated cell sorting (FACS) of adipocyte-specific transgenic reporters. One of the greatest challenges to achieving high yield and quality during such protocols is the substantial amount of lipid contained in adipose tissue. The present protocol describes an optimized procedure for isolating mature adipocytes that leverages heptane to separate lipids from the targets of interest (RNA/chromatin). The resulting RNA has high integrity and generates high-quality RNA-seq results. Likewise, the procedure improves nuclei yield rate and generates reproducible ChIP-seq results across samples. Therefore, the current study provides a reliable and universal murine adipocyte isolation protocol suitable for whole-genome transcriptome and epigenome studies.
Introduction
Obesity is typically understood as a disease of excess fat accumulation that contributes to a heightened risk of type 2 diabetes, cardiometabolic disease, and several forms of cancers1,2,3. While the current understanding of obesity is heavily rooted in genetics (from both human and rodent studies), some 30%-70% of metabolic disease predisposition is non-genetic in origin4,5,6,7,8 and remains ill-defined.
Adipose tissue plays a critical role in obesity and other metabolic diseases9,10. Adipose tissue comprises mature adipocytes and the stromal vascular fraction, including preadipocytes, endothelial cells, and immune cells. It is still unclear how each cell type contributes to obesity and how adipocyte dysregulation contributes to obesity. Reproducible and effective isolation and purification protocols for mature adipocyte epigenome studies are of interest to the field.
Mature adipocytes have long been isolated for gene expression analyses11 and epigenome studies12,13. There are two main strategies for isolating adipocytes. The first is to use enzymatic digestion to separate the mature adipocytes from the rest of the cell types in the stromal vascular fraction11,14. The second is to dounce the adipose tissue to release intact nuclei and then recover the nuclei based on a fluorescent reporter by fluorescence-activated cell sorting (FACS)12,13, which requires specialized transgenic reporter models. The technical challenge in each case is that mature adipocytes contain high concentrations of lipids (Figure 1), which reduces the quality and/or yield of total RNA15,16 and nuclei17. Here, an optimized enzymatic digestion procedure is described for isolating mature adipocytes, in which the advantage of the heptane is to quickly and efficiently dissolve and remove lipids18 prior to RNA extraction or the nuclei isolation steps by Nuclei Extraction by SONication (NEXSON)19. The protocol ensures excellent recovery and quality of total RNA for genome-wide studies and significantly improves the yield of intact nuclei for reproducible ChIP-seq.
Protocol
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC), protocol number: 18-10-028. 12-week-old male C57BL/6J mice were euthanized with CO2 and dissected to collect the fat pads from the epididymal white adipose tissue (eWAT).
1. Adipocyte isolation
- Prepare the digestion buffer (Table 1) and warm to 37 °C in a water bath.
- Put the fat pad from the epididymal white adipose tissue (eWAT) in a petri dish. Cut the tissue into small pieces (5 mm x 5 mm) using forceps and a scalpel in 5 mL of DMEM.
NOTE: A Petri dish that does not contain a cell adhesion coating is preferred. It is also important to hold the fat pad gently to avoid damaging the cells. The more the adipose tissue is handled, the more the yield is compromised. A scalpel/forceps method is preferable to cutting the fat pads with scissors or forceps alone as they can damage the adipocytes. - Drain the excess DMEM without disturbing the tissue debris.
NOTE: Do not pick the tissue debris off the Petri dish's surface. - Add 5 mL of digestion buffer to the Petri dish, swirl to release tissue fragments from the dish, and pour the entire content into a 50 mL centrifuge tube. Repeat the process until all the tissue fragments are transferred to the centrifuge tube.
- Add the remaining digestion buffer to the centrifuge tube so that the final volume is ~20 mL.
- Incubate the tissue fragments in a 37 °C shaking water bath at 100 rpm for 20-30 min, or until the tissue fragments are smaller than 1 mm x 1 mm.
- Add 0.4 mL of 0.5 M EDTA and 0.2 mL of 0.5 M EGTA to the centrifuge tube, and continue shaking at 37 °C for 10 min.
- Transfer the cell suspension with a 10 mL pipette through a fine matrix filter (420 µm, see Table of Materials) on top of another 50 mL centrifuge tube to remove the undigested tissue.
- Centrifuge the 50 mL centrifuge tube at 200 x g for 5 min at room temperature to separate pure adipocytes (floating on the top) from the stromal vascular fraction (pellet at the bottom) (Figure 2A).
- Pour the floating adipocyte layer into a new 50 mL tube, being careful not to disrupt the pellet.
NOTE: Avoid using pipette tips to transfer floating adipocytes because they can stick to the pipette tip wall (hence, reducing yield). Some digestion buffer will carry over into the new tube; use a syringe and needle to remove residual digestion buffer from under the adipocyte layer. - Use the purified adipocytes for total RNA extraction (step 2.) and/or ChIP-seq (step 3. and step 4.) immediately.
NOTE: The user can decide to use the whole adipocyte extract for step 2. or step 3., depending on the research purpose. The user can also split the entire adipocyte extract into two portions and continue step 2. and step 3. in parallel to obtain analysis results from the biologically identical samples.
2. Total RNA extraction
NOTE: Perform this step in a chemical hood.
- Add RNA isolation reagent (1 mL) (see Table of Materials) to the purified adipocytes in the 50 mL centrifuge tube (step 1.11.).
- Pipette up and down using a 1 mL pipette tip to thoroughly dissolve the adipocytes in the RNA isolation reagent and transfer to a 2 mL microcentrifuge tube.
- Incubate the tube for 15 min at room temperature.
- Add 0.5 mL of heptane into the centrifuge tube and vortex at full speed for 30 s.
NOTE: The lipids will dissolve in the heptane (Figure 2B). - Centrifuge the tube at 1,000 x g for 10 min at 4 °C.
- Acquire the bottom layer of RNA isolation reagent with a syringe and 30 G needle, and transfer to a new 1.5 mL tube.
- Add 0.1 mL of 1bromo-3chloropropane (see Table of Materials) to the RNA isolation reagent extract and vortex at full speed for 30 s. Incubate the tube at room temperature for 15 min.
- Centrifuge the tube at 12,000 x g for 15 min at 4 °C. Use a pipette to collect the upper aqueous phase and transfer it to a new tube.
- Add 0.5 mL of isopropanol and incubate for 10 min at 4 °C. Centrifuge the tube at 12,000 x g for 10 min at 4 °C.
NOTE: The RNA will be precipitated as a pellet at the bottom of the tube. - Use a pipette to remove the supernatant, being careful to avoid touching the pellet.
- Add 1 mL of 75% EtOH and vortex at full speed briefly.
- Centrifuge the tube at 7,500 x g for 5 min at 4 °C. Carefully remove the supernatant with a pipette tip. Do not disturb the pellet.
- Air-dry the pellet at room temperature for 10 min or until the pellet becomes clear/transparent.
- Dissolve the pellet in 50 µL of nuclease-free water.
3. Adipocyte fixation for ChIP-seq
NOTE: Perform step 3. in a chemical hood.
- Add 0.3 mL of 0.5 M EDTA, 0.2 mL of 0.5 M EGTA, 14.8 mL of DMEM, and 10 mL of heptane into the 50 mL tube containing isolated adipocytes (step 1.11.).
- Add 0.7 mL of 16% formaldehyde (final 0.7%) and rotate on a tube rotator (see Table of Materials) at 10 rpm for 5 min at room temperature.
NOTE: Set up a timer and count for 5 min. If processing multiple samples, add formaldehyde sequentially at 30 s intervals to enable precise and comparable timing of fixation across all samples. - Add 1.78 mL of 1.25 M glycine (final 0.125 M) and mix on the rotator at 10 rpm for no more than 5 min at room temperature.
NOTE: Formaldehyde remains active even after adding glycine. Therefore, it is important to separate the layers by centrifugation (step 3.4.) immediately after the 5 min incubation step. If there is more than one sample, add glycine to each sample to stop the reaction sequentially in 30 s time gaps. - Next, centrifuge at 200 x g for 5 min at room temperature.
NOTE: There must be three obvious and discrete liquid layers (Figure 2C). The middle white layer contains the fixed adipocytes. - Use a 1 mL pipette tip to transfer the adipocyte (white) layer to a fresh 15 mL centrifuge tube, minimizing the amount of fixative and heptane carried into the new tube.
- Fill the tube with 10 mL (room temperature) DMEM supplemented with the protease inhibitor cocktail (PIC, see Table of Materials) tablet. Mix thoroughly by inversion.
- Centrifuge the tube at ≤200 x g for 5 min at room temperature. Keep the centrifugation force at 100 x g to minimize cell bursting.
- Repeat steps 3.5.-3.7. 1x before moving forward to step 3.9.
- Use a 1 mL pipette tip to transfer the adipocytes (white) layer into a 1.5 mL or 2 mL microcentrifuge tube.
- Centrifuge the tube at 100-200 x g for 5 min at room temperature, and use a 1 ml pipette tip to remove the heptane (top layer) and the DMEM (bottom layer).
- Store the fixed adipocytes at −80 °C for up to 6 months until use.
4. Nuclei extraction and chromatin shearing
NOTE: This procedure is adapted from Arrigoni et al.19.
- Turn on the sonicator (see Table of Materials) and set the peak power to 75 W, the duty factor to 2%, and 200 cycles/burst, with the water bath chiller set to 20 °C.
- Add protease inhibitor cocktail (PIC) to the Farnham lab buffer and the shearing buffer (Table 1).
NOTE: Please check out a few more suggested sonicators and parameters in Arrigoni et al.19. - Resuspend the fixed adipocytes (from step 3.) with 750-800 µL of ice-cold Farnham lab buffer in a 1 mL sonication tube (see Table of Materials).
NOTE: It is recommended not to fill the tube to its full capacity. Otherwise, the adipocytes will float to the topmost part of the tube, which reduces the sonication efficiency. - Sonicate the sample for 2.5 min.
NOTE: Check the nuclei extraction on a phase-contrast microscope to determine if further sonication is needed. The nuclei must be round and intact, as shown in Figure 2D. - Before proceeding further, set the sonicator peak power to 140 W, the duty factor to 5%, and 200 cycles/burst, with the water bath chiller set to 4 °C.
- Use a pipette to transfer the supernatant to a 1.5 mL microcentrifuge tube. Centrifuge the tube at 1,000 x g for 5 min at 4 °C.
- Carefully remove the supernatant from the pellet (nuclei) and keep the pellet.
- Wash the pellet with 1 mL of Farnham lab buffer on ice.
- Centrifuge the tube at 1,000 x g for 5 min at 4 °C, use a pipette to remove the supernatant, and keep the pellet.
- Repeat steps 4.8.-4.9. 1x before moving forward to step 4.11.
NOTE: The isolated and washed nuclei can be stored at −80 °C for 3 months until further use. - Resuspend the isolated nuclei in 1 mL of shearing buffer, and transfer them into a new 1 mL sonication tube. Ensure that there are no bubbles in the tube.
- Sonicate the nuclei in the sonicator with a peak power of 140 W, a duty factor of 5%, and 200 cycles/burst for 12 min to shear the chromatin at 4 °C.
- Transfer the lysate into a 1.5 mL microfuge tube.
NOTE: The sheared chromatin is released from the nuclei and is contained in the buffer (lysate). - Centrifuge the tube at 10,000 x g for 5 min at 4 °C to pellet insoluble debris. Transfer the supernatant (chromatin) into a new 1.5 mL microcentrifuge tube.
- The sheared chromatin can be stored at 4 °C for 10 days or at −80 °C for 3 months until further use.
NOTE: Additional quality check: Aliquot 25 μL of the sheared chromatin to de-crosslink and remove the RNA with a DNase-free RNase following the previously published report19. For the present study, the resulting DNA was purified with a DNA purification kit as per the manufacturer's instructions, the DNA was quantified with a DNA quantification kit on the DNA quantification instrument, and the fragment size was evaluated by the automated electrophoresis instrument (see Table of Materials). The sheared chromatin size should be in the range of 100-800 bp. The rest of the shear chromatin can proceed for downstream ChIP-Seq application.
Results
Adipocytes were isolated from six fat pads, the heptane-based RNA extraction was performed (step 2.), and the resulting RNA was analyzed on the automated electrophoresis instrument. The calculated RNA integrity number (RIN) for all samples was >8 (Figure 3A), indicating a high-quality and reproducible RNA preparation. The total RNA prep kit was then used to prepare RNA libraries, and each sample was sequenced on the next-generation sequencer to reach a read depth of ≥40 million reads. The Phred score for all samples (Figure 3B) and per sequence (Figure 3C) was ≥30, indicative of high-quality DNA sequences20. Thus, heptane removal supports the isolation of high-yield, high-quality RNA suitable for RNA sequencing.
In the formaldehyde fixation step, three heptane-containing nuclear isolation preparations and one control preparation without heptane were also performed. The sheared chromatin was then analyzed on the automated electrophoresis instrument. In both cases, the chromatin was sheared to a 100-800 bp size range (Figure 4A), ideal for downstream ChIP-seq procedures19. Importantly, ~5 times more chromatin was obtained from the heptane-treated samples (11.5 ng/µL, 9.42 ng/µL, and 9.6 ng/µL) relative to the untreated samples (2.2 ng/µL). H3K4me3 and H3K27me3 ChIP-seq were performed following Arrigoni et al.19. As shown in Figure 4B, the signal tracks from three independent heptane-treated samples were comparable to the track of the heptane-untreated sample for both histone marks. Heptane treatment does not interfere with the quality of ChIP-seq but significantly improves nuclei (and sheared chromatin) yield.
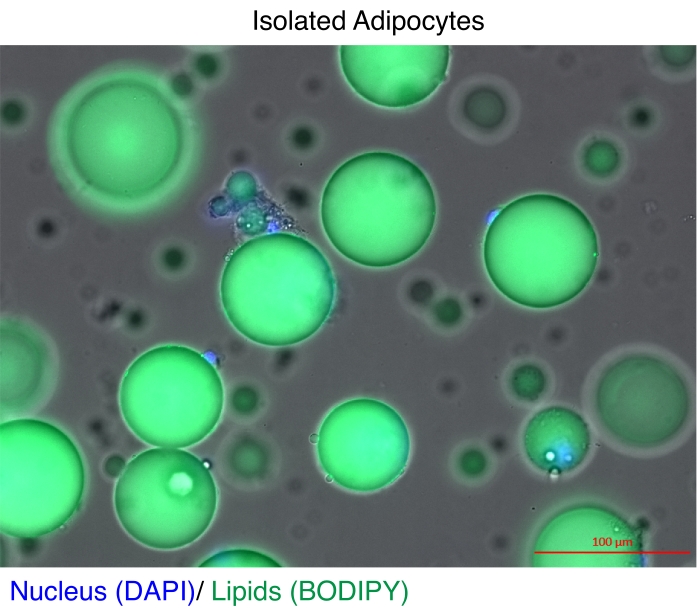
Figure 1: Isolated adipocytes. Isolated adipocytes were stained with DAPI (blue) to stain the nucleus and BODIPY (green) for the lipids. The cytosolic lipid droplets comprise up to 95% of the volume of the adipocytes and pose a technical challenge for achieving high yields during RNA and chromatin extractions. Scale bar = 100 µm. Please click here to view a larger version of this figure.
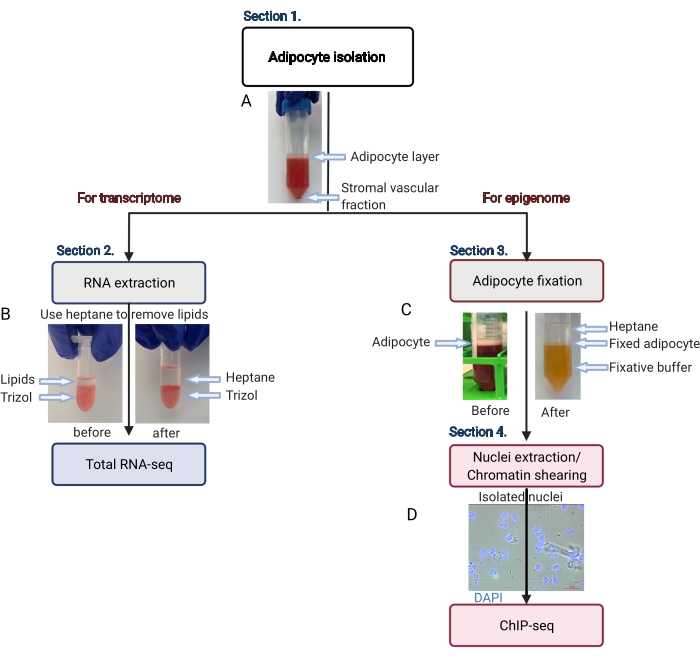
Figure 2: Schematic flow of the adipocyte isolation for transcriptome and epigenome analysis. The entire workflow is depicted, from adipocyte isolation to transcriptome or epigenome application. The key steps and representative results are shown. (A) The isolated adipocytes float on the top layer, separating from the stromal vascular fraction as a pellet at the bottom of the tube. (B) The use of heptane to remove lipids before RNA extraction with RNA isolation reagent. (C) The use of heptane to remove lipids during adipocyte fixation. (D) The representative image of isolated adipocyte nuclei must be intact and round. Scale bar = 20 µm. The scheme was created with BioRender.com. Please click here to view a larger version of this figure.
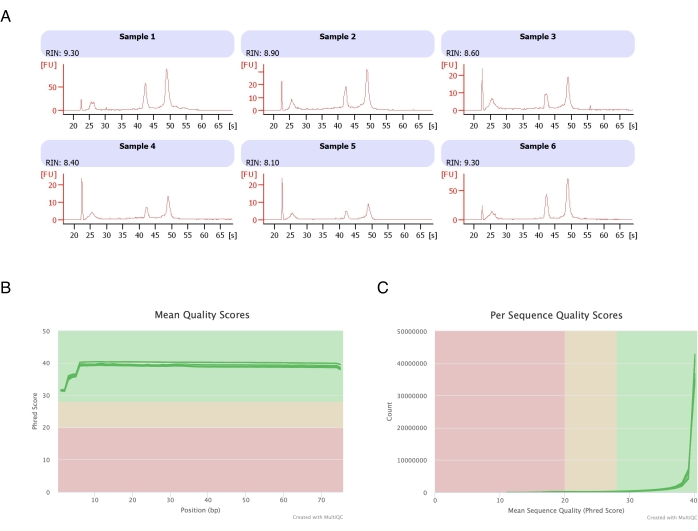
Figure 3: Representative electropherogram of RNA integrity and quality scores after RNA-seq. (A) Samples 1-6 represent six replicates of heptane-treated adipocytes, and their RNA integrity analysis was run on the automated electrophoresis instrument. The RNA integrity number (RIN) was based on 18S and 28S ribosomal RNA ratios and represents the RNA quality. Scale from 1 (much degraded) to 10 (the least degraded). (B,C) The sequencing read quality score was determined by multiQC analysis (B) for the mean quality score on bases (C) and for the quality score per sequence. Please click here to view a larger version of this figure.
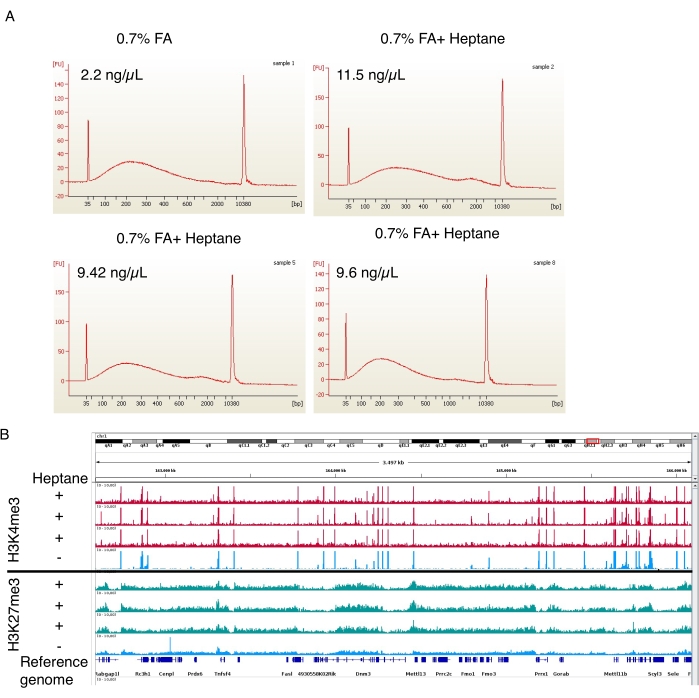
Figure 4: Representative electropherogram of sheared chromatin and enrichment peaks from ChIP-seq. (A) Representative profiles from the automated electrophoresis instrument showing the chromatin size distributions of the control (sample 1, top left) and the heptane-treated adipocyte chromatin preparations (samples 2, 5, and 8, top right and bottom two). The chromatin concentrations in the final preparations are indicated on the top left of each image. (B) Genome browser screenshot made using the Integrative Genomics Viewer (IGV). The upper part of the plot shows H3K4me3 ChIP-seq profiles for three (red) heptane-treated samples and one (blue) control. The lower panel shows the same for the ChIP-seq of H3K27me3. Please click here to view a larger version of this figure.
| Buffer | Composition |
| Digestion buffer (for 3000 mg or less of fat pad/ 20 mL) | 20 mL of Dulbecco's Modified Eagle Medium (DMEM) |
| 0.3 g of fatty acid-free BSA | |
| 0.1 g collagenase type 2 | |
| Farnham lab buffer | 5 mM PIPES (pH 8) |
| 85 mM KCl | |
| 0.5% IGEPAL | |
| Shearing buffer | 10 mM Tris-HCl pH 8 |
| 0.1% SDS | |
| 1 mM EDTA |
Table 1: Composition of the different buffers used in the present study.
Discussion
The adipocyte isolation protocol presented here is based on well-accepted enzymatic digestion methods11,14 to separate mature adipocytes (floating) from the white adipose tissue's remaining stromal vascular fraction. It provides a straightforward and universal approach to purifying mature adipocytes in any mouse model. As demonstrated above, the protocol is suitable for downstream use for whole transcriptome and ChIP-seq analyses. It provides sufficient yield to generate multiple epigenomic profiles (e.g., several histone modifications plus RNA-seq) from the same individual fat pad.
To ensure the high yields that enable the preparation of such matched epigenome data, a critical step is using heptane to dissolve lipids away from intact adipocytes and from adipocytes that lyse during the process. In our experience, this step substantially increases reproducibility and yield by preventing RNA and nuclei from being lost to the lipid layer. Continuous rotation of the tubes containing adipocytes undergoing fixation is equally important as it enhances the homogeneity with which the lipids are removed from the adipocytes. Instead of the 1% formaldehyde fixative buffer reported in much of the ChIP-seq literature21,22, it was found that reducing the concentration to 0.7% formaldehyde is necessary to avoid over-fixation. The chromatin shearing time was also optimized to 12 min to achieve an optimal chromatin size range (100-800 bp) for ChIP-seq.
The protocol was optimized for bulk transcriptome and epigenome studies. The current protocol still has its own limitations for single-cell transcriptome and epigenome applications. Considering the cell heterogeneity reported in the adiposity field23,24, this isolation method could be further developed to be adapted for single-cell assays. In such contexts, adipocyte-restricted markers such as boron-dipyrromethene (BODIPY)25 and perilipin-1 (PLIN1)26, ASC-127, or adipocyte-specific nuclei markers could be valuable in enabling the quantitation of additional, functional, relevant cellular parameters28. Apart from the application to genomic studies, this protocol might also improve adipocyte proteomic studies, which have an extra hurdle due to the high lipid content in adipocytes29.
This protocol can also be used to successfully extract human adipocyte nuclei (data not shown), extending its application to research on human obesity.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We are indebted to the MPI-IE optical imaging, sequencing, bioinformatic core, and personnel. This work was supported by funding from the MPG, the Van Andel Research Institute, the European Union's Horizon 2020 research, NIH (R01HG012444 and R21HG011964), the Marie Skłodowska-Curie grant agreement No 675610, and the Federal Ministry of Education and Research under the Project Number 01KU1216 (Deutsches Epigenom Programm, DEEP).
Materials
| Name | Company | Catalog Number | Comments |
| 16% Formaldehyde Solution (w/v). Methanol-free | ThermoFisher SCIENTIFIC | 28908 | |
| 1-bromo-3-chloropropane | SIGMA | B9673 | |
| 5 M NaCl | invitrogen | AM9759 | |
| Automated electrophoresis instrument (2100 Bioanalyzer Instrument) | Agilent Technologies | G2939BA | |
| BODIPY | ThermoFisher SCIENTIFIC | D3922 | |
| Collagenase Type 2 | Worthington Biochemical Corp. | 43D14184B | |
| Complete, EDTA-free, Protease inhibitor cocktail (PIC) | Roche | 4693159001 | EDTA free |
| DAPI | ThermoFisher SCIENTIFIC | D1306 | |
| DMEM (+ GlutaMAX) | life technologies | 61965-026 | |
| DNA purification kit (PCR purification kit) | Qiagen | 28104 | |
| DNA quantification instrument (Qubit 4 Fluorometer) | Fisher SCIENTIFIC | Q33238 | |
| DNA quantification kit (DNA high sensitivity assay) | Agilent Technologies | 5067-4626 | |
| EDTA | Fisher chimical | E478-500 | |
| EGTA | Fisher chimical | O2783-100 | |
| EtOH | PHARMCO | 111000200 | |
| Fatty acid-free BSA | SERVA | 11932.01 | bovine albumin fraction V, fatty acid-free lyophilized |
| Glycine | SIGMA | G7126-100G | |
| Heptane | SIGMA | H2198-1L | |
| High Sensitivity RNA Analysis | Agilent Technologies | 5067-1513 | |
| Igepal | SIGMA | 18896-50ML | |
| KCl | SIGMA | P3911-25G | |
| Matrix filter (420um) | Tisch Scientific | ME17238 | |
| Next-Generation Sequencer (HiSeq 3000 Sequencer) | Illumina | ||
| Nuclease-free water | Invitrogen | 4387936 | |
| PIPES | ACROS organics | 5625-37-6 | |
| Proteinase K | ThermoFisher SCIENTIFIC | EO0491 | |
| RNA isolation reagent (Trizol) | ThermoFisher SCIENTIFIC | 15596026 | |
| RNase A, DNase-free | ThermoFisher SCIENTIFIC | EN0531 | |
| Rotator (Thermo Scientific Tube Revolver Rotator) | ThermoFisher SCIENTIFIC | 88881001 | |
| SDS, Sodium dodecyl sulfate | SIGMA | 8170341000 | |
| Sonication tube (1 mL milliTUBE with AFA Fiber) | Covaris | 520135 | |
| Sonicator (Evolution Focused-ultra-sonicator (S220 or E220)) | Covaris | 500217 or 500239 | |
| Total RNA Prep kit ( Illumina Stranded Total RNA Prep kit) | Illumina | 20040525 | |
| Tris-HCl | Fisher bioreagents | BP153-500 |
References
- Albuquerque, D., Stice, E., Rodríguez-López, R., Manco, L., Nóbrega, C. Current review of genetics of human obesity: From molecular mechanisms to an evolutionary perspective. Molecular Genetics and Genomics. 290 (4), 1191-1221 (2015).
- Weihrauch-Blüher, S., Schwarz, P., Klusmann, J. H. Childhood obesity: Increased risk for cardiometabolic disease and cancer in adulthood. Metabolism. 92, 147-152 (2019).
- Weihrauch-Blüher, S., Wiegand, S. Risk factors and implications of childhood obesity. Current Obesity Reports. 7 (4), 254-259 (2018).
- Gluckman, P. D. Epigenetics, the life-course and metabolic disease. Nature Reviews Endocrinology. 8 (2), 74-76 (2012).
- Loh, M., Zhou, L., Ng, H. K., Chambers, J. C. Epigenetic disturbances in obesity and diabetes: Epidemiological and functional insights. Molecular Metabolism. 27, 33-41 (2019).
- Sayols-Baixeras, S., et al. DNA methylation and obesity traits: An epigenome-wide association study. The REGICOR study. Epigenetics. 12 (10), 909-916 (2017).
- Xia, Q., Grant, S. F. A. The genetics of human obesity. Annals of the New York Academy of Sciences. 1281 (1), 178-190 (2013).
- Yokoi, N. Epigenetic dysregulation in pancreatic islets and pathogenesis of type 2 diabetes. Journal of Diabetes Investigation. 9 (3), 475-477 (2018).
- Frayn, K. N. Obesity and metabolic disease: Is adipose tissue the culprit. Proceedings of the Nutrition Society. 64 (1), 7-13 (2005).
- Kawai, T., Autieri, M. V., Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. American Journal of Physiology-Cell Physiology. 320 (3), 375-391 (2021).
- Cat, A. N. D., Briones, A. M. Hypertension: Methods and Protocols. Touyz, R. M., Schiffrin, E. L. , Springer. New York. 283-295 (2017).
- Ambati, S., et al. Adipocyte nuclei captured from VAT and SAT. BMC Obesity. 3, 35(2016).
- Roh, H. C., et al. Simultaneous transcriptional and epigenomic profiling from specific cell types within heterogeneous tissues in vivo. Cell Reports. 18 (4), 1048-1061 (2017).
- Jacobsen, M. J., et al. Epigenetic and transcriptomic characterization of pure adipocyte fractions from obese pigs identifies candidate pathways controlling metabolism. Frontiers in Genetics. 10, 1268(2019).
- Janke, J., Engeli, S., Gorzelniak, K., Sharma, A. M. Extraction of total RNA from adipocytes. Hormone and Metabolic Research. 33 (4), 213-215 (2001).
- Cirera, S. Highly efficient method for isolation of total RNA from adipose tissue. BMC Research Notes. 6 (1), 472(2013).
- Rajbhandari, P., et al. Single cell analysis reveals immune cell-adipocyte crosstalk regulating the transcription of thermogenic adipocytes. eLife. 8, 49501(2019).
- Buddrick, O., Jones, O. A. H., Morrison, P. D., Small, D. M. Heptane as a less toxic option than hexane for the separation of vitamin E from food products using normal phase HPLC. RSC Advances. 3 (46), 24063-24068 (2013).
- Arrigoni, L., et al. Standardizing chromatin research: A simple and universal method for ChIP-seq. Nucleic Acids Research. 44 (7), 67(2016).
- Ewels, P., Magnusson, M., Lundin, S., Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 32 (19), 3047-3048 (2016).
- Schmidt, D., et al. ChIP-seq: Using high-throughput sequencing to discover protein-DNA interactions. Methods. 48 (3), 240-248 (2009).
- Hoffman, E. A., Frey, B. L., Smith, L. M., Auble, D. T. Formaldehyde crosslinking: A tool for the study of chromatin complexes. Journal of Biological Chemistry. 290 (44), 26404-26411 (2015).
- Rondini, E. A., Granneman, J. G. Single cell approaches to address adipose tissue stromal cell heterogeneity. Biochemical Journal. 477 (3), 583-600 (2020).
- Corvera, S. Cellular heterogeneity in adipose tissues. Annu Review of Physiology. 83, 257-278 (2021).
- Katz, L. S., Geras-Raaka, E., Gershengorn, M. C. Heritability of fat accumulation in white adipocytes. American Journal of Physiology-Endocrinology and Metabolism. 307 (3), 335-344 (2014).
- Hansen, J. S., de Maré, S., Jones, H. A., Göransson, O., Lindkvist-Petersson, K. Visualization of lipid directed dynamics of perilipin 1 in human primary adipocytes. Scientific Reports. 7 (1), 15011(2017).
- Ussar, S., et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Science Translational Medicine. 6 (247), (2014).
- Onogi, Y., Khalil, A. E. M. M., Ussar, S. Identification and characterization of adipose surface epitopes. Biochemical Journal. 477 (13), 2509-2541 (2020).
- Kim, E. Y., et al. Recent advances in proteomic studies of adipose tissues and adipocytes. International Journal of Molecular Sciences. 16 (3), 4581-4599 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved