Method Article
Human Mesenchymal Stem Cell Processing for Clinical Applications Using a Closed Semi-Automated Workflow
In This Article
Summary
Here, we present a protocol to harvest adherent cells from multi-layered flasks in a closed semi-automated manner using a counterflow centrifugation system. This protocol can be applied for harvesting both adherent and suspension cells from other cell expansion platforms with few modifications to the existing steps.
Abstract
Human mesenchymal stem cells (hMSCs) are currently being explored as a promising cell-based therapeutic modality for various diseases, with more market approvals for clinical use expected over the next few years. To facilitate this transition, addressing the bottlenecks of scale, lot-to-lot reproducibility, cost, regulatory compliance, and quality control is critical. These challenges can be addressed by closing the process and adopting automated manufacturing platforms. In this study, we developed a closed and semi-automated process for passaging and harvesting Wharton's jelly (WJ)-derived hMSCs (WJ-hMSCs) from multi-layered flasks using counterflow centrifugation. The WJ-hMSCs were expanded using regulatory compliant serum-free xeno-free (SFM XF) medium, and they showed comparable cell proliferation (population doubling) and morphology to WJ-hMSCs expanded in classic serum-containing media. Our closed semi-automated harvesting protocol demonstrated high cell recovery (~98%) and viability (~99%). The cells washed and concentrated using counterflow centrifugation maintained WJ-hMSC surface marker expression, colony-forming units (CFU-F), trilineage differentiation potential, and cytokine secretion profiles. The semi-automated cell harvesting protocol developed in the study can be easily applied for the small- to medium-scale processing of various adherent and suspension cells by directly connecting to different cell expansion platforms to perform volume reduction, washing, and harvesting with a low output volume.
Introduction
Human mesenchymal stem cells (hMSCs) are a great candidate for clinical applications, both in tissue engineering and in cell therapies, given their therapeutic potential and high self-renewal potential to grow in vitro, which are critical for generating clinically-relevant dosages of cells1,2,3. According to ClinicalTrials.gov, there are over 1,000 clinical trials currently under investigation for various disease conditions4. Given the backdrop of increasing interest in using hMSCs, more clinical trials and market approvals are imminent in the near future5,6. However, the manufacturing of hMSCs has many inherent challenges in terms of batch-to-batch variability, the use of high-risk raw materials, concerns regarding contamination due to many open and manual processes, as the manufacturing involves multiple unit operations, higher labor costs, the cost of scaling out or scaling up, and regulatory hurdles6,7,8,9,10,11,12. These issues remain a significant barrier to current and future market access.
The development of closed, modular, automated manufacturing solutions and using low-risk ancillary reagents would address these challenges. This would also ensure consistent product quality, decrease the likelihood of batch failures due to human error, reduce labor costs, and improve process standardization and regulatory compliance, such as in terms of digital batch record-keeping8,12,13,14. To be able to obtain a clinically relevant dosage of cells, be it autologous or allogeneic, streamlined manufacturing that involves upstream cell expansion and downstream processing in a closed, automated manner is crucial.
For upstream hMSC expansion, the two most common manufacturing methods currently employed are scale-out (2D monolayer) and scale-up (3D microcarrier-based suspension system)15,16,17,18. The most traditional and widely adopted method for hMSC expansion is 2D monolayer-based culture due to the low production cost and ease of setup19.
Multi-layered flasks composed of flat surface trays stacked within a culture vessel are commonly utilized to scale out hMSC production. These systems typically come in 1-layer to 40-layer culture vessels20 and are handled manually inside biosafety cabinets. The processing steps during cell passaging and harvesting involve manually dispensing and decanting the expansion media, dissociation reagent, and wash buffer by pipetting or physically tilting the entire vessel. Besides, handling multiple units is challenging and time-consuming due to their sheer size and weight.
Subsequently, post-harvesting from multi-layered flasks, centrifugation for media exchange, cell wash, and volume reduction are essential steps across the entire cell manufacturing workflow21. Conventional benchtop centrifugation is a mostly open and manual process that involves a multitude of steps, such as transferring the cell suspension into capped tubes or bottles inside a biosafety cabinet, spinning down the cells, manually aspirating the supernatant, cell resuspension with the buffer, and repeated cell washes. This dramatically increases both the risk of contamination due to the opening and closing of the caps and the chances of losing the cell pellet during the manual aspiration/pipetting process22. In the context of handling multi-layered culture systems for adherent-based cells such as hMSCs, the operator would need to go through a laborious process of shuttling between the centrifuge and biosafety cabinet repeatedly and handling a heavy unit at the same time. These manual steps are laborious, pose risks in terms of human errors and contamination, and have to be conducted in a Class B clean room environment, which is costly23. In addition, the conventional manual centrifugation process is not scalable and could cause cellular shear and stress; thus, maximizing cell recovery, viability, and the wash-out efficiency of residual impurities are other major challenges22. Commercial cGMP scale manufacturing of cell therapies requires closed, modular automation solutions to reduce the risk of contamination, ensure consistent product quality, reduce labor and production costs, and increase process reliability24,25. Multi-layered flasks can be handled as a closed system by having a sterile 0.2 µm filter in one of the ports to facilitate sterile gas exchange and a second port aseptically connected via connectors or tube-welded directly to an automated cell processing instrument for cell harvesting. We worked toward closing and automating most steps of WJ-hMSC passaging and harvesting by evaluating an innovative closed counterflow centrifuge intended for the manufacturing of cell, gene, or tissue-based products. This counterflow centrifuge also has the flexibility to perform a variety of cell processing applications, such as cell separation based on size, medium/buffer exchange, concentration, and harvesting for a variety of cell types8,26,27,28. The instrument uses a closed single-use kit that can be sterile-connected using tube welding or aseptic connectors to transfer bags or can be connected directly to any expansion platform of choice.
In this study, we designed a custom tubing assembly to allow closed sterile connections between the single-use counterflow centrifugation kit and the multi-layered flask. We optimized a protocol to enzymatically detach, wash, and harvest WJ-MSCs from the multi-layered flask in an entirely closed and semi-automated manner within a single run. The harvested WJ-hMSCs were characterized for purity (surface marker analysis) and potency (CFU-F, trilineage differentiation, and cytokine secretion profiles) to ensure that the final product met the critical quality attributes (CQAs) for lot release.
Protocol
1. Preparation of the culture media and coating the culture vessels
- Media preparation
- Composition of the classic serum-containing medium: Prepare the classic serum-containing medium by mixing αMEM (445 mL), fetal bovine serum (FBS) (50 mL), and 100x penicillin-streptomycin (5 mL).
- Prepare the complete SFM XF medium.
- Make a 500 mL bottle by aseptically adding 5 mL of SFM XF supplement (100x), and 5 mL of 100x L-alanyl-L-glutamine (see Tables of Materials) to the SFM XF basal medium (500 mL).
- Make a 2 L media bag by aseptically adding 20 mL of custom MSC SFM XF supplement (100x) and 20 mL of 100x L-alanyl-L-glutamine (see Tables of Materials) to the SFM XF basal medium bag (2 L) by connecting a 50 mL syringe to the appropriate port of the bag.
NOTE: Before using in culture, add growth factors or cytokines (not supplied with the medium) to the complete SF XF medium: PDGF-BB (20 ng/mL), FGF basic (4 ng/mL), and TGFβ (0.5 ng/mL).
- Coating the cell culture vessels with vitronectin for use with serum-free media
- Thaw a stock of vitronectin (VTN-N; 0.9 mg/mL) at 4 °C.
- Use sterile Dulbecco's buffered saline without calcium and magnesium (DPBS) to dilute the thawed VTN-N to a working concentration of 5 µg/mL.
NOTE: Dilute the VTN-N immediately before use, and do not store the diluted vitronectin solution. - Add 1 mL/10 cm2 of the diluted VTN-N solution to the corresponding culture vessel; the final concentration is 0.5 µg/cm2. For example, add 7.5 mL of diluted VTN-N solution to a T-75 flask (75 cm2); add 17.5 mL of diluted VTN-N solution to a T-175 flask (175 cm2); add 250 mL of diluted VTN-N solution to a standard four-layer multi-layered flask (2,528 cm2); and add 630 mL of diluted VTN-N solution to a 10-layer multi-layered flask (6,320 cm2).
- Under sterile conditions, incubate the vessels for 1 h at room temperature (RT).
NOTE: The coated culture vessel can be stored in 4 °C for up to 1 week. Wrap the culture vessel with laboratory film to prevent it drying out. Before use, pre-warm the culture vessel to room temperature for at least 1 h. - Aspirate the VTN-N solution, discard, and immediately add a sufficient volume of culture medium to prevent the coated vessel surface from drying.
NOTE: It is not necessary to rinse off the culture vessel after the removal of the VTN-N.
2. WJ-hMSC expansion
- Thaw WJ-hMSCs (p2) rapidly by placing the cryovial in a 37 °C water bath; slowly swirl the vial until the contents start to defrost.
- Upon thawing, seed the WJ-hMSCs at 5,000 cells/cm2 in a T-175 flask (without VTN-N coating), and incubate in the classic serum-containing medium at 37 °C in a humidified atmosphere of 5% CO2 for cell expansion. After two passages (p4), bank down the expanded cells in the desired cryopreservation medium as a working cell bank (WCB).
- Carry out cell counting using an automated cell counter following the viability and cell count method to determine the total cell count, cell viability, and cell size29.
- From p4, culture the WJ-hMSCs at 5,000 cells/cm2 in T-75 flasks in either classic serum-containing medium or SFM XF medium (use VTN-N-coated flasks with SFM XF medium).
- Maintain the cells in both culture media for a total of three passages (12 days), and measure the cell expansion (fold increase) at each passage by dividing the number of viable cells counted at the end of the culture of each passage by the number of viable cells at the beginning of the culture (day of cell seeding).
3. Seed train scale-out expansion
- WJ-hMSC expansion in a T-175 flask
- Thaw the WJ-hMSCs at p4 rapidly by placing the cryovial in a 37 °C water bath; slowly swirl the vial until the solution starts to defrost.
- Seed 5,000 cells/cm2 into a VTN-N-coated T-175 flask. Allow the cells to grow in an incubator at 37 °C with 5% CO2.
- Replace the spent culture medium every 2-3 days with freshly prepared pre-warmed complete SFM XF medium for optimal performance and cell growth.
- Handling the multi-layered flasks (Figure 1A, B)
- All aseptic connections need to be done in a sterile environment.
- Unpack the multi-layered flask inside a biological safety cabinet.
- Connect the pre-sterilized air filter (0.2 µm) to one port to allow pressure to be released during the fluid transfer using the counterflow centrifuge.
- Fit the multi-layered flask connector from the custom tubing set into the other port (Figure 1B).
- Weld the PVC line of the custom tubing set to the 2 L PVC transfer bag containing complete SFM XF medium.
NOTE: Mix the medium bag completely before adding it to the multi-layered system by gravity flow. Ensure that the PVC transfer bag is hanging higher than the multi-layered flask. - Place the multi-layered flask on its long side, with the air filter at the top (Figure 1B).
- Open the clamp on the PVC tubing to start filling the multi-layered flask. Ensure that the medium is level between the trays during filling.
- Once the medium has fully leveled out in all the trays, turn the multi-layered flask onto its short side with the ports upright.
- Close the clamp on the PVC line, and remove the transfer bag by replacing the tubing connection with the MPC blue closure cap.
NOTE: Do not remove the air filter, as this allows gas exchange during cell expansion. - Turn the multi-layered flask into the incubation position.
NOTE: The filter and the tubing connection should be facing upward. - Empty the multi-layered flask by connecting it to an aspirator bottle; place it above the aspirator bottle, and the liquid will flow out.
NOTE: Tilt the multi-layered flask on its side to completely drain out the spent medium by gravity. Alternatively, the spent medium can be poured into a waste bottle using a custom tubing assembly. - Repeat steps 3.2.5-3.2.11 to replenish the fresh medium.
- Subculture of WJ-hMSCs in the multi-layered flask (T-175 > 4-layer > 10-layer)
- Pre-warm cell dissociation reagent (TrypLE) and complete SFM XF medium inside a 37 °C incubator before use.
- Aspirate the spent medium from the T-175 flask and discard.
- Wash the cell monolayer with pre-warmed DPBS, aspirate, and discard.
- Add TrypLE to each flask, ensure complete coverage of the cell monolayer, and incubate for 5-10 min at 37 °C.
- Transfer the suspension to a sterile 50 mL conical tube.
- Centrifuge the tube at 100-200 x g for 5 min at RT. Aspirate and discard the DPBS, and be careful not to disturb the cell pellet.
- Resuspend the cell pellet in a minimal volume (10 mL) of pre-warm complete SFM XF medium for cell counting.
- Fill a VTN-N-coated four-layer culture vessel with about 800 mL of complete SFM XF medium, as mentioned in section 2 above. Add 5,000 cells/cm2 (i.e., 1.26 x 107 viable cells/flask). Gently swirl the cell suspension to ensure an even distribution.
- Incubate in a 37 °C incubator with 5% CO2 in a humidified atmosphere.
- Replace the spent culture medium every 2-3 days with fresh, pre-warmed complete SFM XF medium for optimal cell growth until the cells reach 60%-80% confluency or are ready to be sub-cultured into the 10-layer multi-layered flask.
- Fill a VTN-N-coated 10-layer multi-layered flask with about 2 L of complete SFM XF medium, as mentioned in section 2. Add 5,000 cells/cm2 (i.e., 3.1 x 107 cells/flask). Gently swirl the cell suspension to ensure an even distribution.
- Incubate in a 37 °C incubator with 5% CO2 in a humidified atmosphere.
- Replace the spent culture medium every 2-3 days with fresh, pre-warmed complete SFM XF medium for optimal cell growth until the cells reach 60%-80% confluency or are ready to be harvested.
4. Closed semi-automated WJ-hMSC dissociation and harvesting using closed counterflow centrifugation
- Single-use counterflow centrifugation kit assembly
- Connect the single-use kit with the single-use PVC transfer bags via tube welding, similar to the configuration shown in Figure 1A.
NOTE: The default flow rate of the single-use kit is 30-165 mL/min. - Attach a 10-layer multi-layered flask containing confluent WJ-hMSCs to the custom tubing assembly inside a biosafety cabinet.
- Transfer the attached 10-layer culture vessel with the custom tubing assembly to a bench, and weld to Line E (3/32 in ID PVC) of the single-use kit, as shown in Figure 1B.
- Make sure to weld a sterile sample port to Line G of the high-flow single-use kit.
- Next, connect the harvest Line H to a sterile Luer fitted with a 50 mL syringe.
NOTE: In all the above steps, ensure that the manual clamps are closed to secure the fluid in each kit bag.
- Connect the single-use kit with the single-use PVC transfer bags via tube welding, similar to the configuration shown in Figure 1A.
- Setting up the instrument run
- Power the instrument "ON" by switching on the toggle switch at the rear of the instrument.
- Connect the laptop to the USB-C port on the instrument using the provided USB-C cable (Figure 1B).
- Run the counterflow centrifuge graphical user interface (GUI) software from the desktop or start menu.
- After signing in, load the protocol by clicking on the Select a Protocol button on the main welcome page.
NOTE: The counterflow centrifugation harvesting protocol (Table 1) was created using the Protocol Builder software and stored locally. - Press the blue unlock button on the instrument, and open the glass door.
- Load the assembled single-use kit onto the counterflow centrifugation system.
- Start by hanging the bags suspended on hanger hooks in an order that best lines them up with the tube ports on the bubble sensor strips, with the 10-layered multi-layered flask placed at an angle, as shown in Figure 1B.
- Line up the kit with the two kit location buttons, stretch the pump tubing around the peristaltic pump, and press the white bulb-shaped connector into place.
NOTE: Make sure the tubing over the pressure sensor is correctly placed in the tubing track. - Attach the centrifuge chamber by lifting the silver lever of the counterflow centrifuge chamber carrier and securing it by returning the lever to its upright position.
- Press the tubing from each port on the kit into the tracks along the bubble sensor strips.
NOTE: Make sure the bags are not tangled so the protocol process can easily be followed. - Close the door by pressing down on the door latch.
NOTE: The pump clamp arm will close, the centrifuge chamber will spin, and the valves will close. Without closing the door, the system will not be able to initiate and run the protocol. - Hit the Initiate button on the GUI. A checklist will appear; the first four items are instrument checks, and the last two items are user checks (ensure the bags and connections match the kit image, and ensure the manual clamps are open).
NOTE: For instrument checks, red Xs instead of blue check marks are shown if something is not right. - Press Confirm to show the protocol inputs screen.
- Set the data entry (Harvest Volume) dialog box value as 45 mL, and press Confirm.
NOTE: The protocol input screen will be prompted if any variable data inputs are set in the user-created protocol. The harvest volume is set as a variable in this protocol, and the user can choose to test different volumes depending on the final cell density requirements. The minimum harvest volume that the system allows is 5 mL. The system allows a maximum of only four data variables to be set for each protocol.
- Running the protocol
- Click on Initiate on the GUI, and press the green Start button on the instrument to start the protocol run (refer to Table 1).
NOTE: The system will initiate the steps according to the protocol starting with the priming sequence by replacing the air in the system with a buffer. - Once the priming is completed (Table 1, step 8) ensure that the spent medium is completely pumped out into the waste bag.
NOTE: Once the 10-layer multi-layered flask is empty, the system will prompt the user in the GUI to confirm if the vessel is empty. Press the "Skip" button on the instrument if the vessel is empty; if not, press the green "Play/Pause" button to continue to drain any residual fluids from the vessel. - In pause steps 15, 19, and 22 (Table 1) make sure to lift up the multi-layered flask and shake to distribute the buffer equally to all the trays. Once completed, place the 10-layer multi-layered flask back in its original draw position for the next steps.
NOTE: For step 19 in Table 1 only, after shaking, make sure to place the multi-layered flask flat, and incubate for 10-15 min at RT to dissociate the cells. - In step 20 and step 23 (Table 1), ensure that the trypsinized cells are transferred completely into the intermediate bag.
- Mix the intermediate bag well manually in step 25 (Table 1).
- In step 26 (Table 1), use a 2 mL Luer syringe to sample through the sampling port.
NOTE: It is recommended to sample at least thrice for accurate cell counts. - In step 29 and step 30 (Table 1), check the stable formation of the fluidized cell bed; it should be similar to the fluidized cell bed as shown in Figure 1C.
NOTE: If the stable fluidized cell bed is not formed similar to that shown in Figure 1C, optimizing the ratio of the g-force to the flow rate (G/F) is critical for achieving a stable fluidized cell bed (high cell recovery) in the chamber during the cell loading and washing steps. The G/F ratio depends on the size of the cells and the density of the medium. A high G/F ratio is needed for a high-density sample/wash buffer, while a low G/F ratio can be used for a low-density sample/wash buffer. - The run is complete at the Ramp to Stop step (step 35 in Table 1), and all the pinch values on the counterflow centrifugation system will close automatically.
NOTE: Lastly, ensure that the manual clamps are closed to secure the fluid in each kit bag before opening the door. - After the protocol run is complete, press the blue unlock button on the instrument, and open the glass door. Take the single-use kit with the harvested concentrate out of the instrument.
- Aseptically seal the harvest line using a hand-carry tube sealer. Carefully transfer the sealed syringe filled with the concentrated cell harvest into the biological safety cabinet for cell counting and cryopreservation.
- Detach the chamber again using the lever, pull the bulb connector out of its fitting and carefully lift the kit away, and dispose into a biohazard bag.
- Clean the instrument using ethanol wipes, and make sure to close the door.
- Close the GUI application first before turning off the switch at the rear of the instrument.
NOTE: The cell harvesting protocol was tested in biological triplicates (n = 3).
- Click on Initiate on the GUI, and press the green Start button on the instrument to start the protocol run (refer to Table 1).
5. Critical quality attributes (CQA) assessment
- Cell identity surface markers (CD73, CD90, and CD105) and non-stromal markers (CD34 and CD45)
- Dilute the harvested WJ-hMSC cell suspension to a concentration of 1 x 106 viable cells/mL by adding an appropriate volume of flow cytometry buffer (DPBS with 1% BSA or 2% FBS).
- Add 100 µL of the cell suspension to each microcentrifuge tube or 96-well plate. Make sure a minimum of 0.1 x 106 cells are present per 100 µL of cell suspension.
- Add fluorophore-conjugated antibody to the sample at an appropriate dilution, as recommended by the antibody vendor.
- Incubate for 20 min in the dark.
- After the incubation, add 100 µL of flow cytometry buffer to wash the samples by centrifugation at 380 x g for 3 min.
- Discard the supernatant, leaving the pellet behind.
- Resuspend the cell pellet in 200 µL of flow cytometry buffer, and subject to flow cytometry analysis30.
- Colony-forming units fibroblastic assay (CFU-F)
- Dilute the cell suspension to a concentration of 1,000 viable cells/mL of complete culture medium.
- Plate ~500 cells per well in a 6-well tissue culture plate in complete culture medium.
- Incubate for 10-14 days at 37 °C in 5% humidified CO2, wash with PBS, and stain with 0.5% Crystal violet in methanol for 30 min at RT.
- Enumerate the colonies in each well.
- Calculate the CFU-F efficiency: Divide the number of colonies formed by the original seeding number to obtain the percentage efficiency of colony formation, and express it as a percentage.
- Trilineage differentiation potential
- Seed 5 x 103 cells/cm2 into osteogenesis and adipogenesis differentiation medium in 12-well plates as per the manufacturer's protocol. For chondrogenesis, prepare 1.6 x 107 viable cells/mL, and generate micro mass cultures by seeding 5 µL droplets of the cell solution in the centers of the wells of 96-well plates, as per the manufacturer's protocol.
- During differentiation, make complete medium changes every 3-4 days.
- After 14 days (for adipogenesis) or 21 days (for osteogenesis and chondrogenesis), monitor the cultures for differentiation by using lineage-specific biologic stains as per the manufacturer's protocol. For adipogenic differentiation, stain the cultures with 0.5% Oil Red O solution. For osteogenesis, perform staining by using a 2% Alizarin Red S solution. For chondrogenic differentiation, stain the micro mass pellets with 1% Alcian Blue.
- Cytokine secretion profiles
- Thaw the cryopreserved cells harvested manually or using the counterflow centrifugation system, and seed 5,000 cells/cm2 in a T-175 flask with SFM XF media.
- After 4 days, collect the spent culture medium, and store it at −80 °C until analysis.
- Quantify the expression of cytokines using a cytokine expression profiles 19-plex panel kit on a multiplex reader.
- Perform the multiplex immunoassays as per the manufacturer's recommendations.
Results
The WJ-hMSC master cell bank (MCB) post-thawing was maintained for three successive passages (p1-p4) in classic serum-containing medium to produce enough working cell banks (WCBs) for the experiments. The p4 WCBs were thawed and expanded in both serum-containing medium and SFM XF medium for three more passages (p4-p7) in T-175 flasks. The WJ-MSCs adapted well when expanded in SFM XF medium and were able to maintain stable proliferation similar to that in serum-containing medium (Figure 2A). However, the cells expanded in SFM XF medium exhibited slightly longer fibroblast-like spindle-shaped morphology, resulting in a slightly larger cell size (Figure 2B) of an average of ~17 µm compared to ~15 µm in serum-containing medium. In both medium conditions across the three passages, the WJ-hMSCs consistently reached their maximum cell density of ~2.3 x 104 cells/cm2 and a population doubling time of ~34 h (Figure 2C,D).
For large-scale WJ-hMSC expansion in a closed system, we carried out a seed train expansion of WJ-hMSCs first in a 4-layer flask and, subsequently, in a 10-layer multi-layered flask. At around 80%-90% confluency after 4 days of culture, we harvested 9.6 x 107 ± 0.9 x 107 and 2.3 x 108 ± 0.2 x 108 cells for the 4-layer and 10-layer stacks, respectively. A higher cell density of 3.6 x 104-3.8 x 104 cells/cm2 was reached compared to in the T-175 flasks, meaning the stacks allowed better cell expansion by up to seven-fold.
Further, the WJ-hMSCs expanded in 10-layer culture vessels were harvested directly using counterflow centrifugation. The sterile connection to the single-use kit was easy to establish for direct fluid transfer at a maximum flow rate of 165 mL/min using the instrument's peristaltic pump. The semi-automated cell harvesting process was achieved by first harvesting the cells using enzymatic dissociation, loading the cells into the counterflow chamber for volume reduction and concentrating, and then washing with wash buffer, which was around 3x the volume of the counterflow centrifugation chamber. Further, the washed cells were then concentrated and harvested to the desired harvest volume preset in the protocol. The processing steps used for the semi-automated cell processing were designed to emulate the manual harvesting workflow. We achieved a 10-fold volume reduction, resulting in the generation of cell concentrations as high as 5.3 million cells/mL. The protocol was able to achieve high cell recovery at ~98% and high cell viability at ~99% consistently for all three independent runs (Figure 3A-C).
We carried out extensive cell characterization assays to determine the critical quality attributes of the cell harvest using counterflow centrifugation compared to manual centrifugation. To test the identity of the WJ-hMSCs, cell surface markers were analyzed by flow cytometry. As shown in Figure 4A, the WJ-hMSCs harvested using both methods displayed characteristic surface marker profiles according to the ISCT regulations, positive expression of CD73, CD90, and CD105, as well as negative expression of CD34 and CD45. Next, to evaluate the clonogenic potential of the WJ-hMSCs, CFU-F assays were carried out. As shown in Figure 4B, cells harvested from counterflow centrifugation displayed a similar CFU-F potential compared with cells harvested by manual centrifugation (21% ± 1% vs. 20% ± 1%, respectively). Furthermore, as shown in Figure 4C, the post-counterflow centrifugation-harvested cells retained the ability to differentiate into adipocytes, osteoblasts, and chondrocytes similar to the cells in the manual centrifugation method. Lastly, we investigated 18 different cytokine secretion profiles of the cells using multiplex immunoassays. As shown in Figure 4D, the cells washed and concentrated using the post-counterflow centrifugation maintained cytokine secretion profiles, and the profiles were comparable to those of the sample taken before washing/concentrating the cells (pre-counterflow centrifugation).
Overall, we have demonstrated efficient hMSC expansion in an SFM XF culture system, and the cells washed and concentrated using the closed, automated counterflow centrifugation system yielded high cell recovery and viability post-wash and could maintain their phenotype and functionality. The closed semi-automated process developed in this study can deliver product quality consistency in terms of final WJ-MSC recovery, as evidenced from three independent runs.
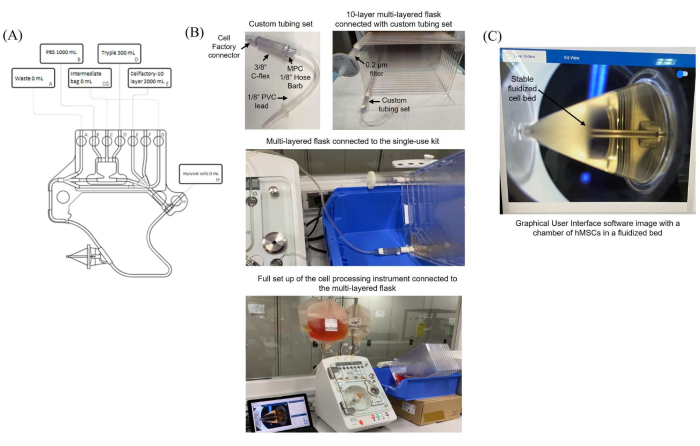
Figure 1: High-flow single-use kit configuration and assembly for the harvest, washing, and concentration of hMSCs. (A) Kit diagram after the bags have been connected in line with the respective tubing. (B) The 10-layer multi-layered flask connected to the high-flow single-use kit with the custom tubing assembly. (C) Visualization of the stable fluidized cell bed formed in the counterflow chamber via the camera function enabled in the graphical user interface of the counterflow centrifugation software. Please click here to view a larger version of this figure.

Figure 2: Comparison of the cell morphology and expansion of hMSCs in serum-containing medium and SFM XF medium. (A) The representative cell morphology of hMSCs in classic serum medium and SFM XF medium. The SFM XF-expanded cells displayed a longer, spindle-shaped, characteristic fibroblast-like morphology, whereas the cells grown in serum-containing medium displayed a more flattened morphology. (B) The average MSC size between the serum-containing medium and SFM XF medium, measured by an automated cell counter (n = 3). It is clear to see that the SFM XF-expanded cells were generally larger than serum-expanded cells across different passages. The total cell yield in different passages (n= 3) (C) in terms of cells per culture surface area and (D) population doubling levels. Similar levels of cell yield were obtained between the SFM XF medium and serum-containing medium across different passages. Data are expressed as the mean ± standard deviation. Please click here to view a larger version of this figure.
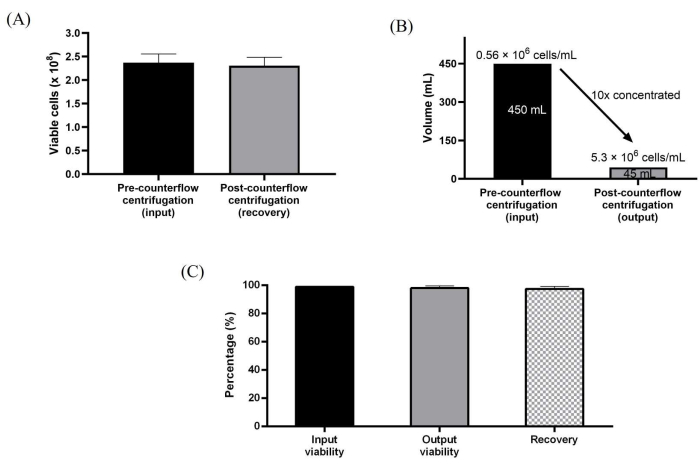
Figure 3: Characterization of cells processed using the counterflow centrifugation system. (A) Total viable cells before and after the washing and concentration. (B) A 10x volume reduction was achieved post-counterflow centrifugation processing. (C) Total recovery and viability of the cells. Data are averaged over three biological replicates (n = 3) of washing and concentration runs. Data are expressed as mean ± standard deviation. Please click here to view a larger version of this figure.

Figure 4: Critical quality attributes analysis. (A) Representative data from flow cytometry. (B) Representative images showing the total CFU. (C) Representative microscopic images of the trilineage differentiation. (D) Cytokine expression analysis results before and after processing the cells on the counterflow centrifugation system (n = 3). Data are expressed as mean ± standard deviation. Please click here to view a larger version of this figure.
Table 1: Sequence of the hMSC harvest by trypsinization, washing, and concentration protocol on the counterflow centrifugation system, including the initial priming steps. Please click here to download this Table.
Discussion
In this work, we have shown the ability to close and semi-automate hMSC dissociation and wash and harvest on the bench using a counterflow centrifugation instrument. One of the critical steps in the entire workflow is making sure the tubings are connected according to the preset protocol defined in the counterflow centrifugation system protocol builder. The setup and operation are simple, and the time it took to process around 2 L of culture from a 10-layer flask from kit assembly to cell harvest was around 60 min. One of the limiting steps in this workflow is the fluid transfer from the multi-layered flask to the transfer bags connected to the counterflow centrifugation instrument. The high-flow single-use kit can only be run at a maximum flow rate of 165 mL/min, and this might be challenging for processing, for example, a 40-layer flask. To quicken the process of fluid transfer, external high-flow rate pumps can be used to transfer the trypsinized contents into a transfer bag first, followed by washing/concentrating and harvesting the cells from the transfer bag using the counterflow centrifugation system. Moreover, this protocol can also be applied for passaging cells from 4-layer to 10-layer multi-layered flasks. Further upstream, the counterflow centrifugation system could also be optimized for the washing of thawed hMSCs and direct harvest and medium formulation into multi-layered flasks to start the seed train. It should be noted that the minimum number of cells required to form the fluidized bed in the counterflow centrifugation chamber is approximately 30 million cells, and the maximum recommended volume to process per batch is 20 L.
Currently, the attachment of the custom tubing assembly to the multi-layered flask in the biosafety cabinet and the autoclaving of the parts of the components are not desirable in a cGMP setting. As an alternative, a custom gamma-sterilized tubing assembly could be outsourced to suppliers. Suppliers providing multi-layered flasks also offer the option of pre-fitting the flasks with desired tubing assemblies, including a 0.2 µm filter, and gamma-sterilization of the entire outfit. This would ensure that the multi-layered flasks and attached tubing are truly closed, meaning the process could be completed on the bench in a Class C clean room setting.
This process utilizing the counterflow centrifugation system is not limited to adherent-based cultured cells in a multi-layered vessel and could be adapted to dynamic (stirred-tank or wave bioreactors) and static (gas-permeable) suspension-based cell expansion platforms. Specifically, for hMSCs expanded in 3D microcarrier cultures, protocols can be optimized on the counterflow centrifugation system to harvest, wash, and formulate the hMSCs dissociated from microcarriers.
Overall, increasing interest in developing translational cellular therapies with improved process robustness and reliability has led to the development of closed, automated cell processing platforms. These systems are imperative, as they reduce the number of handling steps, prevent potential contamination by sterile connections, and reduce manufacturing costs by reducing labor and enhancing the effective use of clean room space21. In line with this, many of the cell therapy product developers who are seeking regulatory approval to translate their therapies are aware of the importance of closing the process and implementing full automation or semi-automation as early as the process development stage14,31,32.
With the use of regulatory-friendly SFM XF medium, and along with ancillary reagents compliant with 21 CFR GMP Part 11 and international quality guidelines, this semi-automated process would be readily suitable for clinical manufacturing. We have shown the reproducibility of the closed process and maintenance of the quality of the WJ-MSCs. Improving the efficiency and safety of culturing adherent-based cells in multi-layered flasks would benefit not only the hMSC therapy field but also companies in cell line banking and adherent virus production.
Disclosures
P.J., A.B., R.L., and J.N. are employees of Thermo Fisher Scientific. A.L. and S.O. have no conflicts of interest.
Acknowledgements
The authors would like to acknowledge support from the Industry Alignment Fund Pre-Positioning (IAF-PP) funding (H18/01/a0/021 and H18/AH/a0/001) from A*STAR, Singapore.
Materials
| Name | Company | Catalog Number | Comments |
| 2L PVC transfer bag | TerumoBCT | BB*B200TM | |
| Alcian blue solution, pH 2.5 | Merck | 101647 | |
| Alizarin-Red Staining Solution | Merck | TMS-008-C | |
| APC anti-human CD73 Antibody | Biolegend | 344015 | |
| APC Mouse IgG1, κ Isotype Ctrl (FC) Antibody | Biolegend | 400121 | |
| Bio-Plex MAGPIX Multiplex Reader | Bio-Rad | ||
| Counterflow Centrifugation System | Thermo Fisher Scientific | A47679 | Gibco CTS Rotea Counterflow Centrifugation System |
| Crystal Violet | Sigma-aldrich | C0775 | |
| CTS (L-alanyl-L-glutamine) GlutaMAX supplement | Thermo Fisher Scientific | A1286001 | |
| CTS Dulbecco's phosphate-buffered saline (DPBS) | Thermo Fisher Scientific | A1285601 | no calcium, no magnesium |
| CTS Recombinant Human Vitronectin (VTN-N) | Thermo Fisher Scientific | A27940 | |
| CTS TrypLE Select Enzyme | Thermo Fisher Scientific | A1285901 | |
| Custom tubing assembly | Saint-Gobain and Colder Product Company (CPC) | N/A | Gamma-sterilized 3/32” ID PVC line fitted with a sterile male MPC (1/8” barb) and sealed on the other end. Autoclave a short C-Flex line fitted with a sterile Cell Factory port connector on one end and a female MPC (3/8” barb) on the other. Connect the PVC and C-Flex lines in a biosafety cabinet |
| Emflon II capsule (0.2um filter) | Pall | KM5V002P2G100 | |
| Fetal Bovine Serum (FBS) | Thermo Fisher Scientific | 12662029 | Mesenchymal stem cell-qualified, USDA-approved regions |
| FGF-basic | Thermo Fisher Scientific | PHG0024 | |
| FITC anti-human CD105 Antibody | Biolegend | 323203 | |
| FITC anti-human CD45 Antibody | Biolegend | 304005 | |
| FITC anti-human CD90 (Thy1) Antibody | Biolegend | 328107 | |
| FITC Mouse IgG1, κ Isotype Ctrl (FC) Antibody | Biolegend | 400109 | |
| Hi-Flow Single Use Kit | Thermo Fisher Scientific | A46575 | Gibco CTS Rotea Hi-flow single-use kit, flow rate of 30 – 165 mL/min |
| Multi-layered systems | Thermo Fisher Scientific | 140360 (4-layers); 140410 (10-layers) | Nunc Standard Cell Factory Systems |
| NucleoCounter NC-3000 | Chemometec | NC-3000 | |
| Oil red O staining solution | Merck | 102419 | |
| PDGF-BB | Thermo Fisher Scientific | PHG0045 | |
| Penicillin-Streptomycin | Thermo Fisher Scientific | 15140122 | |
| PerCP anti-human CD34 Antibody | Biolegend | 343519 | |
| PerCP Mouse IgG1, κ Isotype Ctrl Antibody | Biolegend | 400147 | |
| ProcartaPlex Multiplex Immunoassays | Thermo Fisher Scientific | Custom 19-Plex panel: FGF-2, HGF, IDO, IL-10, IL-1RA, IL-6, IL-8, IP-10, MCP-1, MCP-2 , MIP-1α, MIP-1β, MIP-3α, PDGF-BB, RANTES, SDF-1α, TGFα, TNF-alpha, VEGF-A | |
| Sample port | Thermo Fisher Scientific | A50111 | Gamma-sterilized leur sample port with 2 PVC lines attached |
| StemPro Adipogenesis Differentiation Kit | Thermo Fisher Scientific | A10070-01 | |
| StemPro Chondrocyte Differentiation | Thermo Fisher Scientific | A10071-01 | |
| StemPro Custom MSC SF XF Medium Kit (SFM XF medium) | Thermo Fisher Scientific | ME20236L1 | Contains StemPro MSC SFM Basal Medium and Custom MSC SF XF Supplement (100x) |
| StemPro Osteogenesis Differentiation Kit | Thermo Fisher Scientific | A10072-01 | |
| T175 Nunc EasYFlask | Thermo Fisher Scientific | 159910 | |
| T75 Nunc EasYFlask | Thermo Fisher Scientific | 156472 | |
| TGFβ1 | Thermo Fisher Scientific | PHG9204 | |
| WJ MSCs | PromoCell | (#C12971; Germany) | Human mesenchymal stem cells |
| αMEM media | Thermo Fisher Scientific | 12571063 | With nucleosides |
References
- Zhou, T., et al. Challenges and advances in clinical applications of mesenchymal stromal cells. Journal of Hematology & Oncology. 14 (1), 24 (2021).
- García-Bernal, D., et al. The current status of mesenchymal stromal cells: Controversies, unresolved issues and some promising solutions to improve their therapeutic efficacy. Frontiers in Cell and Developmental Biology. 9, 650664 (2021).
- Mastrolia, I., et al. Challenges in clinical development of mesenchymal stromal/stem cells: Concise review. Stem Cells Translational Medicine. 8 (11), 1135-1148 (2019).
- Jovic, D., et al. A brief overview of global trends in MSC-based cell therapy. Stem Cell Reviews and Reports. 18 (5), 1525-1545 (2022).
- Lechanteur, C., Briquet, A., Bettonville, V., Baudoux, E., Beguin, Y. MSC manufacturing for academic clinical trials: From a clinical-grade to a full GMP-compliant process. Cells. 10, 1320 (2021).
- Fernández-Santos, M. E., et al. Optimization of mesenchymal stromal cell (MSC) manufacturing processes for a better therapeutic outcome. Frontiers in Immunology. 13, 918565 (2022).
- Jossen, V., vanden Bos, C., Eibl, R., Eibl, D. Manufacturing human mesenchymal stem cells at clinical scale: Process and regulatory challenges. Applied Microbiology and Biotechnology. 102 (9), 3981-3994 (2018).
- Jayaraman, P., Lim, R., Ng, J., Vemuri, M. C. Acceleration of translational mesenchymal stromal cell therapy through consistent quality GMP manufacturing. Frontiers in Cell and Developmental Biology. 9, 648472 (2021).
- Levy, O., et al. Shattering barriers toward clinically meaningful MSC therapies. Science Advances. 6 (30), (2020).
- Fričová, D., Korchak, J. A., Zubair, A. C. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson's disease. npj Regenerative Medicine. 5 (1), 20 (2020).
- Childs, P. G., Reid, S., Salmeron-Sanchez, M., Dalby, M. J. Hurdles to uptake of mesenchymal stem cells and their progenitors in therapeutic products. Biochemical Journal. 477 (17), 3349-3366 (2020).
- James, D. How short-term gain can lead to long-term pain. Cell & Gene Therapy Insights. 3 (4), 271-284 (2017).
- Ochs, J., Barry, F., Schmitt, R., Murphy, M. Advances in automation for the production of clinical-grade mesenchymal stromal cells: The AUTOSTEM robotic platform. Cell & Gene Therapy Insights. 3 (8), 739-748 (2017).
- Doulgkeroglou, M. N., et al. Automation, monitoring, and standardization of cell product manufacturing. Frontiers in Bioengineering and Biotechnology. 8, 811 (2020).
- Chen, A. K. -. L., Reuveny, S., Oh, S. K. W. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: Achievements and future direction. Biotechnology Advances. 31 (7), 1032-1046 (2013).
- Couto, P. S., Bersenev, A., Rafiq, Q. A., Fernandes, T. G., Diogo, M. M., Cabral, J. M. S. . Engineering Strategies for Regenerative Medicine. , 33-71 (2020).
- Tsai, A. -. C., Pacak, C. A. Bioprocessing of human mesenchymal stem cells: From planar culture to microcarrier-based bioreactors. Bioengineering. 8 (7), 96 (2021).
- Cherian, D. S., Bhuvan, T., Meagher, L., Heng, T. S. P. Biological considerations in scaling up therapeutic cell manufacturing. Frontiers in Pharmacology. 11, 654 (2020).
- Mizukami, A., Swiech, K. Mesenchymal stromal cells: From discovery to manufacturing and commercialization. Stem Cells International. 2018, 4083921 (2018).
- Hassan, M., et al. Large-scale expansion of human mesenchymal stem cells. Stem Cells International. 2020, 9529465 (2020).
- Li, A., et al. Advances in automated cell washing and concentration. Cytotherapy. 23 (9), 774-786 (2021).
- Mehta, S. Single-use centrifugation solution for volume reduction and cell washing process in cell therapy manufacturing. Cytotherapy. 16, 101 (2014).
- Giancola, R., Bonfini, T., Iacone, A. Cell therapy: cGMP facilities and manufacturing. Muscles Ligaments and Tendons Journal. 2 (3), 243-247 (2012).
- Moutsatsou, P., Ochs, J., Schmitt, R. H., Hewitt, C. J., Hanga, M. P. Automation in cell and gene therapy manufacturing: From past to future. Biotechnology Letters. 41 (11), 1245-1253 (2019).
- Iancu, E. M., Kandalaft, L. E. Challenges and advantages of cell therapy manufacturing under Good Manufacturing Practices within the hospital setting. Current Opinion in Biotechnology. 65, 233-241 (2020).
- Li, A., James, D., Lim, R. The Gibco™ CTS™ Rotea™ system story-A case study of industry-academia collaboration. Gene Therapy. , (2021).
- Li, A., et al. Improving cell viability using counterflow centrifugal elutriation. Cytotherapy. 24 (6), 650-658 (2022).
- Li, A., et al. Automated counterflow centrifugal system for small-scale cell processing. Journal of Visualized Experiments. (154), e60423 (2019).
- Shah, D., Naciri, M., Clee, P., Al-Rubeai, M. NucleoCounter-An efficient technique for the determination of cell number and viability in animal cell culture processes. Cytotechnology. 51 (1), 39-44 (2006).
- Chan, A. K. C., Heathman, T. R. J., Coopman, K., Hewitt, C. J. Multiparameter flow cytometry for the characterisation of extracellular markers on human mesenchymal stem cells. Biotechnology Letters. 36 (4), 731-741 (2014).
- Smith, D., et al. Towards automated manufacturing for cell therapies. Current Hematologic Malignancy Reports. 14 (4), 278-285 (2019).
- Stroncek, D. F., Somerville, R. P. T., Highfill, S. L. Point-of-care cell therapy manufacturing; it's not for everyone. Journal of Translational Medicine. 20 (1), 34 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved