Method Article
Utilizing a Reconfigurable Maze System to Enhance the Reproducibility of Spatial Navigation Tests in Rodents
In This Article
Summary
The present protocol describes a reconfigurable maze, a unique system for testing spatial navigation and behavioral phenotypes in rodents. The adaptability of this maze system enables the execution of various experiments in a single physical environment. The ease of structural rearrangement generates reliable and reproducible experimental results.
Abstract
Several maze shapes are used to test spatial navigation performance and behavioral phenotypes. Traditionally, each experiment requires a unique maze shape, thus requiring several separate mazes in different configurations. The maze geometry cannot be reconfigured in a single environment to accommodate scalability and reproducibility. The reconfigurable maze is a unique approach to address the limitations, allowing quick and flexible configurations of maze pathways in a repeatable manner. It consists of interlocking pathways and includes feeders, treadmills, movable walls, and shut-off sensors. The current protocol describes how the reconfigurable maze can replicate existing mazes, including the T-shaped, plus-shaped, W-shaped, and figure-eight mazes. Initially, the T-shaped maze was constructed inside a single experimental room, followed by modifications. The rapid and scalable protocol outlined herein demonstrates the flexibility of the reconfigurable maze, achieved through the addition of components and behavioral training phases in a stepwise manner. The reconfigurable maze systematically and precisely assesses the performance of multiple aspects of spatial navigation behavior.
Introduction
Spatial navigation is a fundamental ability of an animal to identify a suitable route to a targeted goal. Various cognitive processes, such as decision-making, learning, and memory, are needed during navigation. Utilizing these processes permits experiential learning when determining the shortest route to a goal. Maze tests are used to investigate the behavioral and physiological mechanisms of spatial navigation1. For example, the T-shaped maze2,3, plus-shaped maze4, radial arm maze5,6, and figure-eight maze7 assess spatial navigation behavior, including cognitive variables such as decision-making8 and anxiety9.
Each maze shape has advantages and disadvantages, requiring multifaceted experiments using multiple maze tasks to assess specific learning and memory10,11. For example, the spontaneous alternation task, in which an animal selects between the left and right arm without requiring learning, is a typical spatial working memory task that can be assessed with the T-shaped and Y-shaped mazes12. The plus-shaped and radial arm mazes, which use head direction and external cues, are used to determine goal-oriented navigation ability13. The figure-eight and modified T-shaped mazes, which separate the routes on selection and return, are used to evaluate spatial working memory tasks by analyzing the navigation function by trajectory14,15.
It can be challenging to maintain consistency among mazes when using several mazes in one experiment. Rodents are thought to use visual cues for navigation16,17,18; olfactory19,20 and somatosensory21 modalities may also be used for spatial cognition and may contribute to navigation ability. If a series of maze experiments are conducted using different spaces, layouts, dimensions, and materials, these variables may influence the navigation strategy of the rodents. Spatial navigation studies require the strictest control possible of these variables; however, maintaining a standardized maze apparatus for various shapes or rebuilding the maze for each experiment can be costly. These difficulties prevent a systematic way of conducting a series of experiments within the same laboratory.
To combat configured limitations in previously established maze structures, a maze system that can be configured in various shapes in a single physical environment22 is described here. The "reconfigurable maze" combines standardized parts, providing a highly repeatable, reproducible, flexible, and scalable testing environment. This article describes the ability of a reconfigurable maze to evaluate spatial navigation in rodents.
Protocol
All procedures were approved by the Doshisha University Institutional Animal Care and Use Committees. Three male Long-Evans rats, aged between 24 and 28 weeks (at the start of behavioral training), with body weights of 300-350 g, were used for the present study. The rats were housed individually in home cages (20 cm x 25 cm x 23 cm) on a 12 h light/12 h dark schedule, with the light period starting at 08:00 am. The animals were obtained from a commercial source (see Table of Materials).
1. Maze system components
NOTE: The maze system (including all the components, steps 1.1-1.5) (see Table of Materials) must be mounted in a shielded room covered with copper mesh (4 m x 5 m for rats and 1.8 m × 3.0 m for mice) for simultaneous use of electrophysiological neural activity recording. The maze needs to be elevated at a fixed height from the floor (55 cm for rats and 34 cm for mice).
- Punching board
- Place the aluminum punching board on the shield room floor (dimensions of the punching board: 360 cm x 480 cm x 1.2 cm for rats; 160 cm x 160 cm x 1.2 cm for mice) (Figure 1F,G).
NOTE: The experimenter can stand on the board. - Equip the punching board with a grid of equally spaced holes (for both rats and mice, 25 mm hole spacing and 6 mm hole diameter) (Figure 2C).
NOTE: These holes enable the placement of highly repeatable mazes (Figure 2D).
- Place the aluminum punching board on the shield room floor (dimensions of the punching board: 360 cm x 480 cm x 1.2 cm for rats; 160 cm x 160 cm x 1.2 cm for mice) (Figure 1F,G).
- Tower with baseplate
- Develop a tower with a baseplate made of aluminum to form pathways of a fixed height (the dimensions of the stem part of the tower are 55 cm × 6 cm × 2 cm for rats and 34 cm × 1.3 cm × 1.3 cm for mice) (Figure 1A).
- Use the baseplate to fix the position of the maze parts (the dimensions of the baseplate are 18 cm × 11 cm × 0.5 cm for rats and 12 cm × 7 cm × 0.3 cm for mice).
- Equip the baseplate with protrusions to connect a grid of equally spaced holes in the punching board (the protrusion diameter is 6 mm) (Figure 2B).
- Use the holes to connect components such as feeders, movable walls, and treadmills (see Table of Materials) equipped with towers with baseplates.
NOTE: For rats, the baseplate had four protrusions (length of 8 mm) (Figure 1F) inserted into the holes in the punching board. For mice, the baseplate was too light to support the pathway, so bolts were inserted into the holes (bolt lengths were 14 mm) (Figure 1G).
- Maze pathway
NOTE: The commercially available pathway (49 cm × 10 cm for rats and 39 cm × 5 cm for mice) was made of polyvinyl chloride (thickness of 5 mm for rats and 3 mm for mice) (see Table of Materials).- Construct the smallest part of the maze by placing the pathway in the upper part of the tower (Figure 1B).
- Design the upper part of the tower to conform the dimensions of bottom side of the pathway (dimensions of the upper part of the tower are 48 cm × 8 cm × 1 cm for rats and 21.9 cm × 3.9 cm × 0.3 cm for mice). To fix the pathway to the tower, place it on top.
- Provide side barriers made of polyvinyl chloride to prevent animals from falling (45 mm for rats and 30 mm for mice).
NOTE: Several patterns are available for connecting the pathways in various ways, such as parts with only one side barrier removed. 3D models of the pathway parts are available (https://github.com/TakahashiLab/ReconfigurableMazeParts) and can be printed using a 3D printer (see Table of Materials).
- Accompanying parts
NOTE: The parts required for behavioral experiments can be implemented by attaching a common baseplate with the pathway.- Place feeders on the side of any pathway to change the site of the reward (Figure 1C).
NOTE: Animals poking the feeders are detected by the shut-off sensors (see Table of Materials). - Place movable walls in the gaps between the pathways to force animals to guide the direction of movement (Figure 1D).
NOTE: For rats, when the movable wall is raised, the height of the wall is 90 cm from the floor and 29.5 cm from the side barriers of the pathway. When the movable wall is lowered, the height of the wall is 54 cm from the floor and -5.5 cm from the side barriers of the pathway. For mice, when the movable wall is raised, the height of the wall is 55 cm from the floor and 17 cm from the side barriers of the pathway. When the movable wall is lowered, the height of the wall is 35 cm from the floor and -3 cm from the side barriers of the pathway. - Place treadmills with pathways to force running delays at fixed positions (Figure 1E).
- Place feeders on the side of any pathway to change the site of the reward (Figure 1C).
- Control box
NOTE: Control each part automatically via the control box (Figure 1H) (see Table of Materials).- Use a microcontroller to receive signals from the treadmills and feeders via the control box.
NOTE: The shut-off sensor on the feeder and the number of treadmill rotations can be detected. - Use a microcontroller to send activation signals to the treadmills, feeders, and movable wall actuators according to a set task schedule via the control box. Individually control the dispensing and discarding of pellets, and the raising and lowering of the movable wall.
- Use a microcontroller to receive signals from the treadmills and feeders via the control box.
2. Evaluation of special navigation of rodents in the reconfigurable maze
NOTE: An animal behavior experiment was conducted using the reconfigurable maze (developed in step 1).
- Example construction of a maze
NOTE: An example of how to assemble a T-shaped maze for rats used in the delayed-alternation task experiment is provided in Figure 3.- Insert towers with baseplates into the punching board to form a T-shaped framework (Figure 3A).
- Attach pathways to the upper part of the towers (Figure 3B).
- Replace the pathway in the delayed area with a treadmill (Figure 3C).
NOTE: The treadmill can be replaced by a pathway of the same height and length. - Attach feeders to each edge of the maze (Figure 3D).
- Attach movable walls to the left and right branches (Figure 3E).
NOTE: Ensure the animal's paw and tail do not get caught in the gaps between sections.
- Animals
- Ensure the body weight of the rats remains between 300 and 350 g, and conduct all behavioral experiments during the daytime.
- Task execution
- Start-up and connect the control box, microcontroller, and PC.
- Write a program to set up the task schedule and receive the parameters needed for the experiment.
- Write the program to the microcontroller and execute a task.
NOTE: The example of a set task schedule written in C using a microcontroller board is available in a public repository (https://github.com/TakahashiLab/ReconfigurableMazeExample).
- Behavioral experiment
- Construct the desired maze shape (step 2.1).
- Move the rats from the home cages and place them in the arbitrary position of the maze.
- Allow the rats to freely explore the constructed maze for 10 min to habituate.
- Set up a program to perform the delayed alternation task with the treadmill23,24.
NOTE: The parameters required for the experiment can be obtained automatically by the program settings (e.g., number of poking times, duration of the experiment, treadmill speed, etc.). - Change the shape of the maze if necessary.
- Place the rats at the arbitrary position in the maze and execute the training or test of the delayed alternation task.
NOTE: In the present study, training sessions were conducted with a gradually increasing delay time and test sessions (with a 5 s delay time). - Return the rats to the home cage after each task.
- Wipe the maze thoroughly with 70% ethanol after each rat and wait at least 5 min before using the maze again.
NOTE: The parts of the pathway can be detached from the tower so that they can be thoroughly wiped clean of odors and dirt.
3. Behavioral performance and data analysis
- Animal trajectory
- Record animal behavior during the delayed alternation task with a ceiling-mounted digital video camera (see Table of Materials).
NOTE: By placing the camera on the ceiling, the experimenter can constantly record the animals' movements as they run around the maze during the task. - Track the running trajectories using markerless pose estimation software25 (see Table of Materials) based on images captured at 50 frames/s.
- Record animal behavior during the delayed alternation task with a ceiling-mounted digital video camera (see Table of Materials).
Results
Some parts of the reconfigurable maze used standard maze constructions described in previous studies3,4,7,26,27. Here, the linear track, T-shaped, W-shaped, and figure-eight mazes were reconfigured in the same physical environment (Figure 4A-D). To demonstrate that the reconfigurable maze could smoothly implement the desired behavioral test by gradual and rapid scaling, the protocol utilized for representative results included four training phases (Figure 5A).
In phases I and II, rewards were received by poking Feeder R after poking Feeder A. In phases III and IV, the reward was received by poking Feeder R after poking Feeders A and B, in that order. In phase IV, the poking of Feeder A triggered the rotation of the treadmill, and Feeder B could only be accessed after 5 s of forced running. In the test phase (delayed alternation task), the procedure was similar to that of phase IV, but Feeder R was in the arms at either edge of the T-shaped maze, and rats were rewarded by poking the opposite feeder from the previous phase. Rats were able to move in response to the length and shape of the extending pathway and changing feeder sites (Figure 5B). All phases were performed in 30 trials, with each trial defined as an instance of the rat reaching Feeder R. The task duration spent by the three rats completing 30 trials in each phase is shown in Figure 6A. Repeated measures ANOVA confirmed that the task completion time of rats differed among phases (F (4, 8) = 16.98, p < 0.05, Greenhouse-Geisser corrected28). The rats were able to adapt flexibly to changes in pathway length and reward conditions. In the test phase, which was conducted the following day, all rats asymptotically approached the high percentages of correct choice responses within 3 days (Figure 6B).
Several experimenters constructed the mazes to confirm that such a stepwise maze expansion could be performed rapidly (Figure 6C). In this article, the time of the accompanying parts (treadmill, feeders) were added to the morphing time of the pathway in the previous report22 in order to measure the maze construction time practically. Using the procedure for the delayed alternation task (Figure 5A), five experimenters changed the maze from the phase II shape to the test phase shape. The time converged to 67.80 ± 3.03 s (mean ± SE) on the third trial. The test included experimenters who had used this maze system for several years and those who had rarely used it.
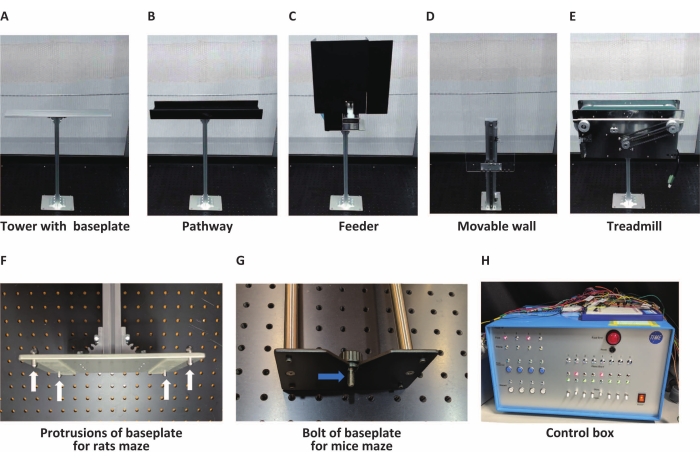
Figure 1: Elements of the reconfigurable maze. (A-E) Tower with baseplate and corresponding parts for rats. (F,G) The fixing method of the baseplate is different for rats and mice. Arrows indicate protrusions (white) and bolts (blue). (H) Signal input/output via the controller for fully automated tasks. Please click here to view a larger version of this figure.
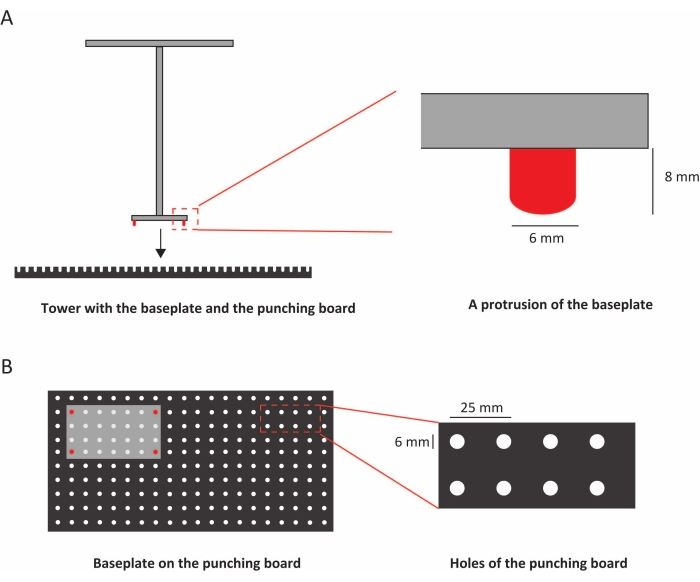
Figure 2: Connecting the punching board with the baseplate. (A) Side view of the baseplate, the punching board, and a close-up photo of a protrusion. (B) Top view of the baseplate and the punching board, and a close-up photo of the holes. Please click here to view a larger version of this figure.

Figure 3: Process of T-shaped maze assembly for the delayed alternation task. (A-E) Images of the reconfigurable maze taken from above. The images of the assembly process are in order from left to right. The red arrows indicate the positions of the newly assembled treadmill (C), feeders (D), and movable walls (E). Please click here to view a larger version of this figure.
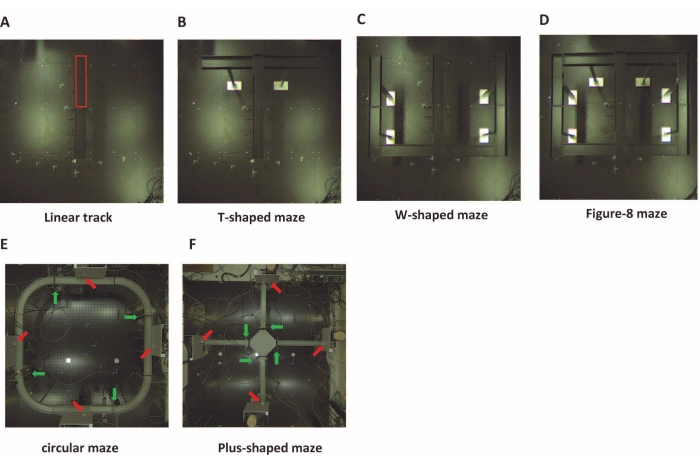
Figure 4: Several maze shapes in a single environment. Images of the reconfigurable maze. (A-D) Reconfigurable maze test for rats. The pathway parts were reconfigured into several shapes in a single environment, with reference to the location of the pathway parts enclosed in red in (A). (E-F) Reconfigurable maze test for mice. These mazes were placed with feeders (red arrows) and movable walls (green arrows) at any location. Please click here to view a larger version of this figure.

Figure 5: Maze expansion and trajectories of a rat. (A) The maze shape changes gradually during the train and test phases of the delayed alternation task. The type of feeder used in the task is indicated by a colored box. (B) Running trajectories of a representative rat. Each trajectory corresponds to the phase in (A). Please click here to view a larger version of this figure.
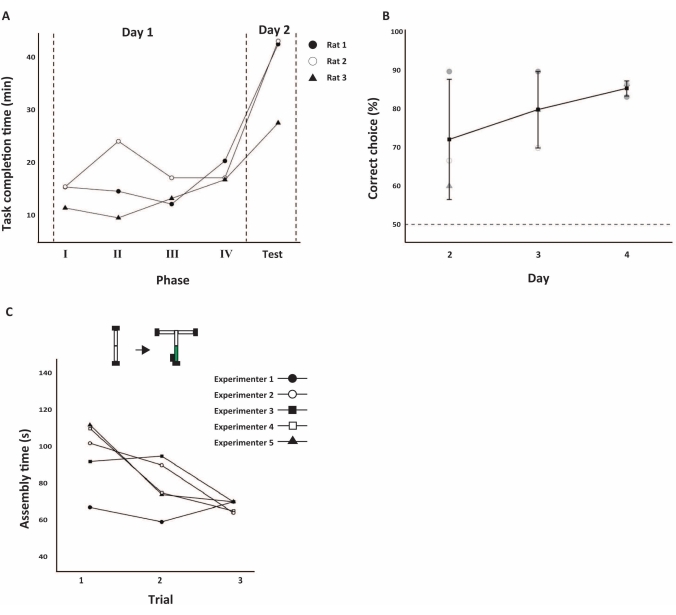
Figure 6: Performance of maze experiments. (A-B) The behavioral performance for 4 days, from the start of training to the end of the test. (A) Task completion time for each training phase and the first day of the test phase (n = 3). (B) The percentages of correct choice responses (mean ± SE) in the delayed alternation test. Dotted lines indicate chance levels. SE: standard error of the mean. (C) Reconfigurable maze assembly time. The linear track was modified into a T-shaped maze (top). The modification included the addition of pathways (white square), feeders (black square), and a treadmill (green square). Five experimenters performed three trials each (bottom). Before the test, the expert user (Experimenter 1) performed one trial as an example. All trials were performed on the same day. Please click here to view a larger version of this figure.
Discussion
The reconfigurable maze enabled us to conduct a variety of maze tasks in a single environment. Equally spaced holes on the floor and an interlocking system coordinated by towers with baseplates guaranteed a high degree of repeatability and reproducibility. In addition, the structure could be easily attached and detached, and the desired maze shape could be configured instantly, functioning as an efficient, flexible, and scalable system.
The reconfigurable maze allowed the animals to learn rapidly. In conventional maze experimental environments, it can be difficult to reconfigure the length and shape of the pathway, and conducting tests that combine multiple mazes is time-consuming. As demonstrated in this study, the reconfigurable maze enables maze extension in a step-by-step manner, where training post-modification of complex behavioral tests is conducted efficiently in a single day (Figure 6A,B). Furthermore, it is easy for the experimenter to make modifications. In this study, the maze assembly time was measured in multiple trials, and the experimenters consistently completed the reconstructions in about 1 to 2 min (Figure 6A).
A major advantage of this maze system is that it allows for fine-tuning the shape of the maze. Because the floor is filled with punching board holes, it is possible to perform flexible maze experiments that would be difficult to achieve with conventional maze systems. In the delayed alternation task performed in this study, the rats initiated the delay and exited the delay area by poking (Figure 5A). Placing two feeders nearby, as we have done here, is difficult in a conventional maze system with a fixed geometry. Additionally, this maze system enables counterbalanced modifications; for example, the position of Feeder B can easily be replaced on the opposite side (Figure 5A). This advantage also allows for the replication of maze configurations across laboratories. Several mazes are used for the delayed alternation task, including the figure-eight maze, the Y maze, and the W maze26,29,30. The reward zone, delay area, and delay method also differ from study to study23,31. With the reconfigurable maze, all of these different mazes can be created in a single physical environment and reproduced in different laboratories. If this system becomes widespread, it could lead to the standardization of maze tasks between laboratories.
The reconfigurable maze supports electrophysiological multiunit recordings, which examine the neural correlates that support spatial navigation22. In hippocampal formation, which is considered to play an essential role in spatial navigation, several types of cells have been reported to encode spatial information, such as cells that fire when passing a specific position32 or when approaching the boundary of the external environment33. These cell types change their firing activity based on alterations in distant landmarks16,17,18. This system is ideal for recording neural activity during spatial navigation experiments because the reconfigurable maze can change only the shape of the maze while maintaining the same environment. The reconfigurable maze maintains strict external environment control, a specification pertinent to neural activity experimentation.
The reconfigurable maze provides an optimal environment for maze experiments, with some caveats. First, the maze is constructed by fitting parts into holes in a punching board, so the angles cannot be changed flexibly. The circular maze (Figure 4E) overcomes this problem to a certain extent, but there are limitations to adding curves and angles to the pathway while ensuring the stability of the maze. In addition, some classical mazes, such as the Morris water maze34 and Barnes maze35, and mazes developed in recent years such as the honeycomb maze36,37, are difficult to construct by combining parts of the reconfigurable mazes. Future efforts should focus on exploring methodologies to merge these maze types with the reconfigurable maze to increase adaptability and cover more cognitive experimentation.
Disclosures
S.T. is an inventor of an examined Japanese patent application publication (No. P7137179, applicant: Doshisha University) pertaining to the reconfigurable maze. F.S., K.I., H.A., and Y.T. declare no conflicts of interest.
Acknowledgements
This work was supported by the Japanese Society for the Promotion of Science, Kakenhi grants 16H06543 and 21H05296 to S.T.
Materials
| Name | Company | Catalog Number | Comments |
| 3D printer | Stratasys Ltd. | uPrint | |
| Arduino Mega 2560 R3 | Elegoo | JP-EL-CB-002 | |
| Camera | Basler | acA640-750uc | |
| Control box | O’Hara & Co., LTD. / Amuza Inc. | FMM-IF | |
| DeepLabCut | Mathis laboratory at Swiss Federal Institute of Technology in Lausanne | N/A | |
| Feeder unit | O’Hara & Co., LTD. / Amuza Inc. | FM-PD | |
| Free maze system for mice | O’Hara & Co., LTD. / Amuza Inc. | FM-M1 | |
| Free maze system for rats | O’Hara & Co., LTD. / Amuza Inc. | FM-R1 | |
| Long-Evans Rat | Shimizu Laboratory Supplies, Co. LTD. | N/A | |
| MATLAB | MathWorks | Matlab2020b | |
| Movable wall for mice | O’Hara & Co., LTD. / Amuza Inc. | FMM-DM | |
| Movable wall for rats | O’Hara & Co., LTD. / Amuza Inc. | FMR-DM | |
| Pathway and tower for mice | O’Hara & Co., LTD. / Amuza Inc. | FMM-SS | |
| Pathway and tower for rats | O’Hara & Co., LTD. / Amuza Inc. | FMR-SS | |
| Pellet dispenser | O’Hara & Co., LTD. / Amuza Inc. | PD-020D/PD-010D | |
| Photo beam sensors unit for rats | O’Hara & Co., LTD. / Amuza Inc. | FMR-PS | |
| Punching board for mice | O’Hara & Co., LTD. / Amuza Inc. | FMM-ST | |
| Punching board for rats | O’Hara & Co., LTD. / Amuza Inc. | FMR-ST | |
| Treadmill for rats | O’Hara & Co., LTD. / Amuza Inc. | FMR-TM |
References
- Olton, D. S. Mazes, maps, and memory. American Psychologist. 34 (7), 583-596 (1979).
- Small, W. S. Experimental Study of the Mental Processes of the Rat. The American Journal of Psychology. 12 (2), 206-239 (1901).
- Jaffard, R., Dubois, M., Galey, D. Memory of a choice direction in a T maze as measured by spontaneous alternation in mice: Effects of intertrial interval and reward. Behavioural Processes. 6 (1), 11-21 (1981).
- Pellow, S., Chopin, P., File, S. E., Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods. 14 (3), 149-167 (1985).
- Olton, D. S., Collison, C., Werz, M. A. Spatial memory and radial arm maze performance of rats. Learning and Motivation. 8 (3), 289-314 (1977).
- Olton, D. S. The radial arm maze as a tool in behavioral pharmacology. Physiology & Behavior. 40 (6), 793-797 (1987).
- Baeg, E. H., et al. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 40 (1), 177-188 (2003).
- Redish, A. D. Vicarious trial and error. Nature Reviews Neuroscience. 17 (3), 147-159 (2016).
- Walf, A. A., Frye, C. A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature Protocols. 2 (2), 322-328 (2007).
- Levin, E. D. Learning about cognition risk with the radial-arm maze in the developmental neurotoxicology battery. Neurotoxicology and Teratology. 52, 88-92 (2015).
- Crawley, J. N., Paylor, R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Hormones and Behavior. 31 (3), 197-211 (1997).
- d'Isa, R., Comi, G., Leocani, L. Apparatus design and behavioural testing protocol for the evaluation of spatial working memory in mice through the spontaneous alternation T-maze. Scientific Reports. 11 (1), 21177(2021).
- Gill, P. R., Mizumori, S. J. Y., Smith, D. M. Hippocampal episode fields develop with learning. Hippocampus. 21 (11), 1240-1249 (2011).
- Takahashi, S. Hierarchical organization of context in the hippocampal episodic code. eLife. 2, 00321(2013).
- Lipton, P. A., White, J. A., Eichenbaum, H. Disambiguation of overlapping experiences by neurons in the medial entorhinal cortex. The Journal of Neuroscience. 27 (21), 5787-5795 (2007).
- Muller, R. U., Kubie, J. L. The Effects of Changes in the Environment on the Spatial Firing of Hippocampal Complex-Spike Cells. The Journal of Neuroscience. 7 (7), 1951-1968 (1987).
- Knierim, J. J. Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. The Journal of Neuroscience. 22 (14), 6254-6264 (2002).
- Fyhn, M., Hafting, T., Treves, A., Moser, M. B., Moser, E. I. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 446 (7132), 190-194 (2007).
- Maaswinkel, H., Whishaw, I. Q. Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behavioural Brain Research. 99 (2), 143-152 (1999).
- Wallace, D. G., Gorny, B., Whishaw, I. Q. Rats can track odors, other rats, and themselves: implications for the study of spatial behavior. Behavioural Brain Research. 131 (1-2), 185-192 (2002).
- Carvell, G. E., Simons, D. J. Biometric analyses of vibrissal tactile discrimination in the rat. The Journal of Neuroscience. 10 (8), 2638-2648 (1990).
- Hoshino, S., et al. The reconfigurable maze provides flexible, scalable, reproducible, and repeatable tests. iScience. 23 (1), 100787(2019).
- Salz, D. M., et al. Time cells in hippocampal area CA3. The Journal of Neuroscience. 36 (28), 7476-7484 (2016).
- Kraus, B. J., et al. During running in place, grid cells integrate elapsed time and distance run. Neuron. 88 (3), 578-589 (2015).
- Mathis, A., et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nature Neuroscience. 21 (9), 1281-1289 (2018).
- Frank, L. M., Brown, E. N., Wilson, M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 27 (1), 169(2000).
- Gothard, K. M., Skaggs, W. E., McNaughton, B. L. Dynamics of mismatch correction in the hippocampal ensemble code for space: interaction between path integration and environmental cues. The Journal of Neuroscience. 16 (24), 8027-8040 (1996).
- Greenhouse, S. W., Geisser, S. On methods in the analysis of profile data. Psychometrika. 24 (2), 95-112 (1959).
- Kraus, B. J., Robinson, R. J., White, J. A., Eichenbaum, H., Hasselmo, M. E. Hippocampal "time cells": time versus path integration. Neuron. 78 (6), 1090(2013).
- Lenck-Santini, P. -P., Save, E., Poucet, B. Place-cell firing does not depend on the direction of turn in a Y-maze alternation task. European Journal of Neuroscience. 13 (5), 1055-1058 (2001).
- Pastalkova, E., Itskov, V., Amarasingham, A., Buzsáki, G. Internally generated cell assembly sequences in the rat hippocampus. Science. 321 (5894), 1322-1327 (2008).
- O'Keefe, J., Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research. 34 (1), 171-175 (1971).
- Lever, C., Burton, S., Jeewajee, A., O'Keefe, J., Burgess, N. Boundary vector cells in the subiculum of the hippocampal formation. The Journal of Neuroscience. 29 (31), 9771-9777 (2009).
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods. 11 (1), 47-60 (1984).
- Barnes, C. A. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. Journal of Comparative and Physiological Psychology. 93 (1), 74-104 (1979).
- Ormond, J., O'Keefe, J. Hippocampal place cells have goal-oriented vector fields during navigation. Nature. 607 (7920), 741-746 (2022).
- Wood, R. A., et al. The honeycomb maze provides a novel test to study hippocampal-dependent spatial navigation. Nature. 554 (7690), 102-105 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved