Method Article
In Vivo Functional Assessment of Rat Masseter Muscle Following Surgical Creation of a Volumetric Muscle Loss (VML) Injury
In This Article
Summary
Volumetric muscle loss (VML) injuries exceed endogenous regenerative ability, resulting in permanent functional deficits. Current VML research primarily focuses on limb and trunk muscles. To extend mechanistic studies of VML to craniofacial muscles, this article describes an in vivo method for longitudinal assessment of masseter muscle function pre- and post-VML injury.
Abstract
Volumetric Muscle Loss (VML) is prevalent in civilian and military populations and represents a debilitating skeletal muscle injury surpassing the body's natural regenerative capacity. These injuries disrupt not only muscle fibers but also nerves, blood vessels, and the extracellular matrix, overwhelming the regenerative capacity of skeletal muscle and leading to severe fibrosis and permanent deficiencies in muscle structure and function. Current clinical management has many limitations, and thus, research is ongoing to develop more effective therapeutic approaches. Notably, however, much of the preclinical emphasis on VML injuries has focused on limb and trunk muscles, with limited investigation into craniofacial muscles. Differences in developmental biology and regenerative capacity between craniofacial and limb/trunk muscles may provide crucial insights that drive more injury-specific VML treatment options. Moreover, evaluation of functional recovery is critical to establishing therapeutic efficacy. In this regard, in vivo testing of muscle contraction with percutaneous nerve stimulation is a minimally invasive method that allows for repeated functional assessment over the course of a study - in the same animal. In light of these considerations, this paper describes a method for the in vivo assessment of muscle function in the rat masseter muscle before and after a VML injury. This protocol is the first published instance to detail the creation and functional evaluation of a biologically relevant craniofacial VML injury in the rat.
Introduction
Traumatic and surgical injuries of soft tissue that involve underlying skeletal muscle remain one of the greatest challenges to tissue reconstruction for both civilians and wounded warriors1. In fact, nearly 20% of battlefield injuries also occur in the craniofacial regions of wounded warriors (head and face)2. Inclusively, extremity trauma and head and neck injuries account for >80% of combat injuries in recent conflicts2. Despite the rather well-known capacity of skeletal muscle to repair, regenerate, and remodel following injury, these more severe injuries, which involve the loss of a substantial portion of muscle tissue, are not capable of healing on their own and are referred to as Volumetric Muscle Loss (VML) injuries. By definition, VML results in permanent aesthetic and functional deficits of either the injured muscle or the muscle unit3.
Interestingly, despite the prevalence of craniofacial VML injury2,4, much of the research to date is focused on limb5,6,7,8 and trunk9,10,11 muscles, with only a handful of reports on VML injuries in craniofacial muscles12,13,14. This presents a potentially important translational research gap, as the extant literature suggests that there are significant differences in both developmental biology, as well as regenerative capacity between craniofacial and limb and trunk muscles15,16. In fact, there are more than 20 muscles on each side of the face — reflecting the fact that the craniofacial region is involved in performing many essential tasks as diverse as blinking, swallowing, and chewing15. Furthermore, even among craniofacial muscles, differences arise with respect to regenerative ability. For example, extraocular muscles appear to regenerate more rapidly than limb muscles17. In contrast, the masseter displays a slower regenerative response than the tibialis anterior (TA)18. These differences may be due, at least in part, to whether the origins of the muscles are branchiomeric or somite-derived — resulting in both different quantities of satellite cells, as well as distinct gene expression profiles of the resident satellite cell populations19. Consideration of the unique features among craniofacial muscles, as well as relative to limb and trunk muscles, may shed important mechanistic insight for improved therapeutic development of customized treatment options that address muscle-specific VML injuries. These muscle-specific differences may also explain known limitations of sheet-like muscles, such as the latissimus dorsi, as donor muscle tissue transfer options for head and neck reconstructive surgeries20,21,22.
Regardless of the location of the VML injury, there are currently no treatments that can restore full form and function. Gold standard clinical management includes autologous tissue transfer as well as rehabilitation; however, the former typically does not improve strength and can result in donor site morbidity3,23, while the latter improves range of motion, with minimal impact on functional recovery or the return of contractile strength, in the muscle1,6. For all these reasons, research is ongoing to develop more effective therapeutic approaches for the regeneration of functional muscle tissue following VML injury. A recent analysis highlighted the critical importance of preclinical studies in rat models of VML injury to advancing regenerative technology solutions for VML repair1.
As noted in prior work5,8,9,10,24,25, assessing functional recovery after VML is critical for evaluating the effectiveness of therapeutic interventions. Specifically, while wound healing and volume recovery are key features of VML repair, measuring force recovery after injury and treatment helps to shed light on the multiscale biomechanical mechanisms responsible for VML deficits, as well as those mechanisms driving functional recovery. As such, establishing a link between tissue repair, volume recovery, and enhanced muscle strength is an absolute requirement for identifying the most efficacious regenerative solutions for VML repair9.
In this regard, there are three main methods for making these functional measurements: (1) in vitro, (2) in situ, and (3) in vivo testing — with each offering its own advantages and disadvantages8. For example, in vitro testing involves the examination of isolated muscle function outside the living organism and provides an opportunity to more directly/specifically manipulate muscle stimulation with a variety of drugs and chemicals that affect muscle excitability and contraction26. Although this method allows for detailed investigations into cellular and molecular aspects of muscle function and recovery27, it removes blood supply and innervation, which limits how well it recapitulates true muscle function8,26. In situ testing assesses muscle function while the muscle is still in its natural anatomical location but isolated from the surrounding tissues25,28. While this method maintains innervation and blood supply, the separation of the muscle can still limit the applicability of findings to the in vivo environment. Additionally, and perhaps most importantly, both in situ and in vitro testing are terminal procedures that preclude longitudinal testing - which is necessary to permit tracking functional recovery over time on the same animal. In contrast, in vivo testing, which involves evaluating muscle function in its native environment, enables a more complete understanding of muscle performance in a physiological context29. In vivo studies are also the least invasive technique and can be performed repeatedly over the time course of a study29. Using percutaneous electrical nerve stimulation, one can collect functional measurements in specific muscles while leaving tendons, vasculature, and innervation intact30. These longitudinal assessments can provide insights into subtle, yet important, changes occurring during different stages of healing in the same animal. This method is commonly employed in small rodent models8,31,32,33; however, it has also been used in larger animal models of pig30 and dog34.
There are currently three published studies on the assessment of functional recovery following craniofacial VML injuries. Rodriguez et al. report on a method for repairing a craniofacial VML injury model in the ovine zygomaticus major muscle. Their method details an in situ testing protocol that can only be implemented at the terminal study time point, and thus, does not permit longitudinal assessment within a single animal13. Kim et al. describe a method for the use of nanomembrane electronics to track continuous electromyogram data of mouse mastication following a VML injury. They reported significantly lower signal from injured mice compared to control, however, they also noted that motion artifacts affected signal analysis and that the mice would occasionally try to scratch off the circuit. Additionally, athymic, nude mice were used to allow secure attachment of the nanomembrane to the skin, which currently prevents wider utilization of this method in more commonly used (and cost-effective) animal models12. Finally, Zhao et al. describe a critically sized VML defect in the mouse masseter, which impairs nutrition in the animals, as reflected in significantly decreased body weight gain, as a functional outcome14. With these studies in mind, this article describes a versatile and minimally invasive method for the in vivo longitudinal assessment of contractile function in the rat jaw before and after a VML injury via stimulation of the trigeminal nerve with subcutaneous electrodes. As the test runs through a series of increasing simulation frequencies (20-200 Hz), a transducer measures the generated force of the muscle. These measurements can allow for the determination of muscle stiffness and maximal tetanus, among others. The protocol below is designed to assess functional recovery of ipsilateral bite force following a traumatic VML injury and, as such, also includes a protocol for the surgical creation of a validated VML injury. This method can be easily implemented on a wide variety of rats and mice — and, with appropriate equipment modifications, is applicable to larger animal models and VML injuries as well30.
Protocol
All animal handling and procedures were approved and done in accordance with the University of Virginia Institutional Animal Care and Use Committee (IACUC) Guidelines. The rats used in these experiments were 12-14 weeks old male Lewis rats weighing 324.8 g ± 12.72 g at the time of surgery. Details of the reagents and equipment used are listed in the Table of Materials.
1. VML injury of masseter muscle
- Equipment preparation
- Prior to surgery, ensure that all the required instruments have been sterilized appropriately: surgical drapes, scalpel, forceps, microscissors, hemostats, sutures, and gauze.
- Power on a heated platform and set it to 37 ˚C.
- Apply sterile ophthalmic ointment to both eyes to prevent eye dryness under anesthesia. Put the animal in the anesthesia induction chamber and deliver 2%-3% isoflurane (following institutionally approved protocols).
- Surgical creation of VML Injury
- Ensure that the animal is properly anesthetized before removing it from the chamber.
NOTE: This can be determined if the animal is non-responsive to a toe pinch. - Weigh and place the animal onto a surgery board in the lateral position on its left side, with its nose securely in the nose cone to allow for continued administration of isoflurane.
- Prepare the animal for surgery by delivering the appropriate analgesic, shaving the right side of the face, and sterilizing the area through three changes of iodine and alcohol swabs. For these studies, extended-release buprenorphine was used for analgesia (0.65 mg/kg body weight).
- Begin the surgery by making an approximately 2 cm incision along the buccal region of the rat. The incision should be in line with the nose and ear of the animal. Use blunt dissection to separate the skin and fascia.
- Make a similar incision along the fascia and gently dissect it away from the underlying masseter.
- Locate the buccal and marginal nerves (Figure 1A). Utilize a sterile surgical marker and ruler to mark the area to be removed. The area should be a 10 mm x 5 mm rectangle in the center of the exposed masseter lying between the facial nerves (Figure 1B).
- Carefully use microscissors to begin tissue removal. Ensure that the final injury reaches a depth of approximately 4 mm with 150 mg of excised tissue (Figure 1C). The removed tissue will be from the full thickness of the superficial masseter and will go partially into the deep masseter.
NOTE: The average mass of tissue removed in these experiments was 146.1 mg ±1.16 mg. - Close the fascia and skin using absorbable (6-0) and non-absorbable (5-0) interrupted sutures, respectively.
- Ensure that the animal is properly anesthetized before removing it from the chamber.
- Cleaning up and monitoring
- After suturing, turn off the anesthesia gas and keep the animal on the heated surface to monitor its return to consciousness. Once the rat begins to regain consciousness, place the animal back in the cage and continue monitoring until it is awake and ambulatory.
- For 3 days following surgery, check on the animal and assess for any signs of pain or discomfort.
- For 7 days following surgery, provide the animal with soft food to reduce strain on the jaw and keep the animals well hydrated/fed.
- Remove the interrupted sutures 7-10 days after surgery, ensuring that the wound is fully closed prior to removal.
- Following the terminal time point of the study, euthanize animals according to IACUC guidelines. Explant masseter muscles and flash freeze by submerging in liquid nitrogen or liquid nitrogen-cooled isopentane.
NOTE: Frozen tissue samples can be stored long-term at -80 °C for future histologic assessment.
2. In vivo functional assessment of jaw
- Equipment preparation
- Check and ensure the proper connection of all equipment.
- In order, power on (1) computer, (2) bi-phase stimulator, (3) dual-mode lever system, and finally, (4) a heated platform to warm it to 37 °C.
NOTE: The user manual states a warmup time of 1 min for the stimulator and lever system to produce accurate reads. Additionally, allow 10-15 min for the platform to fully heat before testing any animals. - Apply sterile opthalmic ointment to both eyes to prevent eye dryness under anesthesia. Place the animal to be tested in the anesthesia induction chamber and supply 2%-3% isoflurane.
- Sterilize the polytetrafluoroethylene coated electrode tips by placing them in 70% ethanol.
- Locate and open the Dynamic Muscle Control (DMC) Software. This will be required to perform the functional assessment.
- Software setup
- In the DMC Software, find Instant Stim in the setup menu and change the parameters to the desired values. In this study, no parameters are changed from their presets (Figure 2A).
- Under the Setup menu, create and select an autosave folder to store the data.
- Near the bottom of the software screen, locate a box titled Autosave Base. Change this to a title specific to the animal being tested, for example, "rat#-timepoint" (Figure 2B).
- Select Sequencer at the top of the software screen. In the new window that opens, select Open Sequence at the bottom of the screen. Select the premade sequence in the file explorer that opens. This will populate the Sequence window with a list of parameters such as frequency, duration of stimuli, and rest time (Figure 2C).
- Click on Load Sequence followed by Close Window.
NOTE: The sequence used in this protocol consists of 9 steps (twitch, 20 Hz, 40 Hz, 50 Hz, 60 Hz, 80 Hz, 100 Hz, 150 Hz, 200 Hz) with 20 s rest between each. Aside from the twitch stimulation, all steps are 500 ms in duration. The sequence protocol should be adjusted to each lab's specific testing goals.
- Click on Load Sequence followed by Close Window.
- To open a new window and enable viewing of real-time data acquisition, select File > Live Data Monitor.
- In the Live Data Monitor window, set the timescale, y-value minimum, and y-value maximum manually or by checking the box to enable autoscale.
- Animal preparation
- Ensure that the animal is in the proper plane of anesthesia before removing it from the anesthesia chamber.
- Place the animal in the supine position with its nose securely in the nose cone to allow for continued administration of isoflurane. Place a securing loop over the upper half of the rat's jaw to hold its nose securely on the platform.
- Shave the neck and face of the animal on the experimental side of the jaw.
- Secure the upper half of the animal by placing and fastening a strap across its arms and chest.
- Adjust the bite lever position using the three rotating knobs located near the rig's platform to control the X, Y and Z planes (Figure 3A). Using the top knob, bring the bite lever down towards the animal and use the other two knobs to adjust the end of the lever to be just over the chin of the animal.
- Use tweezers to hook the hanging loop around the bottom teeth of the animal to secure the head and prevent it from moving (Figure 3B).
NOTE: The loop is located through a hole drilled in the bite lever 3 cm away from the force transducer. The system should be calibrated before first use. - Adjust the height of the lever to increase the tension on the jaw. The reproducible baseline tension used in these experiments was ~0.5 N.
- Electrode placement
- Palpate the jaw of the animal and locate the posterior corner of the mandible. Position electrodes subcutaneously around either side of the corner at approximately 2-3 mm apart. Electrodes should be inserted at a depth of 3-5 mm. Use an alligator clip and stand to hold electrodes in position.
- In the Live Data Monitor window, click on the large orange button labeled Instant Stim to activate it.
- The monitor should begin displaying upward spikes during each stimulation. Adjust electrode placement and lever arm position using the knobs as needed. Only activation of the ipsilateral side of the jaw should be observed (Figure 4).
- On the high-power bi-phase stimulator, there are two knobs near the center, labeled "Range" and "Adjust". Begin turning the Range knob to modulate the amperage. As the amperage increases, the instant stim peaks will begin increasing in magnitude until they plateau - determined as the level at which three consecutive stimulations result in identical contractile responses.
- Avoid turning the amperage too high, that is, no more than 20% above the amperage where maximum peaks were observed — as this can result in the recruitment of neighboring muscles and inaccurate force readings.
- Turn the Adjust knob to modulate the percentage of the current "Range" being used to stimulate the jaw. An increase or decrease in current may be required to optimize the twitch response.
- Confirm that the electrodes are still securely in place and stop the instant stim.
- Below the orange "Instant Stim" button in the live data monitor, locate the grey Start Sequence button and click on it.
- Continue to monitor the curves throughout the duration of the stimulation protocol. As the stimulation frequency increases, so will the maximum force produced. Once tetanus is reached, the force curves will plateau (Figure 5A).
- Cleaning up
- Following completion of the functional stimulation sequence, remove electrodes and wipe them clean with 70% ethanol. If this is the last animal to be tested, the electrodes can be placed back in their covers.
- Turn off the anesthesia gas but keep the animal on the heated surface to monitor its return to consciousness. Once the rat begins to regain consciousness, place it back in the cage and continue monitoring until the animal is awake and ambulatory.
- Turn off the equipment used for data acquisition and wipe down all surfaces.
3. Data analysis
NOTE: This method has been previously described as documenting a method for the in vivo functional testing of the rat TA8. Data analysis is designed to determine the intentions of this study, and protocols may change depending on the goals of the user.
- Locate and open the Dynamic Muscle Analysis software.
- Click on the High Throughput menu and select Force Frequency Analysis to analyze multiple samples simultaneously.
- Select Pick Files and highlight as many data files as desired for analysis. The "Pick Folder" button can also be used if samples are clearly named within the file system.
- Check the box to Remove Baseline. This will automatically remove the baseline force from the maximum force recorded for each trial, providing the absolute maximum values.
- Click on the Analyze button and then click on Export Table to Excel where the data can be saved as a spreadsheet. Ensure that the "Start Cursor" and "End Cursor" values accurately capture the time stamp of the stimulation. These values can be manually set if needed.
- Open the saved spreadsheet.
NOTE: Using the "Maximum" column, the user can calculate the maximum force produced across all frequencies. The force-frequency curve can also be generated (Figure 5).
Results
As described in a prior publication, the tetanic curves can be used to distinguish optimal results from sub-optimal results8. An ideal result is obtained when the muscle is stimulated to its maximal force and maintains that maximum throughout the duration of the tetanic contraction. Summation of the individual twitches into tetanic curves will usually start appearing at, or after, 100 Hz. Figure 5A demonstrates this point as the ideal curve at 150 Hz has a sharp upswing at the start of stimulation, a flat plateau phase at the maximum force value with minimal oscillation, and a sharp, vertical downswing when stimulation ceases. Non-ideal tetanic curves may show oscillations during the plateau phase and may display a negative or positive slope (Figure 5C,D).
The results of this functional testing protocol may be represented differently depending on the goals of the researchers and the experimental design for the study. In the case of this protocol, the absolute maximum force is empirically determined from all frequencies of stimulation and graphed at each timepoint for testing. This allows for comparing baseline maximum force production to maximum force production every 4 weeks after the creation of the described VML injury in the masseter (Figure 1D). For comparison, a cohort of uninjured animals (n = 4) was also tested at each time point (Figure 1E). The animal weights at each time point were also compared (Figure 1F). At 4, 8, and 12 weeks post-VML, respectively, the rats produced an average maximum force of 7.958 N ±1.797 N, 7.183 N ±1.450 N, and 7.823 N ± 0.626 N. One-way Repeated Measures Analysis of Variance (ANOVA) with Fisher's Least Significant Difference (LSD) post hoc pairwise comparisons determined that there were no statistically significant differences among these values at any timepoint post-VML injury, however, they were all significantly different from the average baseline force of 10.031 N ±0.564 N.
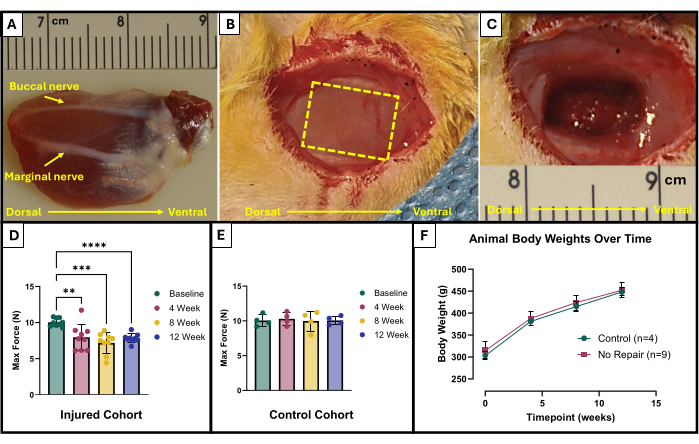
Figure 1: Surgical schematic and functional results for VML-injured masseter muscle. Explanted masseter muscle showing anatomic orientation as well as nerve landmarks for injury creation (A). Animal during surgery before (B) and after (C) VML injury creation. Images are all presented with muscles in the same orientation as (A). The yellow dashed line indicates the region where the muscle is to be excised. Maximum force production at baseline and over time in rats that received a VML injury (n = 9) to the masseter (D) as well as for age-matched control rats that were left uninjured (n = 4) (E). A graph of rat body weights over time (F). (D) represents a one-way Repeated Measures ANOVA with Fisher's LSD post hoc pairwise comparisons, where **= p < 0.01, ***= p < 0.001, ****= p < 0.0001. Rulers in (A) and (C) are in centimeters, with graduations in millimeters. Please click here to view a larger version of this figure.
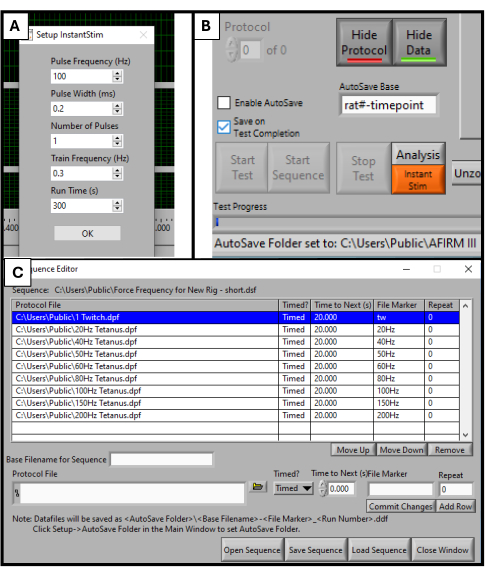
Figure 2: Software initialization for functional testing. Parameter set up for the DMC software. Setting up Instant Stim to desired values (A). Software Graphic User Interface and location of AutoSave Base setting box (B). Selection of 9-step protocol sequence used for testing (C). The values displayed here are example values used for this study, but they may need to be optimized and adjusted depending on the specific use cases of others. Please click here to view a larger version of this figure.
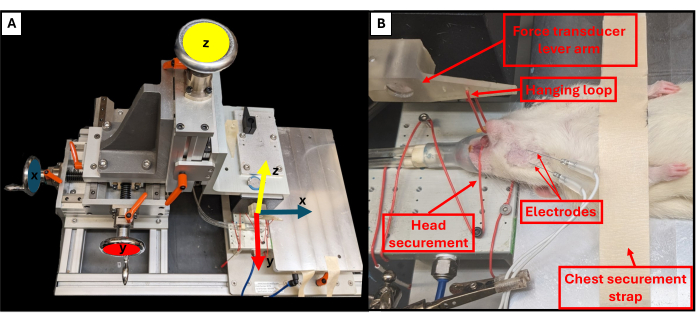
Figure 3: Functional rig axes of motion and proper animal positioning. Schematic illustrates the relationship between each of the three adjustment wheels on the functional testing rig and their corresponding axes of movement for the lever arm (A). Example image of a rat undergoing functional testing, showing the proper supine positioning of the animal on the platform and other important rig components (B). Please click here to view a larger version of this figure.
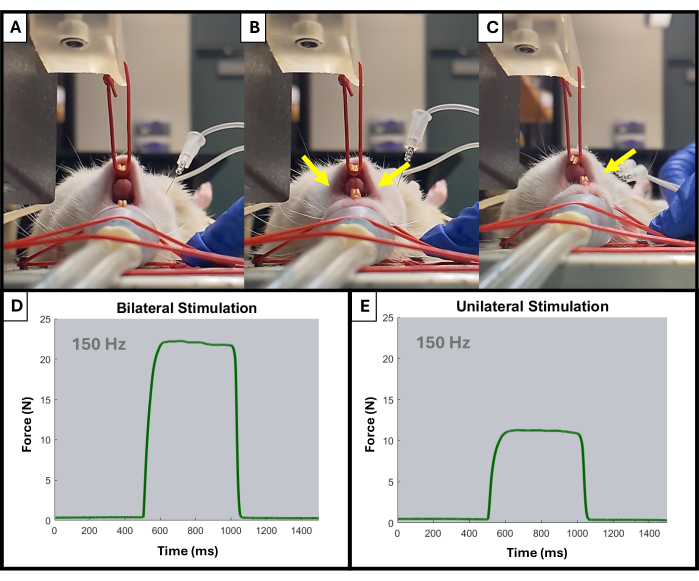
Figure 4: Example of unilateral and bilateral jaw activation during testing. (A) shows the rat's jaw at rest prior to any stimulation. Electrodes placed improperly can result in a bilateral jaw contraction, as indicated by the yellow arrows (B), while properly placed electrodes will result in the desired ipsilateral contraction (C). Yellow arrows indicate points of contraction in the jaw. Also shown are example force-time curves at 150 Hz for bilateral (D) and unilateral (E) muscle activation. Please click here to view a larger version of this figure.
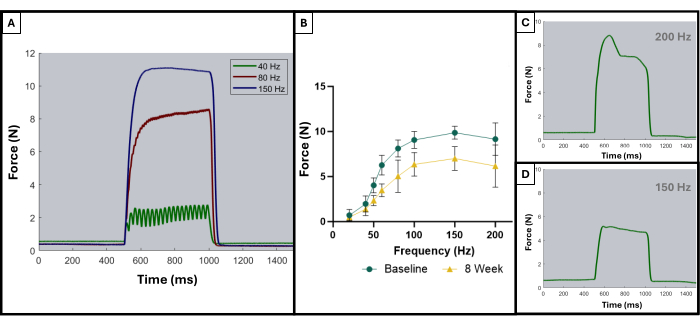
Figure 5: Representative stimulation-response curves for acceptable and unacceptable trials. Example stimulation curves at 40, 80, and 150 Hz (A) to demonstrate what to look for leading up to and during a tetanic contraction. As the stimulation frequency increases, the peak tension recorded is also higher, which can be observed in the force-frequency curves (B) showing the average of the injured animals (n = 9) at baseline and at 8 weeks after VML creation. (C,D) demonstrate representative examples of the shape of a force response that would indicate the need for electrode adjustment due to improper tetanus. Please click here to view a larger version of this figure.
Discussion
This protocol describes a straightforward and reliable method for the in vivo functional testing of the ipsilateral bite force produced in the rat jaw. Also described is a method for the surgical creation of a VML injury in the rat masseter muscle. In combination, these methods provide a biologically relevant animal model to conduct a longitudinal evaluation of functional recovery before and after a traumatic craniofacial injury. While other methods of testing, such as in vitro and in situ, can also provide valuable information, they necessitate the isolation of the tendon and removal of the tissue from its native environment, limiting their applicability to better understanding in vivo functional outcomes8,25,26,27,28. With proper training and practice, a researcher will be able to position an animal quickly for testing and make the necessary adjustments to achieve maximal muscle force production.
There are technical details that should be verified for each animal to ensure proper electrode placement and stimulation. First, the upper incisors should be secured so that the rat's head is fully in contact with the heated platform. This will also keep the rat's nose placed in the nose cone for continuous administration of inhaled anesthetic. If the head is not kept in the proper position, there will be inconsistencies in the force readings as the baseline tension deviates. Additionally, the animal's body should be supine, and its spine should be straight. Keeping the animal in the proper position will help with electrode placement and maintenance in the correct location. Finally, the placement of the electrodes can be difficult, as they must be placed at the proper depth and spacing to stimulate only the experimental side of the jaw. Vigilance and practice are pertinent to becoming adept at electrode placement for reproducible and reliable results. The electrodes will become dull rather quickly, so it is necessary to change them often. The hallmarks of poor electrode placement are activation of accessory muscles, positively/negatively sloped force readings (movement of electrodes during stimulation), or an unfused/oscillating tetanic waveform8 (Figure 5C,D).
Furthermore, there are a few limitations to this method that warrant mentioning and considering in future applications of this system. Firstly, while sufficient for the measurements made in this study, the loop used to secure the upper jaw may not be the most rigid system. Instead, a stereotaxic approach using ear bars could create a better hold of the rat's upper jaw. Additionally, a passive tension value was used as the set position for testing with this method, which may not result in the maximal force generation that would be obtained if the muscle were positioned at its optimal length (Lo). In fact, the Lo could be determined by positioning the animal as described herein and then manipulating jaw occlusion in small (0.5-1 mm) increments while running twitch stimulations - with Lo determined as the optimal testing position where the maximum twitch force was generated. However, the masseter muscle has a rather unique geometry and locomotion35,36, and, therefore, operates over a much wider range of lengths than characteristic of, for example, limb muscles (which typically operate around Lo). However, chewing happens in a narrow range compared to biting. As such, there is no perfect approach to this problem. So, even though setting a baseline tension is not the only way to approach this problem, there is a logical scientific rationale for doing so, as the optimal length of the jaw muscles is beyond the natural opening of the jaw. Regardless, maximal force at a given passive tension still gives important insight into aspects of function - even if they are different from maximal force at Lo. Clearly, further investigation of the force-length relationship of the masseter muscle is warranted. Finally, this method looks at the stimulation of the trigeminal nerve in the rat, which is divided into three branches: V1-V3. While V3, or the mandibular nerve, is the only branch with a motor component, it does innervate multiple muscles, including the masseter, temporalis, pterygoids, and mylohyoid37. This is important to consider as it means this method does not exclusively report masseter force, which may be relevant to other research investigations.
Despite these technical aspects and limitations, this method proves to be robust and consistent. While only ipsilateral testing is described herein, the protocol could be easily modified to include additional testing of the other side of the jaw. Bilateral testing of masseter contraction/function would permit healthy/injured comparisons to be made within a single animal, perhaps elucidating important compensatory changes on the injured and/or control side (uninjured side). Overall, this model provides a powerful tool for assessing longitudinal muscle function, as well as functional recovery, in a minimally invasive way. As such, this system will be applicable to evaluating biomechanical mechanisms that attend VML-mediated functional deficits, as well as for testing the efficacy of various therapeutics for the repair of VML injuries in the craniofacial region.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Myologica LLC for sharing the equipment used to perform functional assessments. Research reported in this publication was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under award number U24 DE029463. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Materials
| Name | Company | Catalog Number | Comments |
| 200 proof ethanol | Decon Labs | Diluted to 70% with deionized water | |
| 25 mm x 27 G monopolar needle electrodes | Chalgren Enterprises | 111-725-24TP | Used to perform functional measurements |
| Alcohol Swabs | BD | 326895 | For sterilzation/cleaning |
| Alligator Clip and Stand/ Helping Hand Sodering Aid | Eclipse Enterprise | 115584 | Holding electrodes in place |
| Bead Sterilizer | 18000-45 | For surgery (resterilizing tools between animals) | |
| Bi-phase Electrical Stimulator | Aurora Scientific | 701C | Deliver electric impulses to animals during function testing |
| Bite lever | Custom | Cuttomized 3D printed (please contact the authors for details) | |
| Compressed Oxygen Gas | Praxair | UN1072 | |
| Cotton tipped aplicators | Fisher | 22363157 | For surgery |
| Dual Mode Muscle Lever System | Aurora Scientific | 309C | Used to perform functional measurements |
| Dynamic Muscle Data Acquisition and Analysis System | Aurora Scientific | 615A | Used to collect and analyze functional measurements |
| Face mask | High Five | AM101 | For surgery |
| Forceps | Integra Miltex | 6-100 | For surgery |
| Gauze | Medline | PRM21408C | For surgery |
| Hair Clippers | Phillips | MG3750 | Fur removal |
| Hairnet | VWR | 75829-204 | For surgery |
| Isoflurane | Covetrud | 29405 | |
| Isoflurane evaporizer funnel fill | Vet Equip | 911103 | |
| Isoflurane Vaporizer | Kent Scientific | VetFlo-1231 | Delivery of anesthesia |
| Large Rodent/Small Animal Apparatus | Aurora Sceintific | 807B | Used with 309C motor for functional measurements |
| Microscissors | FST | 91500-09 | For surgery |
| Needle Driver | FST | 1200-13 | For surgery |
| Povidone-Iodine | Medline | MDS093943 | For sterilzation/cleaning |
| Prolene Sutures 5-0 | Ethicon | 8698G | Suturing skin |
| Scalpel | Personna Medical | 73-8030 | For surgery |
| Scalpel Blade | Glass Van | 1834 | For surgery |
| Surgical drapes - ACT material | N/A | N/A | cut to 8 x 11 in and autoclaved prior to surgery |
| Surgical Gloves | Encore | 5711103PF | For surgery |
| Surgical gown | VWR | 414004-467 | For surgery |
| T/Pump Heating/Cooling Pump | Braintree Scientific, Inc | TP-700 | Heating animal platforms for surgery and function testing - set to continuous therapy time at 38/100 temperature |
| VaporGuard Activated charcoal filter | Vet Equip | 931401 | |
| Vicryl Sutures 6-0 | Ethicon | J492G | Suturing fascia |
References
- Kulwatno, J., Goldman, S. M., Dearth, C. L. Volumetric muscle loss: A bibliometric analysis of a decade of progress. Tissue Eng Part B: Rev. 29 (3), 299-309 (2023).
- Owens, B. D., et al. Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 64 (2), 295-299 (2008).
- Grogan, B. F., Hsu, J. R. Volumetric Muscle Loss. J Am Acad Orthop Surg. 19, S35-S37 (2011).
- Lew, T. A., Walker, J. A., Wenke, J. C., Blackbourne, L. H., Hale, R. G. Characterization of craniomaxillofacial battle injuries sustained by United States service members in the current conflicts of Iraq and Afghanistan. J Oral Maxillofac Surg. 68 (1), 3-7 (2010).
- Dienes, J., et al. Semisynthetic hyaluronic acid-based hydrogel promotes recovery of the injured tibialis anterior skeletal muscle form and function. ACS Biomater Sci Eng. 7 (4), 1587-1599 (2021).
- Aurora, A., Garg, K., Corona, B. T., Walters, T. J. Physical rehabilitation improves muscle function following volumetric muscle loss injury. BMC Sports Sci Med Rehabil. 6, 41 (2014).
- Wu, X., Corona, B. T., Chen, X., Walters, T. J. A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies. BioRes Open Access. 1 (6), 280-290 (2012).
- Mintz, E. L., Passipieri, J. A., Lovell, D. Y., Christ, G. J. Applications of in vivo functional testing of the rat tibialis anterior for evaluating tissue engineered skeletal muscle repair. J Vis Exp. 116, e54487 (2016).
- Passipieri, J. A., et al. In silico and in vivo studies detect functional repair mechanisms in a volumetric muscle loss injury. Tissue Eng Part A. 25 (17-18), 1272-1288 (2019).
- Corona, B. T., et al. Further development of a tissue-engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng Part A. 18 (11-12), 1213-1228 (2012).
- Chen, X. K., Walters, T. J. Muscle-derived decellularised extracellular matrix improves functional recovery in a rat latissimus dorsi muscle defect model. J Plast Reconstr Aesthet Surg. 66 (12), 1750-1758 (2013).
- Kim, H., et al. Real-time functional assay of volumetric muscle loss injured mouse masseter muscles via nanomembrane electronics. Adv Sci (Weinh). 8 (17), 2101037 (2021).
- Rodriguez, B. L., et al. A tissue engineering approach for repairing craniofacial volumetric muscle loss in a sheep following a 2, 4, and 6-month recovery. PLoS One. 15 (9), e0239152 (2020).
- Zhao, N., et al. A critical size volumetric muscle loss model in mouse masseter with impaired mastication on nutrition. Cell Prolif. 57 (6), e13610 (2024).
- Cheng, X., Shi, B., Li, J. Distinct embryonic origin and injury response of resident stem cells in craniofacial muscles. Front Physiol. 12, 690248 (2021).
- Noden, D. M., Francis-West, P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 235 (5), 1194-1218 (2006).
- Stuelsatz, P., et al. Extraocular muscle satellite cells are high performance myo-engines retaining efficient regenerative capacity in dystrophin deficiency. Dev Biol. 397 (1), 31-44 (2015).
- Pavlath, G. K., et al. Heterogeneity among muscle precursor cells in adult skeletal muscles with differing regenerative capacities. Dev Dyn. 212 (4), 495-508 (1998).
- Ono, Y., Boldrin, L., Knopp, P., Morgan, J. E., Zammit, P. S. Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev Biol. 337 (1), 29-41 (2010).
- Aladimi, M. T., et al. Factors to consider when deciding on the type of free-flap reconstruction of head and neck soft tissue defects. ORL J Otorhinolaryngol Relat Spec. 79 (4), 230-238 (2017).
- Maxwell, G. P., Leonard, L. G., Manson, P. N., Hoopes, J. E. Craniofacial coverage using the latissimus dorsi myocutaneous island flap. Ann Plast Surg. 4 (5), 410-421 (1980).
- Strübing, F., et al. Scalp reconstruction using the latissimus dorsi free flap: A 12-year experience. J Clin Med. 12 (8), 2953 (2023).
- Carnes, M. E., Pins, G. D. Skeletal muscle tissue engineering: Biomaterials-based strategies for the treatment of volumetric muscle loss. Bioengineering. 7 (3), 85 (2020).
- Mintz, E. L., et al. Long-term evaluation of functional outcomes following rat volumetric muscle loss injury and repair. Tissue Eng Part A. 26 (3-4), 140-156 (2020).
- Westman, A. M., et al. A coupled framework of in situ and in silico analysis reveals the role of lateral force transmission in force production in volumetric muscle loss injuries. J Biomech. 85, 118-125 (2019).
- Park, K. H., et al. Ex vivo assessment of contractility, fatigability and alternans in isolated skeletal muscles. J Vis Exp. 69, e4198 (2012).
- Moorwood, C., Liu, M., Tian, Z., Barton, E. R. Isometric and eccentric force generation assessment of skeletal muscles isolated from murine models of muscular dystrophies. J Vis Exp. (71), e50036 (2013).
- MacIntosh, B. R., Esau, S. P., Holash, R. J., Fletcher, J. R. Procedures for rat in situ skeletal muscle contractile properties. J Vis Exp. (56), e3167 (2011).
- Iyer, S. R., Valencia, A., Hernández-Ochoa, E. O., Lovering, R. M. In vivo assessment of muscle contractility in animal studies. Methods Mol Biol. 1460, 293-307 (2016).
- Corona, B. T., Call, J. A., Borkowski, M., Greising, S. M. In vivo measurement of hindlimb dorsiflexor isometric torque from pig. J Vis Exp. (175), e62905 (2021).
- Brightwell, C. R., et al. In vivo measurement of knee extensor muscle function in mice. J Vis Exp. (169), e62211 (2021).
- Lovering, R. M., Roche, J. A., Goodall, M. H., Clark, B. B., McMillan, A. An in vivo rodent model of contraction-induced injury and non-invasive monitoring of recovery. J Vis Exp. (51), e2782 (2011).
- Chiu, C. S., et al. Non-invasive muscle contraction assay to study rodent models of sarcopenia. BMC Musculoskelet Disord. 12 (1), 246 (2011).
- Childers, M. K., Grange, R. W., Kornegay, J. N. In vivo canine muscle function assay. J Vis Exp. (50), e2623 (2011).
- Cox, P. G., Jeffery, N. Reviewing the morphology of the jaw-closing musculature in squirrels, rats, and guinea pigs with contrast-enhanced micro CT. Anat Rec. 294, 915-928 (2011).
- Nordstrom, S. H., Yemm, R. Sarcomere length in the masseter muscle of the rat. Arch Oral Biol. 12 (5), 895-902 (1972).
- Huff, T., Weisbrod, L. J., Daly, D. T. . Neuroanatomy, Cranial Nerve 5 (Trigeminal). , (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved