A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Heat Tolerance Assays Using the Drosophila Activity Monitor System: A Guide to an Executable Application for Data Analysis
In This Article
Summary
This report describes a method for measuring adult Drosophila melanogaster time to knockdown using a Drosophila Activity Monitor (DAM2) in response to an air conduction heat stressor within an incubator chamber. The DAM2 measures activity by recording individual fly movements as they cross an infrared beam. Data analysis is facilitated by a novel executable file created by the authors.
Abstract
The study of heat tolerance in Drosophila melanogaster has been of particular interest to researchers for decades, with a common approach to assessing heat tolerance being to monitor the time to knockdown (TKD) after exposure to an elevated temperature. Classically, flies are housed in individual vials and placed inside a heated water bath. TKD is then monitored manually by researchers. While very well-established, there remain problems of subjectivity and consistent application of a tangible definition of cessation of all movement, including muscular spasms, when implementing these manual assays. We have developed a high-throughput method for automating heat tolerance assays using the TriKinetics Drosophila Activity Monitors (DAM2). To accompany the DAM2 system, we have written a program and created an easy-use executable to automatically read the last time of movement from the activity data generated. This script then writes to a .csv file the time to heat paralysis (TKD) for each fly. Our data show that this automated DAM2 method is consistent and reliable. Meanwhile, activity profiles created from the activity count data are of interest. These activity profiles can be compiled and have the potential to expand heat tolerance assays to include the relatively unstudied behavioral components of heat tolerance. This protocol will describe in detail how to use the DAM2 system and the HoTDAM! software to estimate heat tolerance in D. melanogaster.
Introduction
Ectotherms typically respond to heat stress with increased locomotor activity. This phenomenon has been apparent to researchers for decades, with the characteristic behavioral response described by Cowles and Bogert in 19441. They described how an organism under heat stress will first show increased locomotor movement. As the heat stress builds, short bursts of activity are interspersed with periods of inactivity. The temperature at which the organism can no longer show coordinated movement is the critical thermal maximum (CTmax). Muscular spams follow, and ultimately the organism collapses1,2. This collapse is difficult to define and represents something akin to "heat rigor, coma, or death"2. Here we will use the term physiological collapse to theoretically refer to this blurry endpoint of heat stress.
Drosophila melanogaster and other small insects have been valuable models to study heat stress. To estimate at least a portion of the complex collection of traits that constitute heat tolerance, many researchers have manually observed the time and the temperature at which physiological collapse occurs, representing the time to knockdown (TKD) and CTmax, respectively. While very well established, these manual assay methods suffer some drawbacks. An operational definition of physiological collapse can be difficult to establish and apply appropriately to all cases, especially when observers are less experienced. For example, at what point does the organism go from muscular spasms to collapse? The pattern of muscular spasms and seizure activity prior to collapse can be unpredictable and can complicate accurate observation2,3, threatening accuracy and precision. Meanwhile, the difficulty in observation also limits the number of organisms that can be assayed at one time, limiting scalability.
Since an increase in activity is a consistent response to heat and TKD and CTmax are ultimately the point at which activity ceases, we sought to employ the Drosophila Activity Monitors (DAM2) from TriKinetics to automate heat tolerance assays. We recently published a method for an automated assay, along with easy-to-use software, using the DAM2 system4. The assay was validated by comparing measures of heat tolerance in terms of TKD to a classic manual observation-based TKD assay across several factors. We also explored the locomotor activity component of TKD assays to further characterize the inducible thermotolerance phenotype. We named the assay and accompanying software HoTDAM! (Heat Tolerance assays using the Drosophila Activity Monitoring System). Here we provide a detailed description of the automated heat tolerance assay method using the DAM2 system and the HoTDAM! software. The assay is easy to use and is readily scalable to allow for the measurement of many organisms at one time.
In this manuscript, we performed TKD assays on thermosensory mutant flies (transient receptor potential ankyrin 1; TRPA1) and their genetic controls (white1118; w1118). These organisms were chosen to emphasize the importance of the characteristic increased activity seen during heat stress for the assay. Namely, TRPA1 organisms do not show this escape behavior, illustrating the intrinsic connection between conserved behavioral responses and estimates of heat tolerance, such as TKD. We performed the assay for both females and males, along with implementing a heat-hardening pretreatment. The representative results presented here are data from entirely new assays than those used in the original validation assays published previously.
Protocol
1. Fly husbandry
- Choose the appropriate fly stock/line for the investigation.
NOTE: To illustrate the assay, we used the transient receptor potential TRPA1 knockout stock and its genetic control, the w1118 stock. - Maintain the fly stocks under consistent conditions as appropriate. To follow this protocol, house stocks at 25 ˚C under a 12:12 diurnal cycle on standard food (cornmeal, molasses, and torula yeast).
- Separate males and females with light anesthesia.
NOTE: Depending on the experiment, virgin or mated flies might be used.- Allow adult males and females (separately for both stocks) to mate and oviposit for 5 days. Clear the adults from the bottles.
- As adults begin to eclose several days later, clear the bottles again. After 2 days, separate the males and females using light ether anesthesia and allow them to mature separately at a density of 25 flies per vial.
2. Pretreatment
- Apply the pretreatment or independent variable that will define the experimental groups (genetic, environmental, pharmacologic, or otherwise).
- To follow this protocol, separate the adults by sex, and after 5 days, pretreat half of the adults by immersing a sealed vial containing the organisms in a water bath at 37 ˚C for 1 h. Keep the controls in the incubator at 25 ˚C. Allow the flies to recover for 24 h after pretreatment, prior to the heat tolerance assay.
3. DAM2 system setup
- Load the flies into the DAM2 monitor tubes and cap the tubes at both ends with cotton. If the flies are to be assessed for heat tolerance immediately, do not use any anesthesia while loading the organisms into the monitor tubes to avoid any potential confounding effects associated with anesthesia exposure. Instead, aspirate individual flies from the holding vials into the monitor tubes.
- Put the assay tubes into the activity monitors, taking note as to which slot numbers the different groups are loaded.
NOTE: These may be randomized depending on the experiment if location within the monitor might be considered a confounding variable. - DAM2 data acquisition software
NOTE: The DAM2 system and software are described thoroughly in the DAMSystem3 Software Data Sheet available on the company's website (see the Table of Materials). Refer to the DAM2 instructions for specific troubleshooting and general functionality. Here we provide guidance for using the system in the context of our heat tolerance assay.- The DAM2 system monitors the number of times an individual fly within each tube breaks an infrared beam. The data acquisition software then indexes and resets this count every defined time interval. Under preferences, select the reading interval to be used in the assay. We have found a 15 s reading interval to achieve a good balance between resolution and reading errors.
NOTE: It is recommended that the reading interval be no shorter in seconds than the number of monitors being used. A shorter reading interval will give better temporal resolution to the assay but will also potentially increase the instances of reading errors. The software can fall behind while trying to index the readings. In addition, a newer, faster computer goes a long way in preventing reading errors. - While considering reading errors, ensure that the computer being used to run the DAM2 data acquisition software is set to never sleep or hibernate to not interrupt the assay. Further, ensure that auto-updates (including any institution-controlled network updates) are set to off while collecting data.
- Check that all monitors are connected and communicating with the software. Confirm that each monitor's status is green under the current data tab.
NOTE: See the DAMSystem3 Software Datasheet for details on what problems different color codes refer to. - The data acquisition software will automatically write the activity counts to text files in the Data folder within the system files. To make data analysis easier, delete any text files within this Data folder prior to starting the data acquisition software for any assay.
NOTE: The text files will automatically populate, so it is permissible to remove them from this folder to clear out old data without impeding the program.
- The DAM2 system monitors the number of times an individual fly within each tube breaks an infrared beam. The data acquisition software then indexes and resets this count every defined time interval. Under preferences, select the reading interval to be used in the assay. We have found a 15 s reading interval to achieve a good balance between resolution and reading errors.
4. Heat tolerance assay
- Load the monitors into the assay incubator.
- Start the acquisition software and let the software index for a defined length of time (e.g., 40 indexes or 10 min if the reading interval is set to 15 s) before applying the heat stress.
NOTE: This allows for some acclimation to the tubes and recovery from the movement of the monitors while setting them up. Especially if activity during the assay is being analyzed, this acclimation time will establish a baseline activity prior to the induction of heat stress. Depending on the specific nature of the assay being performed, this acclimation phase could be skipped, with the monitors placed directly into the incubator already at the stress temperature. - If performing a static (TKD) assay, set the incubator to ramp as quickly as possible to the set noxious temperature after the period of acclimation. The response variable will be operationalized as the last recorded time of movement (i.e., last non-zero index) to estimate TKD.
- If performing a dynamic assay, determine the rate at which the temperature will increase after the acclimation period. Here the response variable measured is technically time again. The time at which the last movement is recorded will coincide with a temperature (CTmax), depending on the rate of ramping.
- Monitor activity counts in real time on the DAM system display in the acquisition software or directly examine the text files in the data folder in the DAMSystem3 program files. Copy the text files and open the copy as opposed to the original file within the data folder to avoid causing any problems with live data recording.
- After no movement is seen in any of the flies for several minutes, stop the acquisition software.
NOTE: With our assays, we have found that a few to several minutes of inactivity is indicative of physiological collapse due to heat stress similar to that seen in the classic manual TKD assays. This is, however, dependent on the spontaneous escape behavior. As such, consider possible confounding variables if the specific treatment in the investigation might alter this behavioral response. Further, depending on the specific parameters of the assay (e.g., temperature, treatment), the length of the assay will obviously vary.
5. Data organization and analysis
NOTE: See Supplemental Video S1 for a walkthrough of how to download the executable application from the GitHub, as well basic functionality of the software.
- Once the data has been acquired, scan the text files for errors using the referenced software (see the Table of Materials) and select specific start and stop points for binning activity data. If a 10 min acclimation interval was implemented, start the binning 10 min into the activity recording. See the discussion for further details about binning.
- Open the HoTDAM! analysis software and import the scanned monitor data files by clicking File | load monitor data.
NOTE: The software and executable application are available from GitHub (https://github.com/MatthewR47/HoTDAM) or from the company’s website within Analysis Software section. Note that the executable application is only compatible with Windows operating systems at this time. - Add group designations to indicate which treatment groups correspond to which cell within the DAM2 monitors.
NOTE: The layout of the software interface corresponds to the layout of the monitors. - Click Start Multi-Group Definition to pull up a dialog box to add a group designation that will be applied to multiple cells. Once the group designation is accepted, click on the cells to apply the group designation, then click on Stop Multi-Group Designation.
NOTE: The software organizes and exports DAMSystem3 data to .csv files to be analyzed in statistics software. - Export the TKD (i.e., the last non-zero index) for each fly within the monitors to a .csv file by clicking File | Export Knockdown Data | Export All Monitors or Export Selected Monitors. TKD for each fly will be organized in the output by group designation.
- Export activity data for each fly by clicking on File | Export Activity Data | Export All Monitors or Export Selected Monitors to a .csv file, keeping only the timestamp and count data, making the data file easier to work with, while also assigning designated group labels for each fly.
NOTE: The first several columns in the DAMSystem3 data files correspond to internal data for the monitors (e.g., light monitoring or error messages) during acquisition. Count data are in columns 11-42 (see the DamSystem3 Data Sheet for details). Exporting the activity data with the analysis software will remove all columns but the timestamp and the count data columns. - If performing a CTmax experiment, use the TKD to determine the CTmax by using the rate of temperature ramping.
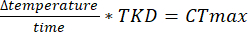
NOTE: The analysis software was written in an object-oriented manner in C# (source code available on GitHub; https://github.com/MatthewR47/HoTDAM) such that aspects of the program can be easily modified to allow for customization to meet specific purposes.
6. Statistics
NOTE: Many different tests can be used to analyze the TKD data, depending on the specifics of the experimental setup.
- Use survival analysis (e.g., Cox regression, Kaplan-Meier) to conceptualize knockdown as an event that each fly within the assay will experience.
NOTE: For a detailed review, see Bradburn's and Clark's 4-paper series discussing survival analysis and its implementation5,6,7,8. - Use ANOVA to assess group differences and compare modalities, especially when validating the automated assay for specific conditions.
- Analyze the TKD data using Kaplan-Meier survival analysis. Split the file by stock such that the analysis is performed separately (e.g., for TRPA1 and w1118 stocks lines). Choose the time variable as TKD (in min), knockdown as the event, pretreatment as the factor, and sex as the strata.
- Perform the log-rank, Breslow, and Tarone-Ware tests to compare pretreatment for each stratum (i.e., sex), for illustrative purposes showing several methods.
- Construct survival plots.
Results
The analyses for TRPA1 and w1118 stocks were performed separately. Percentile TKD times and other descriptives can be found in Table 1.
| Percentilesa | ||||||||
| Sex | Treatment | 25.00% | 50.00% | |||||
Discussion
The heat tolerance assay method we describe here is versatile and scalable. We have previously published a validation study where we compared the HoTDAM! method to a classic, observation-based TKD assay and found the automated assay to give show the same general trend across several factors4 (Figure 3). In other words, in the same way as the classic manual TKD assay, the DAM2 automated assay was able to differentiate organisms by sex, assay temperature, hardening pret...
Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgements
The project described was supported by Institutional Development Award (IDeA) grants from the National Institute of General Medical Sciences of the National Institutes of Health (5P20GM103427 and 1U54GM115458). The UNK Undergraduate Research Fellows Program and the UNMC Medical Student Summer Research Program.
Materials
| Name | Company | Catalog Number | Comments |
| 14 mL polystyrene test tubes | Falcon | 352057 | |
| 30 gallon fish tank | Wal-mart | ||
| 8 oz bottles | Genesee | 32-129F | |
| Constant Climate Chamber | Memmert | HPP750eco | |
| cornmeal | Lab Scientific | FLY801010 | |
| DAM2 Drosophila Activity Monitor | TriKinetics | DAM2 | (DAMSystem3 Data Sheet) https://www.trikinetics.com/Downloads/DAMSystem%20Price%20List%202024.7.pdf |
| DAMSystem data acquisition software | TriKinetics | free download | |
| Drosophila agar | Lab Scientific | FLY80201 | |
| ethanol | Fisher Scientific | BP82011 | |
| Ether | Fisher Scientific | E134-4 | |
| FileScan software | TriKinetics | for scanning for text errors, binning data, and output | |
| FlyStuff Flugs for bottles | Genesee | 49-100 | |
| FlyStuff Flugs for vials | Genesee | 49-102 | |
| FlyStuff vials | Genesee | 32-113RL | |
| HoTDAM software | Github or Trikinetics | https://github.com/MatthewR47/HoTDAM | |
| Immersion circulating heater | PolyScience | MX-CA11B | |
| molasses | Lab Scientific | FLY80084 | |
| propionic acid | Fisher Scientific | A258-500 | |
| Pyrex Glass tubes 5 x 65 mm for DAM2 | TriKinetics | PGT 5x65 | https://www.trikinetics.com/Downloads/DAMSystem%20Price%20List%202024.7.pdf |
| small paint brush | Wal-mart | ||
| SPSS Statistics | IBM | ||
| tegosept | Lab Scientific | FLY55015 | |
| torula yeast | MP Biomedicals | 290308505 | |
| TRPA1 mutant stock | Bloomington Stock center | 26504 | w[1118]; TI{w[+mW.hs]=TI}TrpA1[1] |
| w1118 stock | Bloomington Stock center | 3605 |
References
- Cowles, R. B., Bogert, C. M. A preliminary study of the thermal requirements of desert reptiles. Bull Am Mus Nat Hist. 83, 261-296 (1944).
- Lutterschmidt, W. I., Hutchison, V. H. The critical thermal maximum: history and critique. Can J Zool. 75, 1561-1574 (1997).
- Jørgensen, L. B., Malte, H., Overgaard, J. How to assess Drosophila heat tolerance: Unifying static and dynamic tolerance assays to predict heat distribution limits. Funct Ecol. 33 (4), 629-642 (2019).
- Rokusek, B., et al. HoTDAM! An easy-to-use automated assay expands the inducible thermotolerance phenotype in Drosophila melanogaster: Heat hardening reduces motility. Comp Biochem Physiol A Mol Integr Physiol. 286, 111522 (2023).
- Bradburn, M. J., Clark, T. G., Love, S. B., Altman, D. G. Survival analysis part III: Multivariate data analysis - Choosing a model and assessing its adequacy and fit. Br J Cancer. 89 (4), 605-611 (2003).
- Bradburn, M. J., Clark, T. G., Love, S. B., Altman, D. G. Survival analysis part II: Multivariate data analysis- An introduction to concepts and methods. Br J Cancer. 89 (3), 431-436 (2003).
- Clark, T. G., Bradburn, M. J., Love, S. B., Altman, D. G. Survival analysis part IV: Further concepts and methods in survival analysis. Br J Cancer. 89 (5), 781-786 (2003).
- Clark, T. G., Bradburn, M. J., Love, S. B., Altman, D. G. Survival analysis part I: Basic concepts and first analyses. Br J Cancer. 89 (2), 232-238 (2003).
- Hazra, A., Gogtay, N. Biostatistics series module 9: survival analysis. Indian J Dermatol. 62 (3), 251-257 (2017).
- Stevenson, R. D. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am Nat. 126 (3), 362-386 (1985).
- Grigg, G. C., Beard, L. A., Augee, M. L. The evolution of endothermy and its diversity in mammals and birds. Physiol Biochem Zool. 77 (6), 982-997 (2004).
- Soto-Padilla, A., et al. Thermosensory perception regulates speed of movement in response to temperature changes in Drosophila melanogaster. J Exp Biol. 221 (10), jeb174151 (2018).
- Hoffmann, A. A., Sørensen, J. G., Loeschcke, V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Therm Biol. 28 (3), 175-216 (2003).
- Terblanche, J. S., et al. Ecologically relevant measures of tolerance to potentially lethal temperatures. J Exp Biol. 214 (22), 3713-3725 (2011).
- Rezende, E. L., Castaneda, L. E., Santos, M. Tolerance landscapes in thermal ecology. Funct Ecol. 28 (4), 799-809 (2014).
- MacLean, H. J., Hjort Hansen, J., Sørensen, J. G. Validating the automation of different measures of high temperature tolerance of small terrestrial insects. J Insect Physiol. 137, 104362 (2022).
- Gioia, A., Zars, T. Thermotolerance and place memory in adult Drosophila are independent of natural variation at the foraging locus. J Comp Physiol A. 195, 777-782 (2009).
- Kjærsgaard, A., et al. The effect of developmental temperature fluctuation on wing traits and stressed locomotor performance in Drosophila melanogaster, and its dependence on heterozygosity. Evol Ecol Res. 14 (7), 803-819 (2012).
- Bak, N. K., Rohde, P. D., Kristensen, T. N. Strong sex-dependent effects of malnutrition on life- and healthspan in Drosophila melanogaster. Insects. 15 (1), 9 (2023).
- Bettencourt, B. R., et al. Natural variation in Drosophila stressed locomotion meets or exceeds variation caused by Hsp70 mutation: analysis of behavior and performance. Behav Genet. 393, 306-320 (2009).
- Kjærsgaard, A., et al. Locomotor activity of Drosophila melanogaster in high temperature environments: plastic and evolutionary responses. Clim Res. 43 (1-2), 127-134 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved