电子顺磁共振 (EPR) 光谱学
Overview
来源: 大卫 c 力量, 塔玛拉 m. 力量, 得克萨斯 A 和 #38;
在本视频中, 我们将学习电子顺磁共振 (EPR) 背后的基本原理。我们将使用 EPR 光谱研究 dibutylhydroxy 甲苯 (BHT) 在脂肪族醛氧化中的抗氧化剂的行为。
Principles
EPR 的基本原理:
EPR 是一种光谱技术, 它依赖于类似的物理现象, 如核磁共振 (NMR) 光谱学。当核磁共振测量核自旋跃迁时, EPR 测量电子自旋跃迁。EPR 主要用于研究顺磁性分子, 它们是具有不成对电子的分子。召回一个电子有一个自旋量子数, s = ½, 它有磁性组分ms = ½并且ms =-½。在没有磁场的情况下, 两个m 的状态的能量是等价的。然而, 在外加磁场 (B0) 的存在下, 电子的磁矩与外加磁场对齐, 因此, ms 状态变为退化 (图 1)。ms 状态之间的能量差取决于磁场的强度 (公式 1)。这就是所谓的塞曼效应。
e = m2geμbb0 (公式 1)
其中ge 是g因子, 它是电子的 2.0023, 而µB是玻尔磁。
在给定的磁场 ( B0) 中, 两个 ms状态之间的能量差由公式 2给出。
δ = e½ -e-½ = geμbb0 = hυ (公式 2)
一个电子在两个ms 的状态之间移动, 其发射或吸收的光子与能量ΔE = h节能.公式 2适用于单个的电子。然而, 与在1h 核磁共振中化学位移的方式相类似, 在氢原子的化学环境中, 分子内的电子与孤立电子的行为方式不同。该分子的电场梯度将影响有效磁场, 由方程 3给出。
b伊芙= b0(1-σ) (公式 3)
其中σ是局部场的效应, 它可以是正或负的值。
将公式 3插入公式 2中, 我们可以将给定分子中的未配对电子的g因子定义为g = ge(1-σ), 从而简化了整个等式:
hυ = gμbb0 (公式 4)
在 EPR 实验中, 频率被扫, 最常见的是在微波区域范围从900万兆赫, 和字段保持恒定在大约 0.35 T, 允许计算g。通过实验确定使用 EPR 的g提供有关顺磁性分子的电子结构的信息。
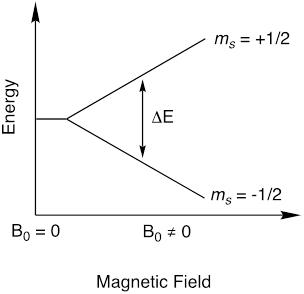
图 1.磁矩的分裂状态, ms, 在磁场的存在。
EPR 的应用:
在本实验中, 我们将使用 EPR 光谱学来研究抗氧化剂的化学成分。O2, 包括地球大气层的 21%, 是一种强氧化剂。尽管它有可能作为氧化剂, O2是基态三连音, 因此只与大多数有机分子相当缓慢地反应。一个重要的, 但通常不受欢迎的, 由 O2介导的反应是氧化。在氧化化学中, O2启动了可快速消耗有机分子的自由基链过程。图 2说明了一种常见的氧化, 其中醛类被氧化为羧酸。
防止氧化化学是很重要的, 以防止分解许多常见的有机材料, 如塑料, 和一个大的领域已开发周围确定有效的抗氧化剂, 以抑制氧化。抗氧化剂能起作用的一种机制是通过与激进中间体的反应来抑制自由基链过程。由于自由基物种具有不成对的旋转, EPR 是理解抗氧化剂化学的宝贵工具。在本实验中, 我们将使用 EPR 光谱学来探讨 BHT 作为抗氧化剂在脂肪族醛氧化中的作用。
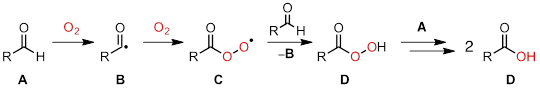
图 2.醛氧化通过一个根本链机制进行。
Procedure
1. 醛的氧化
- 在12毫升闪烁瓶中, 准备醛 (100 毫克) 和 CoCl2· 6H2O (1 毫克) 在 4-氯乙烷 (DCE) (20 毫升) 中的溶液。加入磁性搅拌棒, 用橡皮隔膜装瓶。
- 将1毫升塑料注射器的枪管连接到一根短橡皮管上。将橡皮管插入乳胶气球, 并将气球固定在带橡皮带和电子胶带的管子上。用 O2将乳胶气球充气。
- 将 O2气球的针插入反应瓶中。将第二针插入隔膜, 并用 O2清除反应容器的头部空间。
- 使用搅拌板, 在室温下, 在一个 O2大气压下搅动4小时的反应。
- 使用旋转式蒸发器浓缩反应混合物, 并在 CDCl3中对产生的油性残渣采用1H 核磁共振谱。
2. 用 BHT 作为醛氧化的抗氧化剂
设置两个小瓶如下所述。其中一个将用于分析产品分布, 一个将用于 EPR 光谱学的步骤3。
- 在 4 ml 闪烁瓶中, 准备醛 (100 毫克) 和 CoCl2· 6H2O (1 毫克) 在 DCE (20 毫升) 中的溶液。在溶液中加入 BHT (10 毫克)。加入磁性搅拌棒, 用橡皮隔膜装瓶。
- 将1毫升塑料注射器的枪管连接到一根短橡皮管上。将橡皮管插入乳胶气球, 并将气球固定在带橡皮带和电子胶带的管子上。用 O2将乳胶气球充气。
- 将 O2气球的针插入反应瓶中。将第二针插入隔膜, 并用 O2清除反应容器的头部空间。
- 使用搅拌板, 在室温下, 在一个 O2大气压下搅动4小时的反应。
- 使用旋转式蒸发器浓缩反应混合物, 并在 CDCl3中对产生的油性残渣采用1H 核磁共振谱。
3. 测量 EPR 谱
- 打开 epr 光谱仪, 让仪器预热30分钟. 设置 epr 收购具有以下参数: 中心字段 3345 g, 扫宽100克, 扫时间五十五年代, 时间常数10毫秒, 兆瓦功率5兆瓦, 调制100赫, 调制振幅1克。
- 测量空 epr 管的 epr 频谱, 以确保 epr 管或仪器谐振器没有背景信号。
- 在一个 N2填充的手套箱中准备一个 DCE 中的 BHT 的解决方案。将溶液的 0.5 mL 转化为 epr 管, 并使用步骤3.1 中设置的采集参数测量 BHT 的 epr 谱。
- 利用步骤3.1 中设置的采集参数, 将步骤2中的 BHT 加反应溶液的0.5 毫升转化为 epr 管, 获得和 epr 谱。
Results
醛的氧化提供丁酸。在步骤1中所进行的反应所得到的1H 核磁共振谱表明, 缺乏醛的 C-h 共振和存在的共振预期的丁酸。相比之下, 从步骤 2 (加 BHT) 的反应混合物中得到的核磁共振显示的信号与醛一致, 没有丁酸存在。从这些数据中, 我们观察到醛在醛氧化中起了抗氧化剂的作用。
BHT 在抑制醛氧化中的作用是由 BHT 的 EPR 谱和 BHT 添加到醛氧化反应中得到的。BHT 是一个磁性的有机分子, 这意味着没有不成对的电子。因此, BHT 的 EPR 频谱不显示信号。相反, 氧化反应的 EPR 谱在其中添加了 BHT, 显示了一个强的四内衬模式, 与一个有机的自由基相一致。这一光谱的产生是因为 BHT 的 o H 键较弱, 并且在氧化过程中产生的自由基, H 原子从 BHT 解渴转移到自由基链机制, 并产生一个稳定的 o 型中心基。
Application and Summary
在这个实验中, 我们探讨了抗氧化剂在抑制氧化化学中的作用。本文探讨了利用 EPR 光谱进行抑制的机理, 揭示了 BHT 在氢氧化反应中的作用是通过 H 原子转移淬火活性基中间体。
具有不成对电子的分子可能具有核磁共振的特征, 因此 EPR 光谱学经常提供有关这些物种的有用和互补的信息。EPR 光谱法是一种常用的检测和表征有机自由基的实验技术。此外, 顺磁性无机配合物还经常显示 EPR 谱, 这对表征具有指导意义。实验 EPR 谱描述了未配对电子的g因子, 它提供了关于顺磁中心电子结构的信息。此外, 具有不成对电子的原子核的核自旋以及相邻的原子核也会影响电子的磁矩, 从而导致在 EPR 频谱中产生附加的ms 状态和多条线的分裂。由此产生的超和 super-hyperfine 耦合提供了有关分子电子结构的进一步信息。
除了表征 open-shell 的有机和无机物种外, EPR 光谱的细腻灵敏度对无机系统的应用至关重要, 在这种体系中, 金属因子的浓度较低。EPR 光谱在无机化学中经常被用于提供关于金属离子在酶的心脏的结构和氧化状态的直接信息。
Tags
跳至...
此集合中的视频:

Now Playing
电子顺磁共振 (EPR) 光谱学
Inorganic Chemistry
25.6K Views

用 Schlenk 线技术合成钛 (III) 茂金属
Inorganic Chemistry
31.6K Views

手套和杂质传感器
Inorganic Chemistry
18.7K Views

二茂铁的升华纯化
Inorganic Chemistry
54.7K Views

埃文斯方法
Inorganic Chemistry
68.7K Views

单晶和粉末 X 射线衍射
Inorganic Chemistry
105.1K Views

尔光谱学
Inorganic Chemistry
22.0K Views

路易斯酸碱交互作用在 Ph 值3P BH3
Inorganic Chemistry
39.0K Views

二茂铁结构
Inorganic Chemistry
79.8K Views

群论在红外光谱分析中的应用
Inorganic Chemistry
45.9K Views

分子轨道 (MO) 理论
Inorganic Chemistry
35.5K Views

异体金属-金属粘结 Paddlewheels
Inorganic Chemistry
15.3K Views

染料敏化太阳能电池
Inorganic Chemistry
16.0K Views

载氧钴 (II.) 络合物的合成
Inorganic Chemistry
51.8K Views

自由基聚合反应的光化学引发
Inorganic Chemistry
17.1K Views
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。