A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Enrichment of Astrocyte-Derived Extracellular Vesicles from Human Plasma
In This Article
Summary
This protocol describes the enrichment of astrocyte-derived extracellular vesicles (ADEVs) from human plasma. It is based on the separation of EVs by polymer precipitation, followed by ACSA-1 based immunocapture of ADEVs. Analysis of ADEVs may offer clues to study changes in inflammatory pathways of living patients, non-invasively by liquid biopsy.
Abstract
Extracellular vesicles (EVs) are biological nanoparticles secreted by all cells for cellular communication and waste elimination. They participate in a vast range of functions by acting on and transferring their cargos to other cells in physiological and pathological conditions. Given their presence in biofluids, EVs represent an excellent resource for studying disease processes and can be considered a liquid biopsy for biomarker discovery. An attractive aspect of EV analysis is that they can be selected based on markers of their cell of origin, thus reflecting the environment of a specific tissue in their cargo. However, one of the major handicaps related to EV isolation methods is the lack of methodological consensuses and standardized protocols. Astrocytes are glial cells with essential roles in the brain. In neurodegenerative diseases, astrocyte reactivity may lead to altered EV cargo and aberrant cellular communication, facilitating/enhancing disease progression. Thus, analysis of astrocyte EVs may lead to the discovery of biomarkers and potential disease targets. This protocol describes a 2-step method of enrichment of astrocyte-derived EVs (ADEVs) from human plasma. First, EVs are enriched from defibrinated plasma via polymer-based precipitation. This is followed by enrichment of ADEVs through ACSA-1-based immunocapture with magnetic micro-beads, where resuspended EVs are loaded onto a column placed in a magnetic field. Magnetically labeled ACSA-1+ EVs are retained within the column, while other EVs flow through. Once the column is removed from the magnet, ADEVs are eluted and are ready for storage and analysis. To validate the enrichment of astrocyte markers, glial fibrillary acidic protein (GFAP), or other specific astrocytic markers of intracellular origin, can be measured in the eluate and compared with the flow-through. This protocol proposes an easy, time-efficient method to enrich ADEVs from plasma that can be used as a platform to examine astrocyte-relevant markers.
Introduction
Extracellular vesicles (EVs) are a heterogeneous group of membranous nanoparticles secreted by all types of cells, carrying proteins, lipids, and nucleic acids1. Microvesicles (100-1000 nm), exosomes (30-100 nm), and apoptotic bodies (1000-5000 nm) constitute the main EV types, as distinguished by their site of origin2,3. EVs regulate important physiological processes, such as antigen presentation and immune responses4, receptor recycling, metabolite elimination5, and cellular communication6. The regulation of these processes may occur by direct binding between proteins enriched in the EV cell membrane and targets in recipient cells, and/or through the internalization and release of their cargo in the cytoplasm of the recipient cell7. While EVs perform essential cellular functions, they have gained increasing interest from a pathological perspective in the fields of cancer and neurology. Indeed, several studies have shown EVs can help promote tumor cell migration8,9 or seed toxic protein aggregates in neurodegenerative diseases, such as Alzheimer's disease10,11.
EVs can be selected and enriched from biofluids based on cell surface markers related to their cell of origin, thus reflecting the environment of a specific tissue in their cargo12,13,14,15,16,17,18,19,20. In addition, given their presence in blood, cerebrospinal fluid (CSF), saliva, urine, and breast milk, EVs represent an excellent, non-invasive tool for diagnosis, and can be considered a liquid biopsy for biomarker discovery. This is of special interest in neurology, given the difficulties of studying brain analytes in accessible fluids other than CSF.
Astrocytes have gained rising interest, as they are at the intersection of neuro-vascular communication21. Under physiological conditions, they are responsible for the preservation of the blood-brain barrier, the recycling of neurotransmitters, the supply of nutrients and growth factors to neurons and other glial cells22,23,24 as well as neuro-immune defense, given their metabolic plasticity from pro-inflammatory to anti-inflammatory states and vice versa25,26,27. An important mechanism by which astrocytes accomplish their regulatory functions is by communication through EVs28,29. Reactive astrocytosis is a key hallmark of several neurodegenerative diseases, such as Alzheimer's disease,30 multiple system atrophy (MSA), progressive supranuclear palsy (PSP)31, and amyotrophic lateral sclerosis (ALS)32. Astrocyte reactivity may lead to altered EV cargo, release of inflammatory mediators, and aberrant cellular communication, thus facilitating the spread of pathology and leading to neurodegeneration10,11. Therefore, studying astrocyte derived EVs (ADEVs) and changes in their cargo is an attractive resource to examine neurodegenerative processes in a non-invasive manner.
Currently, several methodologies exist for the isolation of EVs, each with its corresponding advantages and disadvantages33. It is essential to consider which method is more suitable for a specific use, depending on the final application of interest. In the neurology field, and more specifically, in astrocyte studies, polymer-based precipitation followed by immunocapture has been the predominantly used method12,18,19,20,34. However, even when applying the same approach, there remains heterogeneity between studies in the different steps applied for EV isolation. Therefore, there is a need for a clear, step-by-step standardized methodology to facilitate astrocyte EV studies and study reproducibility. Polymer-based precipitation facilitates biomarker screening given that it is a fast, simple procedure that does not require complex equipment, leading to a high yield of EVs without affecting their biological activity35.
The present protocol describes a detailed, simple, two-step method for the enrichment of ADEVs from human plasma. It is based on a polymer-based precipitation of the total EV fraction, followed by an immunocapture of astrocyte EVs. Given the important functions of astrocytes, analysis of ADEVs may shed light for the discovery of biomarkers and brain inflammatory pathways that can be studied in a non-invasive manner.
Protocol
The research described in this protocol has been conducted with human plasma samples from healthy adult donors of both sexes (age range 65.9-81.3 years, 45.5% females), from the Sant Pau Initiative on Neurodegeneration (SPIN) cohort, Barcelona, Spain36. Participants gave informed consent. The study has been conducted following the international ethical guidelines for medical research contained in the declaration of Helsinki and the Spanish law. The Sant Pau Research Ethics Committee (CEIC) reviewed and approved the protocol for the collection and storage of human plasma samples from the SPIN cohort (#16/2013).
1. Enrichment of astrocyte EVs from human plasma
NOTE: This protocol involves the use of human plasma samples. All details about reagents and laboratory material used in this protocol are included in the Table of Materials. No special equipment is required for this procedure, however, please review the safety considerations of each reagent, as specified individually by each manufacturer.
- Sample collection and storage
- Collect blood in lavender EDTA vacutainer tubes, following standardized protocols for the collection of plasma36,37. Centrifuge at 2000 x g for 10 min at 4 °C within 30-120 min after collection for plasma separation. Separate the plasma, which is the upper clear liquid that appears as supernatant, in 500 µL aliquots.
- To remove cell debris, centrifuge the aliquots at 3000 x g for 15 min at room temperature. Recover the supernatant. Store the plasma at -80 °C until analysis. As is common practice for biofluid analyses, aliquot the samples before storage and avoid more than three freeze-thaw cycles.
- Total EV enrichment
NOTE: All reagents used must be filtered through a 0.22 µm filter.- Thaw 500 µL of plasma samples. Add thrombin in a 1:100 proportion to remove coagulation factors. Mix by inversion three times and let it sit for 5 min at room temperature.
- Complete the volume to reach 1 mL with Dulbecco's phosphate-buffered saline (DPBS) working solution: DPBS + 3x concentrated protease inhibitor cocktail (diluted from 10x protease inhibitor cocktail stock). Mix by inversion.
- Centrifuge the samples at 6000 x g for 20 min at 4 °C. Recover the supernatant. Complete the volume with DBPS working solution to reach 1 mL.
- Add 252 µL of the EV precipitation solution and mix by inversion three times. Incubate at 4 °C for 60 min.
- Centrifuge the samples at 1500 x g for 20 min at 4 °C to precipitate the total EV fraction (pellet). Collect 1 mL of the supernatant. Label the fraction as EV-depleted plasma to use as a negative control of EV enrichment markers (No EVs) and store at -80 °C.
- Centrifuge the remaining pellet at 1500 x g for 5 min at 4 °C. Discard the remaining supernatant. Resuspend the pellet in 500 µL of ultrapure water containing concentrated protease and phosphatase inhibitors (final concentration 3x, diluted from a stock of 100x, see Table of Materials).
- Pipette up and down vigorously to loosen the pellet, avoiding foaming; vortex and agitate on a rotatory tube shaker for 30 min at room temperature until complete resuspension. Ensure complete pellet resuspension, since it is a critical step for the subsequent immunocapture step.
- Immunocapture of ADEVs
NOTE: ADEVs carry astrocyte markers. The selected marker for ADEV enrichment via immunocapture is GLAST. GLAST, which stands for glutamate-aspartate transporter (UniProtKB-P43003), is the most abundant glutamate transporter predominantly expressed by astrocytes in the cerebellum and cerebral neocortex38. The anti-GLAST (ACSA-1, astrocyte cell surface antigen-1) antibody is specific for an extracellular epitope of GLAST and has been developed for the identification of astrocytes. It is the most widely used target for the immunocapture of ADEVs from human plasma12,20,34.- Add 10 µL of anti-GLAST (ACSA-1) biotinylated antibody to each sample containing the total EV preparation. Mix well and gently. Incubate for 1 h at 4 °C on a rotatory tube shaker.
- Add 10 µL of magnetic microbeads conjugated to monoclonal anti-biotin antibodies (mouse IgG1). Mix well and gently. Incubate for 1 h at 4 °C on a rotatory tube shaker.
- Place a micro column in the magnetic field of a MACS separator. Prepare the column by rinsing it with 500 µL of PBS-0.5% bovine serum albumin (BSA). Discard the flow-through.
- Load the EV/ACSA-1/microbead suspension onto the column. Do not push with the plunger at this step. Collect the flow-through. Label this as non-astrocytic EVs (No ADEVs) and keep as a control if needed.
- Wash the column twice with 500 µL of PBS-0.5% BSA. Discard the flow-through.
- Remove the column from the magnetic separator and place it in a low-adhesion 1.5 mL collection tube. Pipette 500 µL of PBS-0.5% BSA on the column. Push firmly with a plunger into the column and collect the eluate.
- Label as ACSA-1+ EVs (ADEVs). Store non-lysed EVs at -20 °C for short periods or at -80 °C for longer periods (>6 months).
NOTE: Anti-ACSA-1 antibodies will bind to the GLAST-1/EAAT-1 epitope. Magnetic microbeads will bind with affinity to the anti-ACSA-1-EV complex. MACS micro columns contain an optimized matrix to generate a strong magnetic field when placed in a magnet, which is required to retain the labeled ADEVs.
For a schematic representation of the ADEV enrichment procedure, see Figure 1.
- Lysis of EVs
NOTE: EVs must be completely lysed for the detection of intravesicular markers. The use of two sequential methods, such as chemical followed by mechanical lysis, is recommended.- Perform a chemical lysis of EVs by adding protein extraction reagent (see Table of Materials) in a proportion of 100 µL sample per 150 µL extraction reagent. Add 100x protease and phosphatase inhibitors to reach a 1x final concentration. Vortex vigorously and let sit at room temperature for 15 min.
- Perform a mechanical lysis by a two-step procedure: pass the chemically lysed EV solution through a syringe twice (G29 needle). Then, sonicate the samples for 45 s in an ultrasonic cold-water bath. Freeze the lysed EVs at -80 °C.
- Thaw the samples at 37 °C for 5 min. Freeze again at -80 °C for the storage of lysed EVs. Thaw again for 5 min at 37 °C before analysis.
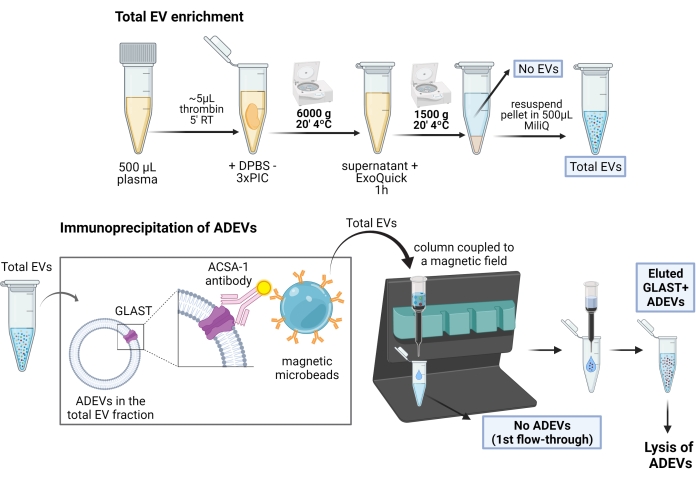
Figure 1: Schematic representation of the two-step procedure for the enrichment of astrocyte derived EVs. In the first step, EVs are enriched from defibrinated human plasma by polymer-based precipitation and centrifugation steps. After total EV resuspension, astrocyte EVs are then selected by immunocapture with biotinylated anti-GLAST (ACSA-1) antibodies and anti-biotin magnetic microbeads. Abbreviations: ACSA-1 = astrocyte cell surface antigen-1; ADEVs = astrocyte-derived extracellular vesicles; DPBS = Dulbecco's phosphate-buffered saline; EVs = extracellular vesicles; GLAST = glutamate-aspartate transporter; No ADEVs = non-astrocytic extracellular vesicles; No EVs = No extracellular vesicles (EV-depleted plasma); PIC = protease inhibitor cocktail; RT = room temperature. Figure created with BioRender. Please click here to view a larger version of this figure.
2. Protocol validation
- Characterization of total EV fraction prior to immunocapture
- Use lysed total EVs (resuspended pellet after polymer-precipitation) and diluted plasma samples for Western blot analysis. Supplement the samples with 4x Laemmli sample buffer (5 µL for 15 µL of sample), boil for 10 min at 90 °C, and load onto 10% stain-free acrylamide gels.
- Perform SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in Tris/Glycine/SDS buffer at 80 V for 30 min, followed by 200 V for 40 min. Following the SDS-PAGE, remove the gel from the glass plates and visualize the total protein content after a 1 min UV excitation.
- Transfer the proteins from the gel to a methanol-activated 0.2 µm PVDF membrane through a semi-dry system at 25 V, 2.5 A for 10 min (see Table of Materials for details about equipment). After the transfer, block the membrane in blocking buffer for 5 min at room temperature and subsequently incubate overnight at 4 °C with the primary antibodies to Alix (1:1000), CD9 (1:1000), or Calnexin (1:1000); see Table of Materials for details.
- Wash the membrane in Tris-buffered saline with 0.1% Tween 20 (TBST) three times for 10 min and incubate for 1 h at room temperature with an anti-rabbit HRP secondary antibody diluted at 1:7500 in blocking buffer. Wash the membrane in TBST four times for 10 min. Perform all washes and incubation steps with gentle agitation of the membrane on a bench rocker.
- Mix in a 1:1 ratio the chemiluminescent solution with the peroxide buffer and incubate the membranes for 5 min at room temperature. Expose and acquire bands using a chemiluminescence imaging system (see Table of Materials for details).
- Validation of the enrichment of astrocyte-specific markers in ADEV fraction
NOTE: Glial fibrillary acidic protein (GFAP) is the main intermediate filament protein in astrocytic cells and an essential element of their cytoskeleton during development. GFAP can be detected in brain tissue and biofluids being a well-known astrocyte marker39. Therefore, this marker was quantified using a commercial biomarker detection technology to demonstrate the enrichment of astrocyte-derived markers in the ADEV fraction. This biomarker technology is an ultrasensitive technique that allows the measurement of single protein molecules with a sensitivity of up to 1,000 times greater than conventional immunoasays. This technique is based on the use of paramagnetic microparticles coupled with antibodies designed to bind to specific targets40,41 (see Table of Materials).- Use lysed EV samples for biomarker analysis. To test the enrichment of astrocyte markers, compare GFAP levels in the eluate (ADEVs) versus the flow-through (No ADEVs) using a commercial ultrasensitive immunoassay.
- Dilute the samples in a 1:4 ratio (25 µL of EVs + 75 µL of assay buffer), prepare the calibrators, and conduct the assay in the biomarker detection equipment, as specified by the manufacturer's instructions (see Table of Materials). The samples can be diluted directly on the plate.
- Test the samples and calibrators in duplicate. The quantification range for the GFAP assay was 1.37-1000 pg/mL. Use the manufacturer's software analysis platform to calculate GFAP concentrations from the calibration curve.
- To estimate the coefficient of variation (CV) of the protocol for the enrichment of astrocyte markers, measure the GFAP levels in ADEV preparations obtained from identical replicates of pooled human plasma samples and calculate the CV as standard deviation/mean x 100. In this protocol, the CV was calculated using N = 10 identical plasma samples.
- Validation of the enrichment of EV markers in ADEV fraction
- To validate the enrichment of EV markers, measure Alix and CD81 levels by ELISA (see Table of Materials) in lysed undiluted EV samples, following manufacturer's recommendations. Test samples and calibrators in duplicate.
- Read the plates at 450 nm and 570 nm using a microplate reader. Use a linear calibration curve to calculate Alix and CD81 concentrations. The quantification range for the Alix assay was 47-3000 pg/mL and for CD81 it was 0.156-10 ng/mL.
- Characterization of the size and morphology of ADEVs
- Nanoparticle tracking analysis
- Use nanoparticle tracking analysis (NTA) to measure the particle concentration and size distribution of fresh non-lysed EV preparations.
- Dilute fresh, non-lysed ADEV suspensions (10 µL) with filtered PBS according to the detection range of the instrument (20-100 particles/frame), using the supplied software, record three 60 s videos with settings as follows: syringe flow rate at 30 a.u., camera level at 13, and the detection threshold at 5. Correct the particle concentrations for the input sample volume, volume of EV resuspension, and dilution necessary for NTA reading.
NOTE: It is recommended to pre-visualize a sample containing the EV vehicle solution to verify the matrix effect and the purity of the medium in which EVs are resuspended.
- Cryo-electron microscopy
- Use cryo-electron microscopy (Cryo-EM) to confirm the presence and morphology of Evs in fresh, non-lysed ADEV suspensions (4 µL). Vitrify the samples using a commercial plunge freezer on Holey carbon grids with the following settings: 3.9 µL of sample, wait time 10 s, blot time 2 s.
- Plunge into liquid ethane-nitrogen in a cryo-workstation. Transfer the grids to a cryoholder maintained at -179 °C for transmission electron microscope analysis. Examine the EVs with a transmission electron microscope operating at an accelerating voltage of 200 kV and equipped with a CCD camera. Acquire micrographs using software.
- Nanoparticle tracking analysis
- Analysis of lipoprotein co-precipitation
- Determine human ApoB levels in undiluted plasma, lysed total EV samples, and lysed ADEV samples with an immunoturbidimetric assay using a commercial kit adapted for a commercial autoanalyzer (see Table of Materials for further details).
- Analysis of inflammatory markers in ADEVs
- Use a bead-based assay (see Table of Materials) to quantitate the concentration of 25 inflammatory markers: eotaxin, IFN Alpha2, IFN Gamma, IL-1 Alpha, IL-1 Beta, IL-3, IL-6, IL-8, IL-10, IL-12 (p40), IL-12 (p70), IL-15, IL-17A, IL-17E/IL-25, IL-17F, IL-18, IP-10, MCP-1, MCP-3, MDC, MIP-1 Alpha, MIP-1 Beta, TGF Alpha, TNF Alpha, TNF Beta.
- Use lysed undiluted EV preparations (25 µL) and incubate the samples with the beads overnight at 4°C, following the manufacturer's instructions. Wash the plate, incubate with 25 µL of detection antibodies, and incubate for 1 h with agitation at room temperature.
- Add 25 µL of Streptavidin-PE to each well and incubate for 30 min at room temperature. Add 150 µL of sheath fluid and incubate for 5 min under agitation. Read the plate on the plate reader attached to the system.
NOTE: This technique is based on immunoassays using antibodies that bind to the surface of fluorescent-coated magnetic microbeads. This technology internally codes microspheres by their color with different dyes. Each type of microsphere is coated with a specific capture antibody, such that multiple conjugated beads capture the analytes embedded in samples, allowing the quantitative multiplex detection of dozens of analytes simultaneously. The system uses the bead-based multiplexed immunoassay platform for signal detection43.
3. Data analysis
- Perform statistical analyses with commercial software. Assess data normality with the Shapiro-Wilk test. Test two-group comparisons with the Mann-Whitney U test and three-group comparisons with the Kruskal-Wallis test and Dunn's post-hoc correction. Set the significance at p < 0.05.
Results
The isolation of ADEVs from plasma collected from healthy donors was successfully accomplished. A polymer-based precipitation method was employed to obtain the total EV fraction, followed by an immunocapture with magnetic microbeads to obtain ADEVs.
Western blot analysis of the total EV fraction prior to the immuno-capture step indicated the lack of calnexin (cellular contamination marker) and the presence of Alix and the transmembrane protein CD9 in the EV preparations (F...
Discussion
EVs have gained strong interest in biomedical research due to their diagnostic and therapeutic potential. Currently, one of the major handicaps related to EV isolation methods is the lack of methodological consensus and standardized protocols. This study provides a detailed protocol for the enrichment of astrocyte EVs from human plasma via polymer-based precipitation and GLAST immunocapture.
Different methodologies exist for the isolation of EVs from body fluids, each with their own a...
Disclosures
Dr. Belbin reported receiving personal fees from ADx NeuroSciences outside the submitted work. Dr. Alcolea reported receiving personal fees for advisory board services and/or speaker honoraria from Fujirebio-Europe, Roche, Nurtricia, Krka Farmacéutica, Zambon S.A.U., and Esteve outside the submitted work. Dr. Lleó has served as a consultant or at advisory boards for Fujirebio-Europe, Roche, Biogen, Grifols, and Nutricia outside the submitted work. Dr. Fortea reported receiving personal fees for service on the advisory boards, adjudication committees, or speaker honoraria from AC Immune, Novartis, Lundbeck, Roche, Fujirebio, and Biogen outside the submitted work. Drs. Alcolea, Belbin, LLeó, and Fortea report holding a patent for markers of synaptopathy in neurodegenerative disease (licenced to ADx, EPI8382175.0). No other disclosures were reported. All other authors have nothing else to disclose.
Acknowledgements
The authors would like to acknowledge the help of Soraya Torres, Shaimaa El Bounasri El Bennadi, and Oriol Sanchez Lopez for sample handling and preparation. We would also like to acknowledge the collaboration of José Amable Bernabé, from the ICTS "NANBIOSIS", unit 6 (Unit of CIBER in Bioingineering, Biomaterials & Nanomedicine) of the Barcelona Materials Science Institute, Marti de Cabo Jaume from the Electron Microscopy Unit at Universitat Autonoma de Barcelona, Dr. Marta Soler Castany and Lia Ros Blanco from the Flow Cytometry Platform at Sant Pau Biomedical Research Institute (IIB-Sant Pau), as well as Dr. Joan Carles Escolà-Gil from the Pathophysiology of lipid-related diseases group at IIB-Sant Pau for help with the NTA, cryo-EM, Luminex, and ApoB determinations, respectively.
The authors acknowledge financial support from the Jérôme Lejeune Foundation (Project #1941 and #1913 to MFI and MCI), Instituto de Salud Carlos III (PI20/01473 to JF, PI20/01330 to AL, PI18/00435 to DA, and INT19/00016 to DA), the National Institute of Health (1R01AG056850-01A1, R21AG056974, and R01AG061566 to JF), the Alzheimer's Association and Global Brain Health Institute (GBHI_ALZ-18-543740 to MCI), the The Association for Frontotemporal Degeneration (Clinical Research Postdoctoral Fellowship, AFTD 2019–2021) to ODI, and the Societat Catalana de Neurologia (Premi Beca Fundació SCN 2020 to MCI). This work was also supported by the CIBERNED program (Program 1, Alzheimer Disease to AL and SIGNAL study. SS is a recipient of a Postdoctoral grant “Juan de la Cierva-Incorporación” (IJC2019-038962-I) by the Agencia Estatal de Investigación, Ministerio de Ciencia e Innovación (Gobierno de España).
Materials
| Name | Company | Catalog Number | Comments |
| Anti-Alix primary antibody for Western blotting | EMD Millipore | ABC40 | |
| µMACS Separator | Miltenyi Biotec | 130-042-602 | The µMACS Separator is used in combination with µ Columns and MACS MicroBeads. |
| Anti-calnexin primary antibody for Western blotting | Genetex | GTX109669 | |
| Anti-CD9 primary antibody for Western blotting | Cell Signaling | 13174 | |
| Blocker BSA (10%) 200 mL | Thermo Fisher | 37525 | |
| Bransonic 1510E-MT Ultrasonic bath | Branson | ||
| COBAS 6000 autoanalyzer | Roche Diagnostics | Analyzer for immunoturbidimetric determination of ApoB; commercial autoanalyzer | |
| cOmplete Protease Inhibitor Cocktail (EDTA-free) | Roche | 11873580001 | |
| Digital Micrograph 1.8 | micrograph software | ||
| Dulbecco's PBS Mg++, Ca++ free 500 mL | Thermo Fisher | 14190144 | |
| EveryBlot Blocking Buffer | BioRad | 12010020 | |
| Exoquick (exosome precipitation solution 5 mL) + Thrombin | System Bioscience | EXOQ5TM-1 | ExoQuick 20 mL can also be purchased (EXOQ20A-1) |
| Gatan 895 USC 4000 | camera | ||
| GeneGnome XRQ chemiluminiscence imaging system | Syngene | ||
| Human CD81 antigen (CD81) ELISA kit | Cusabio | CSB-EL004960HU | |
| Human Programmed cell death 6-interacting protein (PDCD6IP) ELISA kit | Cusabio | CSB-EL017673HU | |
| Immun-Blot PVDF Membrane | BioRad | 1620177 | |
| JEOL 2011 transmission electron microscope | JEOL LTD | Equipped with a CCD Gatan 895 USC 4000 camera (Gatan 626, Gatan, Pleasanton, USA) | |
| Lavender EDTA BD Vacutainer K2E tubes | Becton dickinson | 367525 | |
| Leica EM GP | Leica Microsystem | commercial plunge freezer | |
| Low binding microtubes 1,5 mL | Deltalab | 4092.3NS | |
| MACS µ Columns with plungers | Miltenyi Biotec | 130-110-905 | µ Columns with plungers are especially designed for isolation of exosomes from body fluids |
| MACS Multistand | Miltenyi Biotec | 130-042-303 | |
| MAGPIX plate reader | Luminex Corporation | 80-073 | Luminex's xMAP multiplexing unit (Luminex xPonent v 4.3 software) |
| MicroBead Kit100 μL Anti-GLAST (ACSA-1)-Biotin, human, mouse, rat – small size; 100 μL Anti-Biotin MicroBeads | Miltenyi Biotec | 130-095- 825 | |
| MILLIPLEX MAP Kit Human cytokine/Chemokine/Growth Factor Panel A magnetic bead panel | EMD Millipore | HCYTA-60K-25 | |
| M-PER Mammalian Protein Extraction Reagent 25 mL | Thermo Fisher | 78503 | For certain applications like Western blot, more aggressive lysis buffers can be used (e.g. RIPA) |
| MultiSkan SkyHigh Microplate Spectrophotometer | Thermofisher | A51119500C | |
| NanoSight NS300 | Malvern Panalytical | NTA; 3.4 version | |
| Pierce Halt Protease and Phosphatase Inhibitor Cocktail | Thermo Fisher | 78441 | |
| Polypropylene syringe (G29) | PeroxFarma | 1mL syringe; 0.33x12mm-G29x1/2" | |
| Secondary anti-rabbit antibody | Thermo Fisher | 10794347 | |
| Simoa GFAP Discovery Kit | Quanterix | 102336 | |
| Simoa, SR-X instrument | Quanterix | SR-X Ultra-Sensitive Biomarker Detection System; commercial biomarker detection technology | |
| Specific Protein Test Apolipoprotein B - APOB (100 det) COBAS C/CI | Roche Diagnostics | 3032574122 | |
| SuperSignal West Femto | Thermo Fisher | 34095 | Ultra-sensitive enhanced chemiluminescent (ECL) HRP substrate |
| Trans-Blot Turbo Transfer System | BioRad | 1704150 |
References
- Pathan, M., et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Research. 47, 516-519 (2019).
- Raposo, G., Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. Journal of Cell Biology. 200 (4), 373-383 (2013).
- Gustafson, D., Veitch, S., Fish, J. E. Extracellular vesicles as protagonists of diabetic cardiovascular pathology. Frontiers in Cardiovascular Medicine. 4, 71 (2017).
- Raposo, G., et al. B lymphocytes secrete antigen-presenting vesicles. The Journal of Experimental Medicine. 183 (3), 1161-1172 (1996).
- Harding, C. V., Heuser, J. E., Stahl, P. D. Exosomes: Looking back three decades and into the future. Journal of Cell Biology. 200 (4), 367-371 (2013).
- Valadi, H., et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 9 (6), 654-659 (2007).
- Van Niel, G., D'Angelo, G., Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology. 19 (4), 213-228 (2018).
- Hood, J. L., San Roman, S., Wickline, S. A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Research. 71 (11), 3792-3801 (2011).
- Rak, J. Microparticles in cancer. Seminars in Thrombosis and Hemostasis. 36 (8), 888-906 (2010).
- Ledreux, A., et al. Small neuron-derived extracellular vesicles from individuals with down syndrome propagate tau pathology in the wildtype mouse brain. Journal of Clinical Medicine. 10 (17), 3931 (2021).
- Winston, C. N., et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimer's and Dementia: Diagnosis, Assessment and Disease Monitoring. 3, 63-72 (2016).
- Goetzl, E. J., Schwartz, J. B., Abner, E. L., Jicha, G. A., Kapogiannis, D. High complement levels in astrocyte-derived exosomes of Alzheimer's disease. Annals of Neurology. 83 (3), 544-552 (2018).
- Fiandaca, M. S., et al. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer's and Dementia. 11 (6), 600-607 (2015).
- Mustapic, M., et al. Plasma extracellular vesicles enriched for neuronal origin: A potential window into brain pathologic processes. Frontiers in Neuroscience. 11, 278 (2017).
- Hamlett, E. D., et al. Neuronal exosomes reveal Alzheimer's disease biomarkers in Down syndrome. Alzheimer's and Dementia. 13 (5), 541-549 (2017).
- Goetzl, E. J., et al. Abnormal levels of mitochondrial proteins in plasma neuronal extracellular vesicles in major depressive disorder. Molecular Psychiatry. 26 (12), 7355-7362 (2021).
- Goetzl, E. J., et al. Neuron-derived exosome proteins may contribute to progression from repetitive mild traumatic brain injuries to chronic traumatic encephalopathy. Frontiers in Neuroscience. 13, 452 (2019).
- Goetzl, E. J., et al. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer's disease. FASEB Journal. 30 (11), 3853-3859 (2016).
- Goetzl, E. J., et al. Traumatic brain injury increases plasma astrocyte-derived exosome levels of neurotoxic complement proteins. FASEB Journal. 34 (2), 3359-3366 (2020).
- Nogueras-ortiz, C. J., et al. Astrocyte- and neuron-derived extracellular vesicles from Alzheimer's disease patients effect complement-mediated neurotoxicity. Cells. 9 (7), 1618 (2020).
- Haydon, P. G., Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiological Reviews. 86 (3), 1009-1031 (2006).
- Abbott, N. J., Rönnbäck, L., Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews Neuroscience. 7 (1), 41-53 (2006).
- Rothstein, J., et al. Antisense knockout of glutamate transporters reveals a predominant role for astroglial glutamate transport in excitotoxicity and clearance of extracellular glutamate. Neuron. 16 (3), 675-686 (1996).
- Vasile, F., Dossi, E., Rouach, N. Human astrocytes: structure and functions in the healthy brain. Brain Structure and Function. 222 (5), 2017-2029 (2017).
- Balu, D. T., et al. Neurotoxic astrocytes express the D-serine synthesizing enzyme, serine racemase, in Alzheimer's disease. Neurobiology of Disease. 130, 104511 (2019).
- Sofroniew, M. V. Astrocyte barriers to neurotoxic inflammation. Nature Reviews Neuroscience. 16 (5), 249-263 (2015).
- Yun, S. P., et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nature Medicine. 24 (7), 931-938 (2018).
- Bianco, F., et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO Journal. 28 (8), 1043-1054 (2009).
- Datta Chaudhuri, A., et al. Stimulus-dependent modifications in astrocyte-derived extracellular vesicle cargo regulate neuronal excitability. Glia. 68 (1), 128-144 (2020).
- Serrano-Pozo, A., Gómez-Isla, T., Growdon, J. H., Frosch, M. P., Hyman, B. T. A phenotypic change but not proliferation underlies glial responses in Alzheimer disease. American Journal of Pathology. 182 (6), 2332-2344 (2013).
- Tong, J., et al. Low levels of astroglial markers in Parkinson's Disease: relationship to α-synuclein accumulation. Neurobiology of Disease. 82, 243-253 (2015).
- Kamo, H., et al. A distinctive distribution of reactive astroglia in the precentral cortex in amyotrophic lateral sclerosis. Acta Neuropathologica. 74 (1), 33-38 (1987).
- Coumans, F. A. W., et al. Methodological guidelines to study extracellular vesicles. Circulation Research. 120 (10), 1632-1648 (2017).
- Winston, C. N., Goetzl, E. J., Schwartz, J. B., Elahi, F. M., Rissman, R. A. Complement protein levels in plasma astrocyte-derived exosomes are abnormal in conversion from mild cognitive impairment to Alzheimer's disease dementia. Alzheimer's and Dementia: Diagnosis, Assessment and Disease Monitoring. 11, 61-66 (2019).
- Niu, Z., et al. Polymer-based precipitation preserves biological activities of extracellular vesicles from an endometrial cell line. PLoS ONE. 12 (10), 1-21 (2017).
- Alcolea, D., et al. The Sant Pau Initiative on Neurodegeneration (SPIN) cohort: A data set for biomarker discovery and validation in neurodegenerative disorders. Alzheimer's and Dementia: Translational Research and Clinical Interventions. 5, 597-609 (2019).
- O'Bryant, S. E., et al. Guidelines for the standardization of preanalytic variables for blood-based biomarker studies in Alzheimer's disease research. Alzheimer's and Dementia. 11 (5), 549-560 (2015).
- Kim, K., et al. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: Opportunities for developing novel therapeutics. Journal of Cellular Physiology. 226 (10), 2484-2493 (2011).
- Middeldorp, J., Hol, E. M. GFAP in health and disease. Progress in Neurobiology. 93 (3), 421-443 (2011).
- Mora, J., et al. Next generation ligand binding assays-review of emerging technologies' capabilities to enhance throughput and multiplexing. AAPS Journal. 16 (6), 1175-1184 (2014).
- Chunyk, A. G., et al. A multi-site in-depth evaluation of the quanterix simoa from a user's perspective. AAPS Journal. 20 (1), 1-12 (2018).
- Lötvall, J., et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. Journal of Extracellular Vesicles. 3 (1), 26913 (2014).
- Hansen, E. O., et al. Millipore xMap® Luminex (HATMAG-68K): An accurate and cost-effective method for evaluating Alzheimer's biomarkers in cerebrospinal fluid. Frontiers in Psychiatry. 12, 1-9 (2021).
- Kowal, J., et al. Proteomic comparison defines novel markers to characterize heterogenous populations of extracellular vesicle subtypes. Proceedings of the National Academy of Sciences. 113 (8), 968-977 (2016).
- Gauthier, S. A., et al. Enhanced exosome secretion in Down syndrome brain - a protective mechanism to alleviate neuronal endosomal abnormalities. Acta Neuropathologica Communications. 5 (1), 65 (2017).
- Cataldo, A. M., et al. Endocytic pathway abnormalities precede amyloid β deposition in sporadic alzheimer's disease and down syndrome: Differential effects of APOE genotype and presenilin mutations. American Journal of Pathology. 157 (1), 277-286 (2000).
- Cataldo, A. M., et al. Down syndrome fibroblast model of Alzheimer-related endosome pathology: Accelerated endocytosis promotes late endocytic defects. American Journal of Pathology. 173 (2), 370-384 (2008).
- Théry, C., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles. 7 (1), 1535750 (2018).
- Van Deun, J., et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nature Methods. 14 (3), 228-232 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved