Se requiere una suscripción a JoVE para ver este contenido. Inicie sesión o comience su prueba gratuita.
Method Article
Ensayo de aspiración con micropipeta de fluorescencia para investigar la mecanodetección de glóbulos rojos
En este artículo
Resumen
La exploración del comportamiento celular bajo estrés mecánico es fundamental para los avances en mecánica celular y mecanobiología. Presentamos la técnica de aspiración con micropipeta de fluorescencia (fMPA), un método novedoso que combina la estimulación mecánica controlada con un análisis exhaustivo de la señalización intracelular en células individuales. Esta técnica investiga nuevos estudios en profundidad de la mecanobiología de células vivas.
Resumen
Los ensayos de aspiración con micropipetas han sido durante mucho tiempo una piedra angular para la investigación de la mecánica de las células vivas, ya que ofrecen información sobre las respuestas celulares al estrés mecánico. En este artículo se detalla una adaptación innovadora del ensayo de aspiración de micropipetas acopladas a fluorescencia (fMPA). El ensayo fMPA introduce la capacidad de administrar fuerzas mecánicas precisas mientras se monitorean simultáneamente los procesos de mecanotransducción de células vivas mediados por canales iónicos. La sofisticada configuración incorpora una micropipeta de vidrio de borosilicato diseñada con precisión conectada a un depósito de agua finamente regulado y un sistema de aspiración neumática, lo que facilita la aplicación de presión controlada con incrementos tan refinados como ± 1 mmHg. Una mejora significativa es la integración de imágenes de epifluorescencia, que permite la observación y cuantificación simultáneas de los cambios morfológicos celulares y los flujos de calcio intracelular durante la aspiración. El ensayo fMPA, a través de su combinación sinérgica de imágenes de epifluorescencia con aspiración de micropipetas, establece un nuevo estándar para el estudio de la mecanodetección celular en entornos mecánicamente desafiantes. Este enfoque multifacético es adaptable a varias configuraciones experimentales, proporcionando información crítica sobre los mecanismos de mecanodetección de una sola célula.
Introducción
Los descubrimientos en desarrollo en el mundo de los comportamientos celulares han acentuado el papel de los estímulos mecánicos, como la tensión, el esfuerzo cortante del fluido, la compresión y la rigidez del sustrato, en el dictado de actividades celulares dinámicas como la adhesión, la migración y la diferenciación. Estos aspectos mecanobiológicos son de suma importancia para dilucidar cómo las células interactúan y responden a sus entornos fisiológicos, impactando en diversos procesos biológicos 1,2.
Durante la última década, los ensayos de aspiración basados en micropipetas se han destacado como una herramienta versátil en el estudio de diversas respuestas celulares a estímulos mecánicos. Esta técnica ofrece información valiosa sobre las propiedades mecánicas intrínsecas de las células vivas a nivel de una sola célula, incluido el módulo elástico celular, la rigidez y la tensión cortical. Estos ensayos permiten la medición de diversos parámetros mecánicos, como la tensión de la membrana celular, la presión ejercida sobre la membrana celular y la tensión cortical (resumida en la Tabla 1). El estudio de las fuerzas aspiracionales ha enriquecido nuestra comprensión de cómo influyen en las funciones y procesos celulares, particularmente en el ámbito de la dinámica de la membrana, incluida la fragmentación, la elongación y la gemación 3,4.
| Parámetro mecánico | Descripción | Enfoques seminales |
| Rigidez celular | Medición de la rigidez mecánica y elasticidad de una célula. | Aspiración de la membrana celular y análisis de la respuesta de deformación a la presión negativa20,21. |
| Fuerza de adhesión | Evaluación de la fuerza con la que las células se adhieren a las superficies. | Aplicación de succión controlada para separar las células adheridas de un sustrato2,22. |
| Tensión de la membrana | Evaluación de la tensión o tensión dentro de las membranas celulares. | Medición de la deformación de la membrana en respuesta a la presión aplicada23,24. |
| Propiedades viscoelásticas | Caracterización del comportamiento viscoso y elástico combinado de una célula. | Análisis de la respuesta de deformación dependiente del tiempo a la aspiración23,25. |
| Deformabilidad | Determinación de la facilidad con la que una célula puede cambiar de forma. | Evaluación del grado de deformación bajo aspiración controlada20,24. |
| Tensión superficial | Medición de la tensión en la superficie de la célula. | Evaluación de la presión necesaria para formar una protuberancia de membrana de micropipeta26. |
| Interacción célula-material | Estudio de las interacciones entre células y materiales o sustratos. | Aspiración de células en contacto con diferentes materiales y observación de interacciones2,24. |
| Interacción célula-célula | Examen de las interacciones entre células vecinas. | Aspiración de un grupo de células y análisis de sus fuerzas intercelulares27. |
Tabla 1: Parámetros mecánicos caracterizados por el ensayo de aspiración con micropipeta.
La técnica de aspiración basada en micropipetas se ha utilizado ampliamente para estudiar los glóbulos rojos (RBC), evaluando la deformabilidad y diversas características mecánicas de los RBC, lo cual es esencial para comprender su función en el sistema circulatorio. Los glóbulos rojos exhiben una notable adaptabilidad, preservando su versatilidad mecánica contra la deformación cuando navegan a través de la intrincada red capilar y las hendiduras interendoteliales 5,6. Durante este viaje, los glóbulos rojos deben atravesar pasajes tan estrechos como 0,5-1,0 μm, sometiéndose a una multitud de fuerzas mecánicas, incluida la tensión y la compresión 7,8,9. También tienen una alta sensibilidad al esfuerzo cortante generado por el flujo sanguíneo durante la circulación10. Estos procesos promueven la activación de mecanismos reguladores que involucran la entrada de calcio, un evento de señalización crucial con roles bien establecidos en las respuestas celulares a los estímulos mecánicos11,12. Los complejos mecanismos que gobiernan la mecanodetección mediada por calcio siguen siendo temas de investigación en curso.
En este contexto, el fMPA se presenta como un enfoque eficaz para revelar el alcance de la movilización de calcio bajo fuerzas mecánicas controladas con precisión, lo que permite la aplicación simultánea de la modulación mecánica (utilizando el sistema de aspiración de micropipetas) y la visualización de la intensidad del calcio (utilizando indicadores fluorescentes). Imita particularmente el escenario fisiológico cuando los glóbulos rojos viajan a través del estrechamiento de los vasos sanguíneos. Vale la pena señalar que el sistema fMPA que desarrollamos puede generar presión con una resolución de 1 mmHg. La cámara de alta velocidad implementada puede alcanzar una resolución temporal de 100 ms y una resolución espacial a nivel submicrónico. Estas configuraciones aseguran la aplicación precisa de fuerzas mecánicas a las células vivas y, al mismo tiempo, capturan la señalización celular resultante. Además, debido a la naturaleza de ingeniería integradora de esta configuración, el ensayo de aspiración de micropipetas se puede adaptar fácilmente para complementar otros equipos o técnicas, lo que permite una mayor exploración de las complejidades de la mecánica celular. Esta versatilidad se erige como una ventaja adicional de este enfoque.
Protocolo
Este protocolo sigue las directrices y ha sido aprobado por el Comité de Ética de Investigación en Seres Humanos de la Universidad de Sydney. Se obtuvo el consentimiento informado de los donantes para este estudio.
1. Aislamiento de glóbulos rojos humanos
NOTA: El paso 1.1 debe ser realizado por un flebotomista capacitado utilizando un protocolo que haya sido aprobado por la Junta de Revisión Institucional.
- Extraiga 5 ml de sangre de la vena cubital mediana con una aguja de mariposa de 19 G.
- Transfiera la sangre recolectada a un tubo de 15 ml que contenga enoxaparina 1:200 para prevenir la coagulación.
- Diluir 5 μL de sangre anticoagulada con enoxaparina en 1 mL de tampón carbonato/bicarbonato (tampón C, pH = 8,5-9; Tabla de Materiales).
- Centrifugar la muestra de sangre diluida a 900 × g durante 1 min para sedimentar los glóbulos rojos. Decantar cuidadosamente el sobrenadante sin alterar el gránulo.
- Realizar dos lavados del gránulo RBC con 1 mL de tampón C (Tabla de Materiales), centrifugando cada vez a 900 × g durante 1 min.
- Posteriormente, lave el gránulo de RBC 2 veces con 1 mL de tampón Tyrode utilizando las mismas condiciones de centrifugación y luego vuelva a suspender el gránulo final en 1 mL de tampón Tyrode para obtener la suspensión de RBC de material lavado.
2. Carga del indicador de calcio
- Ajuste la concentración de la solución madre lavada de glóbulos rojos a 10 × 106 células/ml en el tampón de Tyrode, basándose en el recuento de células obtenido utilizando un contador automático de células (Tabla de materiales).
- Etiquete el calcio dentro de los glóbulos rojos incubando con 16,67 μM Cal-520 AM, un colorante sensible al calcio, mientras agita en un mezclador de tubo giratorio durante 1 h.
- Diluir los glóbulos rojos en el tampón de Tyrode que contiene albúmina sérica bovina (BSA) al 0,5% en una proporción de 1:50. Las células ya están listas para su uso experimental.
3. Fabricación de micropipetas
- Monte el tubo capilar de vidrio de borosilicato (1 mm de diámetro exterior x 0,6 mm de diámetro interior) en el extractor de micropipetas P-1000 para producir dos micropipetas correspondientes con puntas cerradas en el lugar de extracción utilizando el programa de extracción preestablecido. Para esta configuración, utilice los siguientes valores del programa de tracción: calor 516, tracción 150, velocidad 75, tiempo 250 y presión 500.
NOTA: Los parámetros de calentamiento y tracción establecidos en el programa de tracción se pueden personalizar y dependen de la configuración deseada del diseño experimental12. CHECKPOINT (véase el cuadro complementario S1). - Abra la punta cerrada montando una de las micropipetas cerradas obtenidas después de tirar de la fresa de micropipetas. Ajuste la temperatura de calentamiento a aproximadamente 50-60 °C.
- Localice la micropipeta con un ocular de 10x. Acerque la micropipeta a la perla de vidrio de borosilicato utilizando las perillas para el ajuste.
- Cambie el ocular a 30x antes de colocar la micropipeta lo más cerca posible de la perla de vidrio de borosilicato sin doblar la punta de la pipeta.
- Ablande la perla de vidrio de borosilicato con calor pisando el pedal calefactor. Inserte suavemente la punta de la micropipeta cerrada sin procesar en el cordón ablandado hasta que se haya alcanzado el punto final deseado, el diámetro de apertura.
- Suelte el pedal y deje que la cuenta de vidrio se enfríe. Asegúrese de que la punta de la micropipeta permanezca siempre dentro de la perla.
NOTA: La inserción adicional de la punta conduce a diámetros de apertura más grandes. - Extraiga suavemente la micropipeta, lo que dará lugar a un corte recto claro en la micropipeta cerrada. Confirme que el diámetro final del capilar es de 1 μm.
NOTA: PUNTO DE CONTROL (véase el cuadro complementario S1)
4. Preparación de la cámara celular
- Utiliza un lápiz de diamante para dividir un cubreobjetos de vidrio estándar de 40 mm x 22 mm x 0,17 mm en tres tiras iguales.
- Adhiera una pieza del cubreobjetos de vidrio cortado al fondo de un soporte de cámara casero con grasa para aspiradora.
NOTA: El soporte de la cámara consta de dos cuadrados de metal (cobre/aluminio) que están unidos por un mango curvo. La distancia entre los bloques metálicos debe ser inferior a 40 mm para que el cubreobjetos cortado se adhiera al soporte para formar una cámara paralela. - Adhiera la segunda pieza del cubreobjetos de vidrio cortado a la parte superior del soporte de cámara casero con grasa de vacío.
NOTA: PUNTO DE CONTROL (véase el cuadro complementario S1) - Inyecte 200 μL de la suspensión RBC marcada entre dos cubreobjetos con una pistola de pipetas de 200 μL (Figura 1).
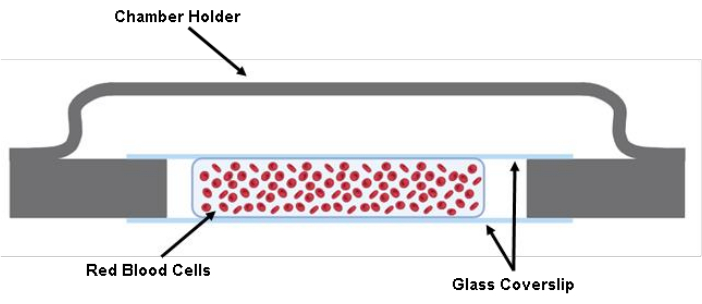
Figura 1: Ilustración de la cámara celular. Dos piezas cortadas de un cubreobjetos de vidrio de 40 mm x 22 mm x 0,17 mm se adhieren al soporte de la cámara con grasa. Entre los dos cubreobjetos de vidrio cortado, se siembran aproximadamente 200 μL de la solución celular en Tyrode's Buffer. Haga clic aquí para ver una versión más grande de esta figura.
5. Conjunto de aspiración de micropipetas
- Monte la cámara celular en la platina de soporte presente en la plataforma del microscopio. Ajuste la posición de modo que la cámara de la celda esté directamente encima del objetivo (Figura 2B).
- Baje el soporte de la micropipeta por debajo del nivel de líquido del depósito de agua conectado.
- Inyecte agua desmineralizada o tampón Tyrode en la micropipeta fabricada y elimine con cuidado todas las burbujas de aire con una jeringa acoplada a una aguja de 34 G (consulte la Tabla de materiales).
- Desenrosque el extremo del soporte de la micropipeta hasta la mitad y deje que el agua gotee del soporte de la micropipeta durante unos segundos.
NOTA: PUNTO DE CONTROL (véase el cuadro complementario S1) - Inserte la micropipeta en la punta del soporte. Apriete el tornillo del soporte para asegurarse de que la micropipeta esté fija.
- Inserte la micropipeta en la cámara celular y ubique la micropipeta y los glóbulos rojos bajo el microscopio. Utilice el micromanipulador para ajustar la posición.
- Baje aún más la punta de la micropipeta para asegurarse de que la punta esté nivelada con el RBC ubicado.
NOTA: PUNTO DE CONTROL (véase el cuadro complementario S1) - Ponga a cero la presión hidráulica en la punta de la micropipeta ajustando la altura del depósito de agua. Luego, levante ligeramente el depósito de agua para generar una presión positiva sutil en la punta.
6. Realice el ensayo de aspiración de micropipetas acopladas a fluorescencia
- Encienda la fuente de luz de excitación fluorescente de 488 nm. No encienda el obturador de fluorescencia en esta etapa para evitar la fotodecoloración (Figura 2C). Encienda la cámara de fluorescencia y la cámara transmitida.
NOTA: Ambas cámaras se utilizan con el software adecuado (consulte la Tabla de materiales). - Configure el tiempo de exposición deseado (100 ms para ambas cámaras en este estudio), la región de interés (ROI) y el tamaño de agrupación (ninguno para este estudio) para ambas cámaras en el software. Abra el panel de adquisición multidimensional para configurar el número de trama de adquisición, 2.000 para este estudio y el directorio de guardado.
NOTA: El número de trama de adquisición depende del número deseado de eventos de aspiración que se van a registrar. Para 1 evento de aspiración, el rango del número de adquisición debe establecerse entre 100 y 500, que es aproximadamente de 10 a 50 s. - Encuentre la micropipeta debajo del campo de visión con el micromanipulador.
- Encienda la pinza de presión neumática, incluida la caja de control y el sistema de abrazadera (Figura 2A). Asegúrese de que la caja de control esté en el modo EXTRNL. Compense cualquier presión de compensación dentro del sistema girando lentamente la perilla.
- Encienda el software separado que controla la abrazadera neumática. El software tiene un panel de control eléctrico para controlar la entrada analógica discreta al sistema de abrazaderas. La presión se controla con un factor de conversión de 20 mV/mmHg.
- Ponga a cero la presión dentro del sistema. Vuelva a colocar con cuidado la micropipeta cerca de los glóbulos rojos. Ajuste la posición del depósito de agua hasta que se note una sutil presión positiva en la punta de la micropipeta.
- Inicie la adquisición en el software de manejo de la cámara. Encienda el obturador de fluorescencia.
- Aspire un RBC escribiendo la magnitud de voltaje calculada en el panel de control para alcanzar la presión deseada.
NOTA: La presión para aspirar un eritromatíes suele estar en el rango de Δp = -5 a -40 mmHg. Debe haber un alargamiento notable de la lengua dentro de la punta de la micropipeta (Figura 2D). - Mantenga la presión durante un período preestablecido; Luego, libera la presión.
- Mueva la micropipeta para recoger la siguiente célula y repita el experimento.
7. Análisis de intensidad de fluorescencia
- Cargue las imágenes de fluorescencia guardadas en el software de análisis.
- Ajuste el umbral de intensidad utilizando la pestaña de ajuste de pantalla . Para ello, introduzca manualmente los valores o utilice el control deslizante para asegurarse de que las imágenes de fluorescencia muestren un contraste claro de la célula en el software de análisis (consulte el Archivo complementario 1-Figura suplementaria S1).
- Desplácese hasta la línea de tiempo en la parte inferior del software. Localice el evento de aspiración designado.
- Haga clic en Agregar nuevas superficies. Definir el ROI del análisis.
NOTA: El software proporciona un proceso guiado de cinco pasos para ajustar y completar la segmentación (consulte el Archivo Suplementario 1-Figura Suplementaria S2 y la Figura Suplementaria S3).
NOTA: Mantenga el ROI lo más pequeño posible para ahorrar recursos computacionales. - Utilice el control deslizante de sustracción de fondo y ajuste el umbral de segmentación con el control deslizante para obtener el mejor resultado de segmentación.
NOTA: Esto significa que, aparte del evento de aspiración, el fondo debe segmentarse con la mayor precisión posible (consulte el Archivo Suplementario 1-Figura Suplementaria S4 y la Figura Suplementaria S5). - Agregue un filtro de área para excluir los ruidos de fondo (consulte el Archivo complementario 1-Figura complementaria S6).
NOTA: Esto se completa en la etapa posterior al proceso. - Seleccione la pestaña de estadísticas | Pestaña Detallado | Pestaña Valores promedio . Desplácese para buscar y seleccionar la media de intensidad (consulte el Archivo Suplementario 1-Figura Suplementaria S7).
- Exporte el rastro de la señal de fluorescencia a lo largo del tiempo a un archivo .csv.
- Abra el archivo csv exportado. Reste las señales de fondo, Fb, de todas las mediciones.
- Calcule el cambio de intensidad de calcio, ΔFmáx., usando la ecuación (1):
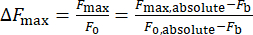 (1)
(1)
Donde ΔFmax es el cambio máximo de intensidad de calcio, Fb es la intensidad de fondo y F0 es la intensidad de reposo.
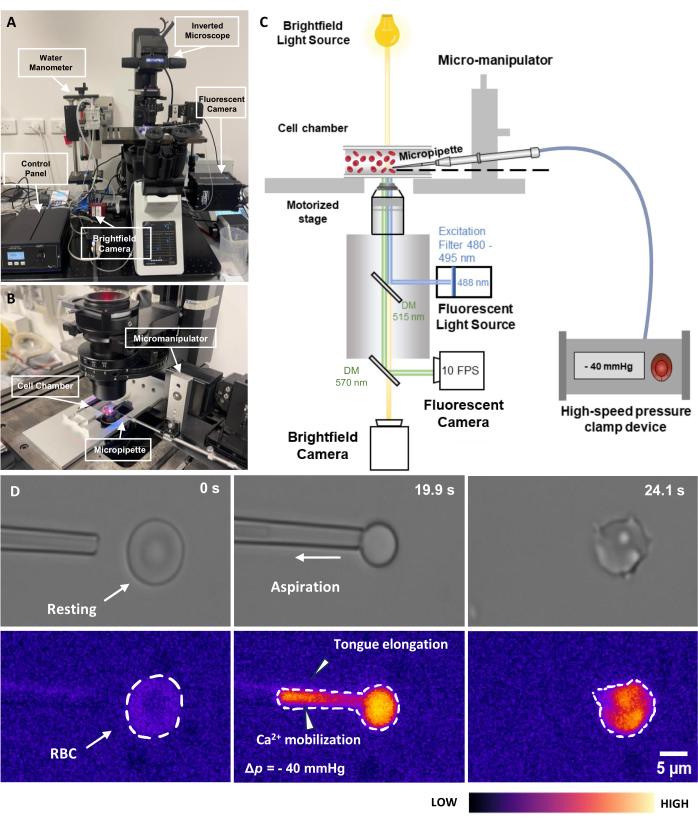
Figura 2: Conjunto de aspiración de micropipetas acopladas a fluorescencia. (A) Una descripción general del sistema de hardware fMPA que incorpora el microscopio invertido combinado con las cámaras de campo claro y fluorescencia. El lado izquierdo de la imagen muestra el manómetro de agua casero y la caja de control que permite ajustar con precisión la presión de la bomba de presión neumática. (B) La etapa del microscopio que representa la cámara de la célula del experimento y el sistema de micromanipulación con una sola micropipeta. (C) Esquema de la configuración del sistema fMPA. Imágenes simultáneas de señales de campo claro (amarillo) y fluorescencia (emisión azul, excitación verde) utilizando dos espejos dicroicos para dirigir las trayectorias de luz desde la fuente de luz de fluorescencia (azul) hasta el objetivo, y luego a las cámaras para obtener imágenes (verde). (D) La fila superior muestra las imágenes de campo claro, mientras que la fila inferior muestra las imágenes de fluorescencia. La izquierda representa la posición de la micropipeta antes de la aspiración cuando el RBC está en reposo. La columna central muestra una instantánea del proceso de aspiración en el que el eritromatíes experimenta una presión negativa de -40 mmHg. A la derecha se muestra la morfología celular después de experimentar la presión de aspiración negativa. Barra de escala = 5 μm. Abreviaturas: fMPA = Aspiración de micropipetas acopladas a fluorescencia; DM = espejo dicroico; RBC = glóbulos rojos. Haga clic aquí para ver una versión más grande de esta figura.
Resultados
Para establecer los ensayos de aspiración de micropipetas, primero construimos una cámara celular personalizada que comprendía dos cuadrados de metal (cobre/aluminio) conectados por un mango. Se colocaron dos cubreobjetos de vidrio de tercer corte (40 mm × 7 mm × 0,17 mm) para crear una cámara llena de 200 μL de glóbulos rojos suspendidos en el tampón de Tyrode. Después de introducir los glóbulos rojos en la cámara, se fijó una micropipeta de borosilicato a medida en un soporte y se colocó cuidadosamente de...
Discusión
Los ensayos de aspiración con micropipetas incorporan una metodología refinada, que despliega una modulación de presión sustancial, una orquestación espacial exacta y un discernimiento temporal fiable para sondear las profundas complejidades de la biomecánica celular. Este estudio pone especial énfasis en la aplicación de fMPA como una herramienta crucial para revelar las respuestas mecanosensibles matizadas que muestran los glóbulos rojos bajo diferentes estímulos. El uso simultáneo de señales de campo claro...
Divulgaciones
Los autores declaran que no tienen intereses contrapuestos que informar con respecto al presente estudio.
Agradecimientos
Agradecemos a Nurul Aisha Zainal Abidin y Laura Moldovan por el reclutamiento adicional de donantes, la recolección de sangre y el apoyo a la flebotomía. Agradecemos a Tomas Anderson y Arian Nasser por organizar el equipo y los reactivos. Esta investigación fue financiada por el Proyecto de Descubrimiento del Consejo Australiano de Investigación (ARC) (DP200101970-L. A.J.); el Consejo Nacional de Salud e Investigación Médica (NHMRC) de Australia Ideas Grant (APP2003904-L. A.J.); Beca de Equipos del NHMRC-L.A.J.; Programa de Desarrollo de Capacidades Cardiovasculares de Nueva Gales del Sur (Beca para Investigadores de Inicio-Mitad de Carrera-L.A.J.); Beca de Innovación en Investigación NSW CVRN-VCCRI; Oficina de Compromiso Global y de Investigación (Premio de Colaboración de la Asociación Sydney-Glasgow-L.A.J.); L.A.J. es becario de nivel 2 (105863) de la Fundación Nacional del Corazón para futuros líderes y miembro de la Fundación de Investigación Médica Snow (2022SF176).
Materiales
| Name | Company | Catalog Number | Comments |
| µManager | Micro-Manager | Version 2.0.0 | |
| 1 mL Syringe | Terumo | 210320D | Cooperate with the Microfil |
| 200 µL Pipette | Eppendorf | 3123000055 | Red clood cell preparation |
| 22 x 40 mm Cover Slips | Knittel Glass | MS0014 | Cell chamber assembly |
| 50 mL Syringe | Terumo | 220617E | Connect to the water tower |
| Calcium Chloride (CaCl2) | Sigma-Aldrich | C1016 | Tryode's buffer preparation - 12 mM NaHCO3, 10 mM HEPES, 0.137 M NaCl, 2.7 mM KCl, and 5.5 mM D-glucose supplemented with 1 mM CaCl2. Final pH = 7.2 |
| Centrifuge 5425 | Eppendorf | 5405000280 | Red clood cell preparation |
| Clexane | Sigma-Aldrich | 1235820 | To prevent clotting of the collected blood. 10,000 U/mL |
| DAQami | Diligent | ||
| Fluorescence light source | CoolLED | pE-300 | Micropipette aspiration hardware system |
| Glass capillary | Narishige | G-1 | Micropipette manufacture |
| Glucose | Sigma-Aldrich | G8270 | Tryode's buffer preparation - 12 mM NaHCO3, 10 mM HEPES, 0.137 M NaCl, 2.7 mM KCl, and 5.5 mM D-glucose supplemented with 1 mM CaCl2. Final pH = 7.2 |
| Hepes | Thermo Fisher | 15630080 | Tryode's buffer preparation - 12 mM NaHCO3, 10 mM HEPES, 0.137 M NaCl, 2.7 mM KCl, and 5.5 mM D-glucose supplemented with 1 mM CaCl2. Final pH = 7.2 |
| High speed GigE camera | Manta | G-040B | Micropipette aspiration hardware system |
| High speed pressure clamp | Scientific Instrument | HSPC-2-SB | Cooperate with the pressure pump |
| High speed pressure clamp head stage | Scientific Instrument | HSPC-2-SB | Cooperate with the pressure pump |
| Imaris | Oxford Instruments | ||
| Inverted Microscopy | Olympus | Olympus IX83 | Micropipette aspiration hardware system |
| Microfil | World Precision Instruments | MF34G-5 | 34 G (67 mm Long) Revome air bubble in the cut micropipette and test the opening of the pipette tip |
| Micropipette Puller | Sutter instrument | P1000 | Micropipette manufacture |
| Milli Q EQ 7000 Ultrapure Water Purification System | Merck Millipore | ZEQ7000T0C | Carbonate/bicarbonate buffer & Tryode's buffer preparation |
| Pipette microforge | Narishige | MF-900 | Micropipette manufacture |
| Potassium Chloride (KCl) | Sigma-Aldrich | P9541 | Tryode's buffer preparation - 12 mM NaHCO3, 10 mM HEPES, 0.137 M NaCl, 2.7 mM KCl, and 5.5 mM D-glucose supplemented with 1 mM CaCl2. Final pH = 7.2 |
| Pressue Pump | Scientific Instrument | PV-PUMP | Induce controlled pressure during experiment |
| Prime 95B Camera | Photometrics | Prime 95B sCMOS | Flourscent imaging |
| Rotary wheel remote unit | Sensapex | uM-RM3 | Control panel for micropipette position adjustment |
| Scepter 3.0 Handheld Cell Counter | Merck Millipore | PHCC340KIT | Automatic cell counter |
| Sodium Bicarbonate (NaHCO3) | Sigma-Aldrich | S5761 | Carbonate/bicarbonate buffer preparation - 2.65 g of NaHCO3 with 2.1 g of Na2CO3 in 250 mL of Mili Q water - Final pH = 8-9. |
| Sodium Carbonate (Na2CO3) | Sigma-Aldrich | S2127 | Carbonate/bicarbonate buffer preparation - 2.65 g of NaHCO3 with 2.1 g of Na2CO3 in 250 mL of Mili Q water - Final pH = 8-9. |
| Sodium Chloride (NaCl) | Sigma-Aldrich | S7653 | Tryode's buffer preparation - 12 mM NaHCO3, 10 mM HEPES, 0.137 M NaCl, 2.7 mM KCl, and 5.5 mM D-glucose supplemented with 1 mM CaCl2. Final pH = 7.2 |
| Sodium Phosphate Monobasic Monohydrate (NaH2PO4 • H2O) | Sigma-Aldrich | S9638 | Tryode's buffer preparation - 12 mM NaHCO3, 10 mM HEPES, 0.137 M NaCl, 2.7 mM KCl, and 5.5 mM D-glucose supplemented with 1 mM CaCl2. Final pH = 7.2 |
| Touch screen control unit | Sensapex | uM-TSC | Control panel for micropipette position adjustment |
| X dry Objective | Olympus | Olympus 60x/0.70 LUCPlanFL | Micropipette aspiration hardware system |
Referencias
- González-Bermúdez, B., Guinea, G. V., Plaza, G. R. Advances in micropipette aspiration: applications in cell biomechanics, models, and extended studies. Biophysical Journal. 116 (4), 587-594 (2019).
- Mierke, C. T. . Physics of Cancer, Volume 3 (Second Edition): Experimental biophysical techniques in cancer research. , (2021).
- Chen, Y., et al. Loss of the F-BAR protein CIP4 reduces platelet production by impairing membrane-cytoskeleton remodeling. Blood. 122 (10), 1695-1706 (2013).
- Shin, J. -. W., Swift, J., Spinler, K. R., Discher, D. E. Myosin-II inhibition and soft 2D matrix maximize multinucleation and cellular projections typical of platelet-producing megakaryocytes. Proceedings of the National Academy of Sciences. 108 (28), 11458-11463 (2011).
- Liapis, H., Foster, K., Miner, J. H. Red cell traverse through thin glomerular basement membrane. Kidney International. 61 (2), 762-763 (2002).
- Wang, H., et al. Fluorescence-coupled micropipette aspiration assay to examine calcium mobilization caused by red blood cell mechanosensing. European Biophysics Journal. 51 (2), 135-146 (2022).
- Danielczok, J. G., et al. Red blood cell passage of small capillaries is associated with transient Ca2+-mediated adaptations. Frontiers in Physiology. 8, 979 (2017).
- Diez-Silva, M., Dao, M., Han, J., Lim, C. -. T., Suresh, S. Shape and biomechanical characteristics of human red blood cells in health and disease. MRS Bulletin. 35 (5), 382-388 (2010).
- Maître, J. -. L., Niwayama, R., Turlier, H., Nédélec, F., Hiiragi, T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nature Cell Biology. 17 (7), 849-855 (2015).
- Ju, L., Chen, Y., Xue, L., Du, X., Zhu, C. Cooperative unfolding of distinctive mechanoreceptor domains transduces force into signals. eLife. 5, e15447 (2016).
- Bogdanova, A., Makhro, A., Wang, J., Lipp, P., Kaestner, L. Calcium in red blood cells-a perilous balance. International Journal of Molecular Sciences. 14 (5), 9848-9872 (2013).
- . Pipette Cookbook 2018 Available from: https://www.sutter.com/PDFs/cookbook.pdf (2018)
- Cahalan, S. M., et al. Piezo1 links mechanical forces to red blood cell volume. eLife. 4, e07370 (2015).
- Sforna, L., et al. Piezo1 controls cell volume and migration by modulating swelling-activated chloride current through Ca2+ influx. Journal of Cellular Physiology. 237 (3), 1857-1870 (2022).
- High speed pressure clamp. ALA Scientific Instruments Available from: https://alascience.com/products/hspc-2sb/ (2023)
- . Teledyne Imaging Prime 95BTM Scientific CMOS Camera Datasheet Available from: https://www.photometrics.com/wp-content/uploads/2019/10/Prime-95B-Datasheet-07172020.pdf (2020)
- Lee, L. M., Lee, J. W., Chase, D., Gebrezgiabhier, D., Liu, A. P. Development of an advanced microfluidic micropipette aspiration device for single cell mechanics studies. Biomicrofluidics. 10 (5), 054105 (2016).
- Weaver, W. M., et al. Advances in high-throughput single-cell microtechnologies. Current Opinion in Biotechnology. 25, 114-123 (2014).
- Zhou, E. H., Lim, C. T., Quek, S. T. Finite element simulation of the micropipette aspiration of a living cell undergoing large viscoelastic deformation. Mechanics of Advanced Materials and Structures. 12 (6), 501-512 (2005).
- Oh, M. -. J., Kuhr, F., Byfield, F., Levitan, I. Micropipette aspiration of substrate-attached cells to estimate cell stiffness. Journal of Visualized Experiments. (67), e3886 (2012).
- Rand, R. P., Burton, A. C. Mechanical properties of the red cell membrane. Biophysical journal. 4 (4), 303-316 (1964).
- Hogan, B., Babataheri, A., Hwang, Y., Barakat, A. I., Husson, J. Characterizing cell adhesion by using micropipette aspiration. Biophysical Journal. 109 (2), 209-219 (2015).
- Henriksen, J. R., Ipsen, J. H. Measurement of membrane elasticity by micro-pipette aspiration. The European Physical Journal E. 14 (2), 149-167 (2004).
- Hochmuth, R. M. Micropipette aspiration of living cells. Journal of Biomechanics. 33 (1), 15-22 (2000).
- Pu, H., et al. Micropipette aspiration of single cells for both mechanical and electrical characterization. IEEE Transactions on Biomedical Engineering. 66 (11), 3185-3191 (2019).
- Guevorkian, K., Maître, J. -. L. Chapter 10 - Micropipette aspiration: A unique tool for exploring cell and tissue mechanics in vivo. Methods in Cell Biology. , 187-201 (2017).
- Biro, M., Maître, J. -. L. Chapter 14 - Dual pipette aspiration: A unique tool for studying intercellular adhesion. Methods in Cell Biology. 125, 255-267 (2015).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoThis article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados