Se requiere una suscripción a JoVE para ver este contenido. Inicie sesión o comience su prueba gratuita.
Method Article
Condensados sintéticos y arquitecturas similares a células a partir de nanoestructuras de ADN anfifílico
En este artículo
Resumen
Presentamos un protocolo para la preparación de condensados biomoleculares sintéticos consistentes en nanoestrellas de ADN anfifílico a partir de sus oligonucleótidos de ADN constituyentes. Los condensados se producen a partir de un solo componente de nanoestrella o de dos componentes y se modifican para mantener la transcripción in vitro de ARN a partir de una plantilla de ADN incrustada.
Resumen
Las gotas y condensados sintéticos se están convirtiendo en constituyentes cada vez más comunes de los sistemas biomiméticos avanzados y las células sintéticas, donde se pueden utilizar para establecer la compartimentación y mantener respuestas similares a las de la vida. Las nanoestructuras de ADN sintético han demostrado un potencial significativo como bloques de construcción formadores de condensado debido a su forma programable, funcionalización química y comportamiento de autoensamblaje. Recientemente hemos demostrado que las "nanoestrellas" de ADN anfifílico, obtenidas mediante el marcaje de uniones de ADN con fracciones hidrofóbicas, constituyen una solución particularmente robusta y versátil. Los condensados de ADN anfifílico resultantes pueden programarse para mostrar arquitecturas internas complejas de múltiples compartimentos, responder estructuralmente a diversos estímulos externos, sintetizar macromoléculas, capturar y liberar cargas útiles, sufrir transformaciones morfológicas e interactuar con células vivas. Aquí, demostramos protocolos para la preparación de condensados de ADN anfifílico a partir de oligonucleótidos de ADN constituyentes. Abordaremos (i) sistemas de un solo componente que forman condensados uniformes, (ii) sistemas de dos componentes que forman condensados de núcleo y (iii) sistemas en los que los condensados se modifican para soportar la transcripción in vitro de nanoestructuras de ARN.
Introducción
Las células sintéticas son dispositivos a escala micrométrica (10-50 μm) construidos de abajo hacia arriba para replicar funciones y estructuras de las células biológicas existentes 1,2. Las células sintéticas a menudo están unidas por membranas construidas a partir de vesículas bicapa lipídica 3,4,5,6,7, polimerosomas 8,9 o proteinosomas10,11, que también se pueden utilizar para establecer la compartimentación interna12,13. Inspiradas en los orgánulos sin membrana que se sabe que sostienen diversas funcionalidades en las células vivas14, estructuras como los coacervados poliméricos, los condensados biomoleculares y los hidrogeles están ganando terreno como alternativas versátiles y robustas para establecer la compartimentación externa e interna en las células sintéticas 15,16,17,18.
Aprovechando el versátil conjunto de herramientas de la nanotecnología de ADN19, se han desarrollado múltiples soluciones para diseñar gotas y condensados sintéticos a partir del autoensamblaje de nanoestructuras artificiales de ADN, cuyo tamaño, forma, funcionalidad, valencia e interacciones mutuas se pueden programar con precisión20. Las gotas o condensados de ADN son biocompatibles y pueden actuar como andamios tanto para las células sintéticas como para los orgánulos, albergando reacciones químicas y biomoleculares21, computando información22,23, capturando y liberando cargas24,25 y sosteniendo respuestas estructurales26.
Entre los diversos diseños de nanoestructuras de ADN formadoras de condensado, las nanoestrellas de ADN anfifílicas, denominadas estrellas C, han demostrado ser robustas y versátiles27. Las estrellas C son motivos ramificados simples que consisten en una unión de ADN fija (generalmente de cuatro vías), de la cual emergen brazos de ADN de doble cadena (ds)28. Luego, los brazos se llenan de fracciones hidrofóbicas, generalmente colesterol, lo que hace que las nanoestructuras sean anfifílicas e impulsan su condensación después de un recocido sencillo de una sola olla. Los condensados C-star ofrecen una programabilidad estructural y funcional precisa, incluida la posibilidad de establecer arquitecturas multicompartimentales29,30, responder estructuralmente a los desencadenantes de ADN y cationes31, sintetizar macromoléculas29, capturar y liberar cargas útiles32 e interactuar con células vivas33. A continuación, describiremos y discutiremos los protocolos para producir condensados de estrellas C a partir de sus oligonucleótidos constituyentes.
El protocolo resume la preparación de condensados unarios (de un componente) y binarios (de dos componentes), utilizando tres diseños diferentes de estrellas C (Figura 1): "No sensible", "Sensible a TMSD" y "Plantillas de ARN". La estrella C "no sensible" (panel A) consta de cuatro "hebras centrales" con secuencias distintas que forman la unión de cuatro vías. Cuatro oligonucleótidos idénticos modificados con colesterol están conectados a la unión, lo que garantiza que una molécula de colesterol esté presente al final de cada brazo. Las estrellas C que no responden constituyen andamios simples e inertes para condensados unarios y binarios. En la estrella C "sensible a TMSD" (panel B), la conexión entre las hebras colesterolizadas y la unión está asegurada por una hebra de "puente de detención", que presenta un dominio de "toehold" de ADN monocatenario (ss) colgante. En presencia de una hebra de ADN invasora con un dominio de punto de apoyo complementario, se puede desencadenar una reacción de desplazamiento de la hebra mediada por un punto de apoyo34, mediante la cual el invasor desplaza el puente de detención, rompiendo la conexión entre la unión y las partes hidrofóbicas y desencadenando el desmontaje de la red de ADN32. Por último, la estrella C de "plantillas de ARN" (panel C) incluye una modificación de "Base" complementaria a una hebra "puente", la última de las cuales enlaza la plantilla de ssDNA transcribible para el aptámero de brócoli29. Los detalles de la secuencia de los oligonucleótidos constituyentes para los tres tipos de diseños de estrellas C mencionados aquí se pueden encontrar en la Tabla Suplementaria 1 y en trabajos anteriores 29,30,32.
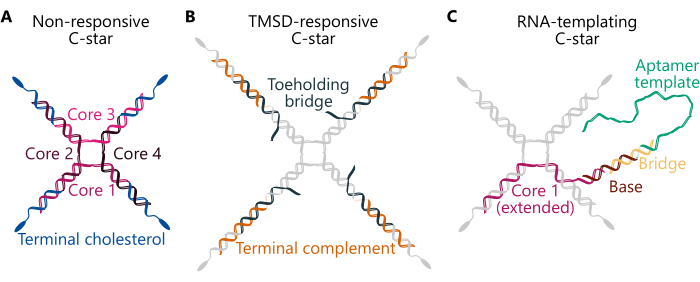
Figura 1. Esquemas de tres diseños diferentes de nanoestrellas de ADN anfifílico (estrellas C). Las secuencias de oligonucleótidos para varios ejemplos de las estrellas C descritas aquí se pueden encontrar en la Tabla Suplementaria 1. (A) Esquema de una estrella C diseñada para formar condensados que no responden, con las hebras de oligonucleótidos componentes "Core 1", "Core 2", "Core 3", "Core 4" (coloreadas en tonos rosados) y "Colesterol terminal" (coloreadas en azul). Cada color único representa una cadena de oligonucleótidos de secuencia única. "Core 1" y "Core 3" son parcialmente complementarios a "Core 2" y "Core 4", pero no complementarios entre sí. (B) Esquema de una estrella C diseñada para desmontarse tras la adición de una hebra invasora a través del desplazamiento de la hebra mediada por un punto de apoyo, como se describe en el trabajo anterior32. Esta estrella C está compuesta por las hebras "Core" y "Terminal cholesterol" (coloreadas en gris), así como una hebra "Terminal complemento" (mostrada en naranja) y una hebra "Toeholding bridge" (mostrada en verde azulado oscuro). Este último contiene un saliente de seis nucleótidos al que una hebra invasora adecuadamente diseñada puede unirse y posteriormente desplazar por completo la hebra del "puente de sostenimiento del pie", lo que provoca la disociación de la unión central de la nanoestrella (compuesta por el "Núcleo 1, 2, 3 y 4") de los dúplex compuestos por las hebras "Complemento terminal" y "Colesterol terminal". (C) Esquema de una estrella C funcionalizada con una plantilla de ADN para un aptámero de ARN. Este también está compuesto por la hebra de "colesterol terminal" y el "Núcleo 2, 3 y 4" (todos mostrados en gris), así como una versión extendida de la hebra "Core 1" (mostrada en rosa), una hebra "Base" (marrón), una hebra "Puente" (amarillo) y la "plantilla de aptámero" (verde). El dúplex de ADN compuesto por las dos últimas hebras forma la región promotora de la polimerasa T7, que marca el sitio de inicio de la transcripción. Haga clic aquí para ver una versión más grande de esta figura.
Los condensados de estrella C se forman tras el recocido térmico de los oligonucleótidos constituyentes, que en el protocolo presentado aquí se lleva a cabo dentro de capilares de vidrio sellados con una sección transversal rectangular de alta relación de aspecto. Estos recipientes ofrecen múltiples ventajas clave: i) El sellado garantiza que la evaporación se evite por completo durante las etapas de recocido (a veces lentas); ii) La parte inferior plana de calidad óptica de los capilares permite obtener imágenes del transitorio de autoensamblaje (o desmontaje); iii) la alta relación de aspecto de los capilares asegura que los condensados pesados se depositen en un área amplia y plana, lo que reduce las posibilidades de coalescencia y agregación en etapas posteriores del transitorio de autoensamblaje que ocurriría en contenedores en forma de cuña (por ejemplo, tubos de microcentrífuga), y produce poblaciones de condensado relativamente monodispersas; iv) Realizar el recocido en un capilar de vidrio alargado minimiza la exposición de la muestra a interfaces hidrofóbicas (aire, plástico o aceite), que se ha observado que perturban el autoensamblaje al reclutar los oligonucleótidos colesterolizados anfifílicos. Una vez completado el protocolo de montaje, los condensados pueden extraerse de los capilares de vidrio para realizar experimentos posteriores que impliquen reactivos adicionales.
Protocolo
NOTA: El protocolo se divide en tres secciones. En la sección 1 se describen los pasos previos, incluida la preparación de oligonucleótidos de ADN y capilares de vidrio. La sección 2 describe la preparación de condensados de estrella C de varios diseños, incluidos diseños de uno y dos componentes, y su extracción de los capilares de vidrio. En la sección 3 se describe el uso de condensados de estrella C de plantillas de ARN de un componente para la síntesis de un aptámero de ARN. El usuario debe seguir las buenas prácticas de laboratorio en todo momento, asegurarse de que se implementen todas las evaluaciones y mitigaciones de riesgos necesarias y usar el equipo de protección personal (EPP) adecuado, incluidos guantes, gafas de seguridad y una bata de laboratorio. La limpieza de los tubos capilares de vidrio requiere su sonicación, primero en una solución de tensioactivo y segundo en isopropanol o etanol. La extracción de condensados de estrella C de los tubos capilares requiere el uso de un bolígrafo de rayado de diamante para marcar y romper el vidrio, con un riesgo asociado de lesiones por fragmentos de vidrio. Los materiales, equipos y reactivos clave utilizados se enumeran en la Tabla de materiales. La mayoría de los oligonucleótidos no funcionalizados son purificados por el proveedor mediante desalinización estándar, con la excepción de las hebras "Núcleo 1 extendido" y "Plantilla de aptámero", que se solicitan con purificación por electroforesis en gel de poliacrilamida (PAGE). Los oligonucleótidos modificados con colesterol son purificados por el proveedor mediante cromatografía líquida de alta resolución (HPLC) en fase inversa.
1. Requisitos previos
NOTA: Las siguientes soluciones deben prepararse en agua ultrapura (Tipo I) y filtrarse utilizando filtros de jeringa de 0,22 μm: Tampón Tris-EDTA (TE), que comprende 10 mM de Tris, 1 mM de EDTA, a pH ~8,0; Tampón TE suplementado con NaCl 2 M; y tampón TE suplementado con NaCl 0,3 M. Las soluciones tampón deben utilizarse dentro de las 2 semanas siguientes a la preparación y almacenarse a 4 °C cuando no estén en uso. Además, se utilizará una solución de detergente óptico alcalino al 1 % en agua ultrapura para la limpieza de los capilares de vidrio.
- Preparación de oligonucleótidos de ADN a partir de un estado liofilizado
- Centrifugar brevemente (2000 x g durante 10-30 s) los tubos de oligonucleótidos de ADN liofilizado para asegurarse de que los gránulos de ADN liofilizado se recojan en el fondo.
NOTA: La centrifugación se realiza a temperatura ambiente (RT) durante 10-30 s. La mini centrífuga utilizada aquí tiene un valor predeterminado de 2000 x g (6000 rpm). - Agregue la cantidad adecuada de tampón TE para reconstituir cada oligonucleótido a 100 μM. Vértice a fondo para asegurar la disolución completa, luego gire los tubos hacia abajo para recolectar todo el líquido según el paso 1.1.1.
- Para cada solución madre, mida la absorbancia a 260 nm y calcule la concentración final utilizando el coeficiente de extinción de la secuencia de oligonucleótidos bajo prueba.
NOTA: El coeficiente de extinción para una secuencia de oligonucleótidos dada generalmente se encuentra en la hoja de datos del proveedor, pero también se puede calcular con herramientas en línea utilizando el modelo de vecino más cercano35 o valores tabulados del coeficiente de extinción de nucleótidos individuales36. - Almacene los oligonucleótidos rehidratados a 4 °C durante períodos cortos de tiempo (hasta 1 semana) o a -20 °C para un almacenamiento a largo plazo (hasta 6 meses).
- Centrifugar brevemente (2000 x g durante 10-30 s) los tubos de oligonucleótidos de ADN liofilizado para asegurarse de que los gránulos de ADN liofilizado se recojan en el fondo.
- Limpieza de tubos capilares de vidrio
- Sonicato en detergente óptico alcalino al 1% para componentes ópticos en agua desionizada.
- Tome el número requerido de tubos capilares de vidrio que se van a limpiar y colóquelos en un recipiente alto y estrecho (por ejemplo, un vaso de precipitados o un tubo de centrífuga de 15/50 ml) de modo que los capilares no queden planos sobre la base del recipiente.
- Agregue el detergente al recipiente, llenándolo justo por encima del nivel de los tubos capilares. Asegúrese de que no haya burbujas de aire atrapadas dentro de los tubos capilares: toque para eliminar, si es necesario. Cubra el recipiente sin apretar (por ejemplo, con papel de aluminio o con la tapa del tubo de centrífuga, asegurándose de que no esté herméticamente sellado).
- Ponga un baño de agua sonicante a 40 °C, coloque el recipiente tapado en posición vertical en el baño y sonique durante 30 min. Asegúrese de que la lámina, si se utiliza, no entre en contacto con el agua del baño de sonicación.
NOTA: El baño de sonicación utilizado en este estudio tiene por defecto una frecuencia de sonicación de 60 Hz y una potencia de sonicación de 150 W.
PRECAUCIÓN: Nunca agregue o elimine elementos de un baño de sonicación mientras corre; Pausar siempre primero el protocolo. - Una vez finalizada la sonicación, apague el elemento calefactor del baño de agua de sonicación, enjuague a fondo los capilares del recipiente con agua desionizada o ultrapura, un mínimo de cinco veces, desechando el agua de enjuague cada vez.
- Sonicato en detergente óptico alcalino al 1% para componentes ópticos en agua desionizada.
- Sonicato en isopropanol o etanol.
- A los tubos capilares de vidrio en el recipiente de limpieza, agregue isopropanol o etanol (mínimo 70%), llenando hasta justo por encima del nivel de los tubos capilares. Al igual que en el paso 1.2.1.2, asegúrese de que no haya burbujas de aire atrapadas en los tubos capilares y cubra el recipiente sin apretar.
- Coloque el recipiente en posición vertical en el baño de sonicación y sonique durante 15-30 min.
PRECAUCIÓN: El isopropanol y el etanol son inflamables, con puntos de inflamación por debajo de RT. Minimice los volúmenes de disolventes utilizados durante la limpieza eligiendo un recipiente del tamaño adecuado, asegúrese de que los recipientes estén cubiertos sin apretar y no deje el baño de sonicación desatendido durante esta etapa. - Una vez finalizada la sonicación, deseche adecuadamente el isopropanol o etanol y seque los capilares bajo nitrógeno, manipulándolos con un pañuelo sin pelusa.
2. Preparación y extracción de condensados C-star (Figura 2)
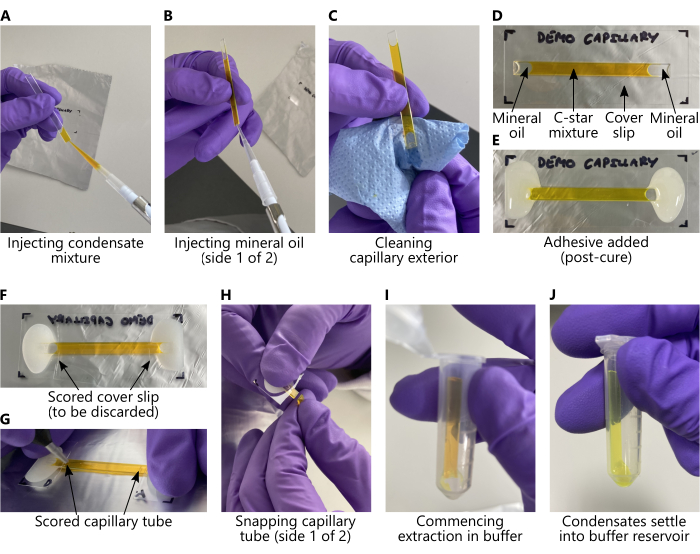
Figura 2: Carga de mezclas C-star y extracción de condensados de tubos capilares de vidrio. En todos los paneles, la mezcla C-star se ha sustituido por una solución acuosa de 25 mM de calceína para mejorar la visibilidad. (A-E) Pasos clave, en orden, que deben tomarse antes del recocido, correspondientes a las secciones 2.1 y 2.2 del protocolo. (De F a J) Pasos clave, en orden, que deben tomarse después del recocido, correspondientes a la sección 2.3 del protocolo. Durante la extracción (paneles (I-J)), los condensados de ADN se sedimentarán desde el capilar hasta el depósito tampón siempre que el tubo de microcentrífuga se almacene verticalmente. Los condensados no serán visibles a simple vista. Haga clic aquí para ver una versión más grande de esta figura.
- Preparación de mezclas C-star para condensados monocomponente
NOTA: Los detalles de la secuencia para varios diseños de componentes individuales se pueden encontrar en la Tabla Suplementaria 1 y en los trabajos anteriores 29,30,32. Los protocolos de recocido lento definidos en los pasos 2.1.9 y 2.2.2 se desarrollaron para garantizar la relajación de las redes de nanoestrellas en morfologías compactas, dadas las propiedades reológicas fuertemente dependientes de la temperatura de las redes de nanoestrellas37.- Los oligonucleótidos y los componentes del tampón variarán en función del diseño de estrella C deseado. Prepare una mezcla de 60 μL de condensados de estrella C de un solo componente a una concentración de 5 μM para la estrella C de cuatro brazos que no responde, la estrella C que responde a TMSD o la estrella C que crea plantillas de ARN.
- Para cada diseño, pipetee los componentes apropiados enumerados en la Tabla complementaria 2 en un tubo de microcentrífuga separado y mezcle bien mediante pipeteo.
NOTA: Se supone que todas las soluciones madre de oligonucleótidos tienen una concentración de 100 μM en TE. - Extraiga todo el 60 μL de solución de C-star y pipetee con cuidado para inyectar la mezcla en el tubo capilar de vidrio limpio y seco como se muestra en la figura 2A, teniendo cuidado de evitar la introducción de burbujas de aire.
- Con una pipeta, inyecte aproximadamente 9-12 μL de aceite mineral en cada extremo del tubo capilar, tapando la muestra de manera que no haya una interfaz libre entre la solución de C-star y el aire (ver Figura 2B). Luego, seque cuidadosamente el tubo capilar con papel de seda para asegurarse de que no haya aceite en el exterior (Figura 2C), teniendo cuidado de no absorber el aceite mineral o la solución de C-star fuera del tubo capilar. El resultado final se muestra en la Figura 2D.
- Prepare un pequeño lote de pegamento epoxi de dos partes para adherir cada extremo del tubo capilar, con el lado plano hacia abajo, a un engobe de cubierta de vidrio. Asegúrese de que las aberturas del tubo estén completamente cubiertas de pegamento, formando un sello continuo, como se muestra en la Figura 2E.
- Dejar curar durante un mínimo de 3 h, pero preferiblemente toda la noche.
- Después de ~ 30 min de curado, inspeccione la capa de pegamento para detectar la presencia de espacios en el sello causados por burbujas de aire. Si estos están presentes, séllelos con una pequeña cantidad de pegamento.
- Envuelva el tubo capilar pegado al cubreobjetos en papel de aluminio, asegurándose de que el tubo se mantenga plano en la parte inferior del cubreobjetos de vidrio. La lámina asegura un buen contacto térmico entre el mismo y el elemento calefactor del termociclador (ver siguiente paso).
- Coloque la muestra envuelta en un termociclador y recociendo siguiendo el siguiente protocolo: Mantenga a 95 °C durante 30 min, luego enfríe de 85 °C a 50 °C a -0,04 °C·min-1, y luego enfríe de 50 °C a RT a -0,5 °C·min-1.
- Almacene los condensados recocidos de estrella C a 4 °C o RT durante períodos prolongados (meses) mientras el capilar permanezca sellado, ya que el recocido térmico esteriliza eficazmente la muestra y desnaturaliza las nucleasas. Mantenga el capilar plano durante el almacenamiento y la manipulación para evitar que los condensados se sedimenten hacia un extremo y se agreguen.
- Preparación de mezclas C-star para condensados binarios
NOTA: Para informar la selección de las poblaciones de estrellas C para sistemas binarios, consulte el trabajo de Malouf et al., que describe las reglas de diseño y el comportamiento de fase esperado de los condensados binarios de estrellas C30.- Para preparar condensados binarios, combine volúmenes de 30 μL de las mezclas C-star descritas en el paso 2.1 anterior hasta un volumen total de 60 μL, mezcle bien, cargue en capilares y siga los pasos 2.1.4-2.1.8.
- Coloque la muestra envuelta en un termociclador y recociendo siguiendo el siguiente protocolo: Mantenga a 95 °C durante 30 min, luego enfríe de 85 °C a 40 °C a -0.01 °C·min-1, y luego enfríe de 40 °C a RT a -0.1 °C·min-1.
- Extracción de condensados de estrella C de tubos capilares.
- Prepare un tubo de microcentrífuga grande (1,5 mL o 2,0 mL) que contenga 60 μL de NaCl 0,3 M en tampón TE.
- Desenvuelva la muestra capilar y use un bolígrafo de rayado de diamante para marcar la parte inferior del cubreobjetos en cada extremo interior del área pegada (Figura 2F). Rompa el cubreobjetos en esta región y deséchelo adecuadamente.
- Limpie a fondo los tubos capilares con etanol y séquelos.
- Marque cada extremo de los tubos capilares con el rotulador de diamante, primero con la parte inferior (Figura 2G), luego voltee y marque los tubos "con el lado derecho hacia arriba".
- Asegúrese de que las líneas de puntuación no se superpongan a la interfaz aceite-agua. Deben estar dentro de la región acuosa para evitar que el aceite permanezca en la muestra extraída.
- Separar los extremos de los tubos capilares (Figura 2H), reteniendo la parte central del tubo y desechando el resto. Coloque el tubo capilar cortado en el tubo de microcentrífuga preparado en el paso 2.3.1, asegurándose de que la parte inferior del tubo capilar entre en contacto con la solución tampón (Figura 2I).
- Los condensados se sedimentarán desde el tubo hacia el depósito amortiguador a través de la gravedad; permita que esto ocurra durante un mínimo de 10 minutos (Figura 2J). Retire el tubo capilar cortado y deséchelo adecuadamente. Este enfoque de extracción suave limita el riesgo de agregación de condensado.
3. Transcripción de un aptámero de ARN a partir de condensados de estrellas C con plantillas de ARN
NOTA: Para la producción del aptámero de ARN de brócoli, se requiere una solución de difluoro-4-hidroxibencilideno imidazolidinona (DFHBI): el polvo de DFHBI se prepara primero como una solución madre a 10 mM en dimetilsulfóxido (DMSO), que luego se diluye a 600 μM en agua libre de ARNasa y DNasa.
- Lavado de condensados C-star con plantillas de ARN
NOTA: Los condensados de estrella C con plantillas de ARN se lavan tres veces para garantizar que se eliminen los oligonucleótidos plantilla no unidos.- Deje que la solución de condensados extraídos se asiente durante al menos 5 minutos y luego elimine aproximadamente la mitad del volumen del sobrenadante.
- Para un volumen de 60 μL de condensados extraídos, elimine entre 25-30 μL del sobrenadante.
- Pipetee desde la parte superior del nivel de líquido para minimizar el número de condensados eliminados durante este paso.
- Añadir el mismo volumen de 0,3 M de NaCl en TE para sustituir el sobrenadante eliminado y mezclar mediante pipeteo.
- Repita los dos pasos anteriores para un total de tres ciclos.
- Deje que la solución de condensados extraídos se asiente durante al menos 5 minutos y luego elimine aproximadamente la mitad del volumen del sobrenadante.
- Preparación de la mezcla de transcripción T7
NOTA: La mezcla de transcripción T7 se prepara utilizando el kit de transcripción CellScript T7-FlashScribe. El paso 3.2.2 es una modificación del protocolo del fabricante, que se puede encontrar aquí38. Aquí describimos la transcripción del aptámero de ARN de brócoli, que induce fluorescencia en DFHBI al unirse. En el caso de otros aptámeros luminosos, sustituya el volumen de DFHBI descrito en el paso 3.2.2 por el fluorógeno adecuado. Para otras transcripciones, elimine este componente por completo.- Prepare una solución madre de 10 mM de DFHBI en DMSO, luego diluya una alícuota hasta una concentración final de 600 μM usando agua libre de RNasa y DNasa.
- Descongele los componentes del kit de transcripción en hielo y, a continuación, pipetee lo siguiente en un tubo de microcentrífuga esterilizado en autoclave a temperatura ambiente utilizando puntas de pipeta estériles: 2 μL de tampón de transcripción T7 10x (incluido en el kit de transcripción), 1,8 μL de 100 mM de ATP, 1,8 μL de 100 mM de CTP, 1,8 μL de 100 mM UTP, 1,8 μL de 100 mM GTP, 2 μL de DTT de 100 mM, 2 μL de DFHIBI de 600 μM, 0,5 μL de inhibidor de RNasa, 2 μL de solución de enzima T7 (incluida en el kit de transcripción).
- Mezcle suavemente la solución pipeteando.
- Utilice la mezcla de transcripción para el paso 3.3 (síntesis de la transcripción de ARN) inmediatamente después de la preparación.
- Síntesis de la transcripción de ARN
- En una cámara adecuada para la obtención de imágenes de microscopía, pipetee 3,3 μL de los condensados lavados preparados a partir de estrellas C con plantillas de ARN y añada el volumen total de la mezcla de transcripción preparada anteriormente.
- Adquisición de imágenes de microscopía durante 18 h, comenzando inmediatamente después de añadir la mezcla de transcripción a los condensados.
NOTA: La configuración de la microscopía dependerá del sistema preparado. Asegúrese de que se utilizan los ajustes adecuados de excitación y emisión para los fluoróforos de la muestra, junto con tiempos de exposición que no provoquen saturación a medida que avanza la transcripción. Al igual que para cualquier lapso de tiempo, se debe encontrar un compromiso entre una potencia láser o LED lo suficientemente alta para una buena señal y, al mismo tiempo, minimizar el riesgo de fotoblanqueo. El fotoblanqueo durante largos períodos de tiempo se minimizará en los sistemas basados en LED en comparación con los sistemas basados en láser. Intervalos de imagen sugeridos: 20 min durante las primeras 2 h y 30 min posteriormente.
Resultados
Después del recocido, los condensados de estrella C se pueden obtener imágenes directamente en el tubo capilar, o después de la extracción, para confirmar su formación. Para todas las variaciones de diseño de la estrella C, se deben observar distintos condensados esféricos o poliédricos de aproximadamente 10-50 μm de diámetro, este último se forma cuando se produce la cristalización28,32. En el caso de los condensados de un solo componente, los conden...
Discusión
El protocolo descrito aquí proporciona un enfoque para la preparación de condensados de uno o dos componentes a partir de nanoestrellas de ADN anfifílico, con variaciones de diseño para introducir diferentes respuestas en los condensados. El protocolo dado produce condensados en una solución tampón de 0,3 M de NaCl en TE, pero las condiciones tampón pueden modificarse modificando adecuadamente los volúmenes enumerados anteriormente. Trabajos previos han estudiado la formación de condensados de estrella C en 0,2 ...
Divulgaciones
No se declaran conflictos de interés.
Agradecimientos
LM, LDM y DT reconocen el apoyo del Consejo Europeo de Investigación (ERC) en el marco del Programa de Investigación e Innovación Horizonte 2020 (ERC-STG n.º 851667 - NANOCELL). LDM agradece el apoyo de una beca de investigación de la Royal Society para becarios de investigación (RGF/R1/180043) y el apoyo de una beca de investigación de la Royal Society University (UF160152, URF/R/221009).
Materiales
| Name | Company | Catalog Number | Comments |
| 0.22 μm syringe filters | Sigma-Aldrich | SLGVR33RB | |
| 24 x 60 mm #1.5 Rectangular cover glasses, Menzel Gläser | VWR | 631-0853 | |
| 2-Propanol | Sigma-Aldrich | 34683 | |
| 6 L Ultrasonic Cleaner with Digital Timer and Heat, 230 VAC | Cole-Parmer | WZ-08895-11 | |
| Araldite Rapid Adhesive 2 Part Epoxy Glue | RS | ARA-400005 | |
| Bio-Rad C1000 thermal cycler | Bio-Rad | 1851197 | |
| Brand Microcentrifuge Tube 2 mL with Locking Lid | Fisher Scientific | 15338665 | 2 mL microcentrifuge tubes for the extraction of C-star condensates |
| Diamond Scribing Pen | RS | 394-217 | |
| Difluoro-4-hydroxybenzylidene imidazolidinone (DFHBI) | Sigma-Aldrich | SML1627 | |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | 472301 | |
| Eppendorf PCR Clean Colorless Safe-Lock Centrifuge Tubes | Fisher Scientific | 0030123301 | 0.5 mL microcentrifuge tubes for the preparation of C-star mixtures |
| Ethanol Absolute 99.8+% | Fisher Scientific | 10437341 | 70% ethanol is sufficient for cleaning purposes |
| Fisherbrand ZX4 IR Vortex Mixer | Fisherbrand | 13284769 | |
| Hellmanex III | Hellma | 9-307-011-4-507 | |
| Hollow Rectangle Capillaries ID 0.40 x 4.00 mm, 50 mm in length | CM Scientific | 2540-50 | |
| Mineral oil | Sigma-Aldrich | 69794 | |
| Mini Centrifuge, 230 V | PRISM(TM) | Z763128 | |
| NaCl | Sigma-Aldrich | S3014 | |
| NanoDrop One Spectrophotometer | Thermo Fisher Scientific | ND-ONE-W | Used to measure absorbance of oligonucleotides for concentration calculations |
| Oligonucleotides | Integrated DNA Technologies | Custom | Oligonucleotide sequences are unique to the C-star design required. |
| ScriptGuard RNase inhibitor | CELLSCRIPT | C-SRI6310K | RNase inhibitor |
| T7-FlashScribe Transcription Kit | Cambio | C-ASF3507 | |
| Tris-EDTA buffer, 100x stock solution | Sigma-Aldrich | 574793 | |
| UltraPure DNase/RNase-Free Distilled Water | Invitrogen | 10977035 | |
| VWR Spec-Wipe 3 Wipers | VWR | 21914-758 |
Referencias
- Buddingh', B. C., Hest, J. C. M. v. Artificial cells: Synthetic compartments with life-like functionality and adaptivity. Acc Chem Res. 50 (4), 769-777 (2017).
- Fanalista, F., et al. Shape and size control of artificial cells for bottom-up biology. ACS Nano. 13 (5), 5439-5450 (2019).
- Dora Tang, T. -. Y., et al. Fatty acid membrane assembly on coacervate microdroplets as a step towards a hybrid protocell model. Nat Chem. 6 (6), 527-533 (2014).
- Deshpande, S., et al. Spatiotemporal control of coacervate formation within liposomes. Nat Commun. 10 (1), 1800 (2019).
- Rubio-Sánchez, R., et al. Thermally driven membrane phase transitions enable content reshuffling in primitive cells. J Am Chem Soc. 143 (40), 16589-16598 (2021).
- Jahnke, K., Huth, V., Mersdorf, U., Liu, N., Göpfrich, K. Bottom-up assembly of synthetic cells with a DNA cytoskeleton. ACS Nano. 16 (5), 7233-7241 (2022).
- Tran, M. P., et al. A DNA segregation module for synthetic cells. Small. 19 (13), 2202711 (2023).
- Mason, A. F., Buddingh', B. C., Williams, D. S., Hest, J. C. M. v. Hierarchical self-assembly of a copolymer-stabilized coacervate protocell. J Am Chem Soc. 139 (48), 17309-17312 (2017).
- Gumz, H., et al. Toward functional synthetic cells: In-depth study of nanoparticle and enzyme diffusion through a cross-linked polymersome membrane. Adv Sci. 6 (7), 1801299 (2019).
- Huang, X., Patil, A. J., Li, M., Mann, S. Design and construction of higher-order structure and function in proteinosome-based protocells. J Am Chem Soc. 136 (25), 9225-9234 (2014).
- Booth, R., Qiao, Y., Li, M., Mann, S. Spatial positioning and chemical coupling in coacervate-in-proteinosome protocells. Angew Chem Int Ed Engl. 58 (27), 9120-9124 (2019).
- Hindley, J. W., et al. Light-triggered enzymatic reactions in nested vesicle reactors. Nat Commun. 9 (1), 1093 (2018).
- Zubaite, G., Hindley, J. W., Ces, O., Elani, Y. Dynamic reconfiguration of subcompartment architectures in artificial cells. ACS Nano. 16 (6), 9389-9400 (2022).
- Hirose, T., et al. A guide to membraneless organelles and their various roles in gene regulation. Nat Rev Mol Cell Biol. 24 (4), 288-304 (2023).
- Guindani, C., Silva, L. C. d., Cao, S., Ivanov, T., Landfester, K. Synthetic cells: From Simple Bio-Inspired Modules to Sophisticated Integrated Systems. Angew Chem Int Ed Engl. 61 (16), e202110855 (2022).
- Adamala, K. P., et al. Present and future of synthetic cell development. Nat Rev Mol Cell Biol. 25 (3), 162-167 (2023).
- Allen, M. E., et al. Biomimetic behaviors in hydrogel artificial cells through embedded organelles. Proc Natl Acad Sci U S A. 120 (35), e2307772120 (2023).
- Cook, A. B., Novosedlik, S., Hest, J. C. M. v. Complex coacervate materials as artificial cells. Acc Mater Res. 4 (3), 287-298 (2023).
- Seeman, N. C., Sleiman, H. F. DNA nanotechnology. Nat Rev Mater. 3, 17068 (2018).
- Takinoue, M. DNA droplets for intelligent and dynamical artificial cells: from the viewpoint of computation and non-equilibrium systems. Interface Focus. 13 (5), 20230021 (2023).
- Liu, W., Lupfer, C., Samanta, A., Sarkar, A., Walther, A. Switchable hydrophobic pockets in DNA protocells enhance chemical conversion. J Am Chem Soc. 145 (13), 7090-7094 (2023).
- Wilner, O. I., Willner, I. Functionalized DNA nanostructures. Chem Rev. 112 (4), 2528-2556 (2012).
- Gong, J., Tsumura, N., Sato, Y., Takinoue, M. Computational DNA droplets recognizing miRNA sequence inputs based on liquid-liquid phase separation. Adv Funct Mater. 32, 2202322 (2022).
- Jeon, B. -. J., Nguyen, D. T., Saleh, O. A. Sequence-controlled adhesion and microemulsification in a two-phase system of DNA liquid droplets. J Phys Chem. 124 (40), 8888-8895 (2020).
- Sato, Y., Sakamoto, T., Takinoue, M. Sequence-based engineering of dynamic functions of micrometer-sized DNA droplets. Sci Adv. 6 (23), 3471 (2020).
- Saleh, O. A., et al. Vacuole dynamics and popping-based motility in liquid droplets of DNA. Nat Commun. 14 (1), 3574 (2023).
- Rubio-Sánchez, R., Fabrini, G., Cicuta, P., Michele, L. D. Amphiphilic DNA nanostructures for bottom-up synthetic biology. Chem Commun. 57 (95), 12725-12740 (2021).
- Brady, R. A., Brooks, N. J., Cicuta, P., Di Michele, L. Crystallization of amphiphilic DNA C-Stars. Nano Lett. 17 (5), 3276-3281 (2017).
- Leathers, A., et al. Reaction-diffusion patterning of DNA-based artificial cells. J Am Chem Soc. 144 (38), 17468-17476 (2022).
- Malouf, L., et al. Sculpting DNA-based synthetic cells through phase separation and phase-targeted activity. Chem. 9 (11), 3347-3364 (2023).
- Fabrini, G., Minard, A., Brady, R. A., Antonio, M. D., Michele, L. D. Cation-responsive and photocleavable hydrogels from noncanonical amphiphilic DNA nanostructures. Nano Lett. 22 (2), 602-611 (2022).
- Brady, R. A., Brooks, N. J., Foderà, V., Cicuta, P., Di Michele, L. Amphiphilic-DNA platform for the design of crystalline frameworks with programmable structure and functionality. J Am Chem Soc. 140 (45), 15384-15392 (2018).
- Walczak, M., et al. Responsive core-shell DNA particles trigger lipid-membrane disruption and bacteria entrapment. Nat Commun. 12 (1), 4743 (2021).
- Zhang, D. Y., Winfree, E. Control of DNA strand displacement kinetics using Toehold exchange. J Am Chem Soc. 131 (47), 17303-17314 (2009).
- . Integrated DNA Technologies OligoAnalyzer Tool Available from: https://www.idtdna.com/pages/products/custom-dna-rna/dna-oligos/custom-dna-oligos (2024)
- Cavaluzzi, M. J., Borer, P. N. Revised UV extinction coefficients for nucleoside-5'-monophosphates and unpaired DNA and RNA. Nucleic Acids Res. 32 (1), e13 (2004).
- Lattuada, E., Caprara, D., Piazza, R., Sciortino, F. Spatially uniform dynamics in equilibrium colloidal gels. Sci Adv. 7 (49), (2021).
- . T7-FlashScribeTM Transcription Kit Available from: https://www.cellscript.com/products/018pl0617CS.pdf (2017)
- Zadeh, J. N., et al. NUPACK: Analysis and design of nucleic acid systems. J Comput Chem. 32 (1), 170-173 (2011).
- Walczak, M., Brady, R. A., Leathers, A., Kotar, J., Di Michele, L. Influence of hydrophobic moieties on the crystallization of amphiphilic DNA nanostructures. The Journal of Chemical Physics. 158 (8), 084501 (2023).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoExplorar más artículos
This article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados