È necessario avere un abbonamento a JoVE per visualizzare questo. Accedi o inizia la tua prova gratuita.
Method Article
Produzione e purificazione in coltura in sospensione di virus adeno-associati mediante centrifugazione a gradiente di densità di iodixanolo per applicazioni in vivo
In questo articolo
Riepilogo
Il virus adeno-associato viene prodotto in coltura cellulare in sospensione e purificato mediante centrifugazione a gradiente di densità a doppio iodixanolo. Sono incluse misure per aumentare la resa totale del virus, diminuire il rischio di precipitazione del virus e concentrare ulteriormente il prodotto finale del virus. I titoli finali attesi raggiungono10-12 particelle virali/mL e sono adatti per l'uso preclinico in vivo .
Abstract
Questo protocollo descrive la produzione e la purificazione di virus adeno-associati ricombinanti (rAAV) mediante centrifugazione a gradiente di densità dello iodio, un metodo sierotipo-agnostico di purificazione dell'AAV descritto per la prima volta nel 1999. I vettori rAAV sono ampiamente utilizzati nelle applicazioni di terapia genica per veicolare transgeni a vari tipi di cellule umane. In questo lavoro, il virus ricombinante è prodotto dalla trasfezione di cellule Expi293 in coltura in sospensione con plasmidi che codificano per il transgene, il capside vettore e i geni helper adenovirali. La centrifugazione a gradiente di densità dello iodixanolo purifica le particelle AAV complete in base alla densità delle particelle. Inoltre, in questa metodologia ormai onnipresente sono incluse tre fasi al fine di aumentare la resa totale del virus, diminuire il rischio di precipitazione a causa della contaminazione delle proteine e concentrare ulteriormente il prodotto virale finale, rispettivamente: precipitazione di particelle virali da terreni cellulari utilizzando una soluzione di polietilenglicole (PEG) e cloruro di sodio, l'introduzione di un secondo ciclo di centrifugazione a gradiente di densità dello iodixanolo, e lo scambio di tamponi tramite un filtro centrifugo. Utilizzando questo metodo, è possibile ottenere costantemente titoli nell'intervallo di10-12 particelle virali/mL di eccezionale purezza per l'uso in vivo .
Introduzione
I vettori virali adeno-associati ricombinanti (rAAV) sono strumenti ampiamente utilizzati per il trattamento di malattie genetiche, tra cui l'atrofia muscolare spinale, la distrofia retinica e l'emofilia A 1,2,3. I vettori rAAV sono ingegnerizzati per mancare dei geni virali presenti nell'AAV4 wild-type, un piccolo virus icosaedrico senza involucro con un genoma lineare di DNA a singolo filamento di 4,7 kb. L'AAV è stato scoperto per la prima volta negli anni '60 come contaminante dei preparati di adenovirus5. Nonostante le sue piccole dimensioni del capside, che limitano la dimensione del transgene che può essere impacchettato a un massimo di 4,9 kb escludendo ITRs6, l'AAV è utile per il rilascio del transgene perché non è patogeno nell'uomo, consente l'espressione del transgene in molti tipi di cellule in divisione e non in divisione e ha effetti immunogenici limitati7.
Come membri del genere dependoparvovirus, la produzione di rAAV si basa sull'espressione di geni helper presenti nell'adenovirus o nel virus dell'herpes simplex8. Sono state sviluppate diverse strategie per produrre rAAV, ma la produzione in cellule HEK293 trasformate con i geni adenovirali E1A/E1B helper è il metodo più consolidato utilizzato oggi9. L'approccio generale alla produzione di rAAV inizia con la trasfezione di cellule HEK293 con tre plasmidi contenenti il transgene all'interno di ripetizioni terminali invertite (ITR), geni AAV rep e cap e geni helper adenovirali aggiuntivi, rispettivamente. Settantadue ore dopo la trasfezione, le cellule vengono raccolte e processate per purificare rAAV contenente il transgene.
Nello sviluppo di nuovi vettori rAAV per scopi terapeutici, un obiettivo importante è la produzione di vettori con una maggiore efficienza di trasduzione. Un aumento dell'efficienza di trasduzione delle cellule bersaglio significherebbe una diminuzione della dose clinica necessaria di rAAV, diminuendo così la probabilità di effetti immunogenici avversi che vanno dalla neutralizzazione mediata da anticorpi a tossicità acute10,11. Per migliorare l'efficacia di trasduzione dei vettori rAAV, è possibile apportare modifiche al genoma impacchettato o al capside. I metodi praticabili per regolare l'efficacia della trasduzione attraverso la progettazione del genoma impacchettato includono l'incorporazione di promotori forti e tessuto-specifici, una selezione ponderata degli elementi di elaborazione dell'mRNA e l'ottimizzazione della sequenza codificante per migliorare l'efficienza della traduzione12. Le alterazioni del capside vengono effettuate con l'obiettivo di aumentare il tropismo per i tipi di cellule umane bersaglio. Gli sforzi verso lo sviluppo di nuovi capsidi vettoriali di trasporto del transgene rAAV sono generalmente caratterizzati da un focus sulla progettazione razionale di capsidi AAV con mutazioni specifiche che hanno come bersaglio specifici recettori cellulari o sull'evoluzione diretta per identificare capsidi con tropismo per specifici tipi di cellule da librerie di capsidi combinatori ad alta complessità senza mirare a un recettore specifico (sebbene alcuni gruppi combinino questi approcci)13, 14,15. Nell'approccio dell'evoluzione diretta, le librerie combinatorie del capside sono costruite utilizzando una particolare spina dorsale del sierotipo con regioni variabili mutate all'esterno del capside16. Le librerie combinatorie di capsidi sono spesso costruite a partire da sierotipi AAV non originati nell'uomo, riducendo il rischio di immunità preesistente durante l'uso clinico10. Pertanto, i metodi di purificazione che possono essere applicati a qualsiasi sierotipo sono ideali per eliminare la necessità di un'ottimizzazione sierotipo-specifica per i sierotipi meno comunemente usati che fungono da spina dorsale per queste librerie.
La centrifugazione a gradiente di densità dello iodixanolo viene utilizzata per purificare titoli elevati di rAAV con elevata infettività17. In questo protocollo, l'rAAV viene prodotto in coltura cellulare in sospensione per ridurre la quantità di lavoro necessaria per produrre grandi titoli di AAV. È inclusa anche una fase di centrifugazione per eliminare il lisato cellulare per ridurre la presenza di proteine contaminanti e diminuire il rischio di precipitazione del virus. Questo protocollo è un metodo economico per produrre preparati di rAAV ad alta purezza adatti all'uso preclinico.
Protocollo
La composizione delle soluzioni e dei tamponi utilizzati in questo protocollo è fornita nella Tabella 1.
| Soluzione | Composizione | |
| Tampone di lisi AAV | 1,2 mL di soluzione 5 M di NaCl | |
| 2 mL di soluzione 1 M Tris-HCl pH 8,5 | ||
| 80 uL di soluzione di 1 M di MgCl2 | ||
| mQ acqua fino a 40 mL | ||
| Soluzione di precipitazione AAV | 40 g PEG 8000 | |
| 50 mL di soluzione 5 M di NaCl | ||
| mQ acqua fino a 100 mL | ||
| Frazione di iodixanolo al 15% | 7,5 mL di OptiPrep | |
| 3 mL di DPBS 10X | ||
| 6 mL di soluzione di NaCl 5 M | ||
| 30 uL di soluzione di 1 M di MgCl2 | ||
| mQ fino a 30 mL | ||
| Frazione di iodixanolo al 25% | 12. 5 ml di OptiPrep | |
| 3 mL di DPBS 10X | ||
| 30 uL di soluzione di 1 M di MgCl2 | ||
| 60 uL di soluzione di rosso fenolo | ||
| mQ fino a 30 mL | ||
| 40% frazione di iodixanolo | 33,3 mL di OptiPrep | |
| 5 mL di DPBS 10X | ||
| 50 uL di soluzione di 1 M di MgCl2 | ||
| mQ fino a 50 mL | ||
| 60% frazione di iodixanolo | 50 ml di OptiPrep | |
| 100 uL di soluzione di rosso fenolo | ||
| Soluzione tampone AAV | 8 mL di NaCl 5 M | |
| 20 uL di Pluronic F-68 al 10% | ||
| PBS fino a 200 mL | ||
Tabella 1: Composizioni delle soluzioni utilizzate in questo protocollo.
1. Tripla trasfezione delle cellule Expi293
- Seme: Expi293 cellule (vedi Tabella dei materiali) ad una densità iniziale di 5 x 105 cellule vitali (vc)/mL.
- Lasciare incubare le cellule a 37 °C con l'8% di CO2 e una velocità dell'agitatore di 125 giri/min fino a raggiungere una densità di 3-5 x 106 vc/mL. Monitora la densità e la vitalità cellulare utilizzando l'esclusione del blu di tripano con un contatore di cellule automatizzato (vedi Tabella dei materiali).
- Quando le cellule raggiungono la densità cellulare desiderata, dividerle da 1 a 10 aggiungendo terreni freschi in modo che il loro volume totale sia dieci volte il loro volume originale. Se necessario, trasferire le cellule in un pallone più grande. Ritorno all'incubazione.
- Continuare ad espandere le cellule come descritto nei passaggi 1.2-1.3 fino a raggiungere una densità di almeno 2 x 106 vc/mL in 250 mL di terreno in un matraccio da 1 L.
- Il giorno prima della trasfezione, diluire le cellule a 2 x 106 vc/mL in 250 mL di terreno, scartando le cellule in eccesso se necessario. Incubare le cellule per una notte.
- Il giorno della trasfezione, centrifugare metà delle cellule a 58 x g per 5 minuti a 18 °C. Risospendere il pellet cellulare nello stesso volume (125 ml) di terreno fresco. La vitalità cellulare dovrebbe essere vicina al 98%.
- Preparare due provette coniche da 50 ml. Etichettare uno "PEI" e l'altro "DNA".
- Nella provetta conica marcata PEI, diluire 1,2 mL di PEI (polietilenimmina, vedere la tabella dei materiali) per un volume totale di 12,5 mL in terreni OptiMEM.
- Nella provetta conica marcata con DNA, preparare una soluzione equimolare di DNA plasmidico per un totale di 500 μg di DNA in un volume totale di 12,5 mL in terreni OptiMEM.
- Aggiungere la soluzione di DNA alla soluzione di PEI, capovolgere più volte per combinare accuratamente e lasciare incubare a temperatura ambiente per 10 minuti.
NOTA: È fondamentale che la soluzione di DNA-PEI sia lasciata incubare per tutti i 10 minuti in modo che il PEI formi complessi carichi con il DNA. - Dopo che la soluzione di DNA-PEI è stata incubata per 10 minuti, utilizzare una pipetta sierologica per applicare lentamente la soluzione di DNA-PEImax alle cellule Expi293. Rimettere le cellule trasfettate nell'incubatrice e lasciarle incubare per 72 ore (Figura 1).
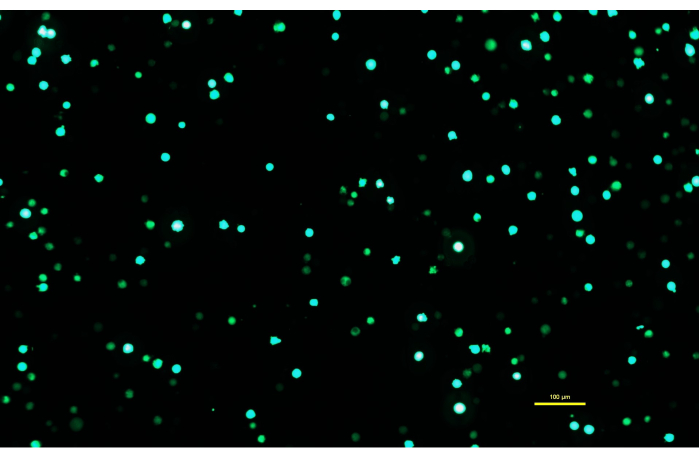
Figura 1: Cellule Expi293 che esprimono GFP due giorni dopo la trasfezione. Dopo la trasfezione con un plasmide contenente un gene per la GFP, le cellule Expi293 esprimono transitoriamente eGFP. La morfologia cellulare è rotonda. L'immagine è stata catturata con un tempo di esposizione di 15 ms. Le immagini al microscopio vengono acquisite utilizzando un microscopio invertito dotato di illuminazione a epifluorescenza e un obiettivo 10x/0,30. Barra della scala = 100 μm. Fare clic qui per visualizzare una versione più grande di questa figura.
2. Purificazione del vettore rAAV
- Dopo 72 ore, trasferire le cellule in sospensione in due provette coniche da 250 mL e centrifugarle a 415 x g per 10 minuti a 4 °C.
- Versare il terreno surnatante in una nuova provetta conica da 500 mL e conservare a 0 °C su ghiaccio per una successiva lavorazione.
- Risospendere ciascun pellet cellulare in 10 mL di tampone di lisi AAV (Tabella 1). Raggruppare i due lisati insieme in una delle provette. Sciacquare l'altra provetta con altri 5 mL di tampone di lisi AAV, quindi aggiungerla ai lisati raggruppati. Congelare a -70 °C.
NOTA: A questo punto l'esperimento può essere messo in pausa. Conservare il terreno surnatante a -70 °C anziché su ghiaccio. - Aggiungere un volume 1:4 di soluzione di precipitazione AAV al mezzo surnatante del passaggio 2.2. Capovolgere più volte per mescolare accuratamente e incubare a 0 °C su ghiaccio per almeno 2 ore o per tutta la notte.
- Centrifugare la soluzione incubata a 3000 x g per 1 h a 4 °C.
- Scartare il surnatante e risospendere il pellet contenente precipitato virale in 5 mL di tampone di lisi AAV.
- Scongelare il lisato cellulare a 37 °C a bagnomaria.
- Raggruppare il precipitato virale con il lisato cellulare. Questo è il lisato grezzo. Sciacquare la provetta di centrifugazione che conteneva il precipitato virale con altri 5 mL di tampone di lisi AAV e raggrupparla con il lisato grezzo.
- Congelare il lisato grezzo a -70 °C, quindi scongelare a 37 °C. Ripeti questo ciclo ancora una volta.
NOTA: Il lisato grezzo non deve essere lasciato a 37 °C per un periodo superiore a quello necessario per lo scongelamento. - Una volta scongelato per la terza volta, aggiungere immediatamente 4 μL di benzonasi al lisato grezzo, capovolgere per mescolare e incubare per 30 minuti a 37 °C.
- Centrifugare il lisato grezzo per 10 minuti a 650 x g a 18 °C.
- Trasferire il surnatante in una provetta conica pulita da 50 ml. Questo è il virus grezzo. Scartare il pellet.
- Centrifugare il virus grezzo per altri 30 minuti a 3000 x g a 18 °C per eliminare le proteine contaminanti.
- Trasferire il surnatante in una provetta conica pulita da 50 ml. Questo è il virus chiarificato.
NOTA: A questo punto l'esperimento può essere messo in pausa. Congelare il virus chiarificato a -70 °C. - Installare la pompa peristaltica multicanale con quattro tubi peristaltici (vedere la tabella dei materiali). Fissare i capillari a entrambe le estremità di ciascun tubo.
- Posizionare i capillari sul lato di ingresso della pompa in un becher riempito con acqua deionizzata. Posizionare i capillari sul lato di uscita della pompa in un bicchiere vuoto. Far funzionare la pompa peristaltica a 25.0 giri/min per lavare il tubo con acqua deionizzata (DI).
- Una volta lavato accuratamente, svuotare il tubo peristaltico facendo funzionare la pompa fino a riempire il tubo solo con aria. Appoggiare tutti i capillari su una salvietta pulita e priva di lanugine.
- Posizionare quattro provette per ultracentrifuga in un rack sul lato di uscita della pompa.
- Utilizzare una pipetta sierologica da 10 mL per erogare con cura 10 mL di virus chiarificato in ciascuna provetta per ultracentrifuga. Fare attenzione a non introdurre bolle d'aria.
NOTA: Il volume di virus chiarificato in ciascuna provetta può essere allungato fino a 12 mL per provetta, se necessario. Ridurre la quantità di iodixanolo al 60% in modo appropriato nel passaggio 2.25 di seguito. - Il virus chiarificato viene ricoperto con frazioni di iodixanolo (Tabella 1) dalla densità più bassa alla densità più alta utilizzando la pompa peristaltica. La frazione del 15% viene aggiunta per prima, seguita dalla frazione del 25%, dalla frazione del 40% e infine dalla frazione del 60%. Trasferire 22 mL (5,5 mL per provetta per ultracentrifuga) della frazione di iodixanolo al 15% in una provetta conica pulita da 50 mL. Posizionare i capillari sul lato di ingresso della pompa nel tubo, facendo attenzione che tutti i capillari tocchino il fondo del tubo.
- Avviare la pompa e lasciare che il tubo si riempia con la frazione di iodixanolo. Quando la frazione di iodixanolo raggiunge le estremità dei capillari sul lato di uscita della pompa, arrestare la pompa.
- Inserire un capillare di uscita in ciascuna provetta di ultracentrifuga con il virus chiarificato, facendo attenzione che i capillari tocchino il fondo di ciascuna provetta di ultracentrifuga.
NOTA: È di fondamentale importanza che non rimanga aria nei capillari. Lo iodixanolo può fluire attraverso il tubo peristaltico a velocità leggermente diverse, quindi assicurarsi che ogni singolo capillare di uscita sia completamente riempito di iodixanolo prima di inserirlo nel virus chiarificato. Potrebbe essere necessario arrestare e avviare la pompa più volte. - Avviare la pompa e lasciare che le provette dell'ultracentrifuga si riempiano. Quando l'ultima frazione del 15% sta per essere immessa nei capillari di ingresso, arrestare la pompa. È fondamentale che nessuna bolla d'aria penetri nel tubo peristaltico.
NOTA: Se le bolle d'aria entrano nei capillari di ingresso, la pompa può essere brevemente fatta funzionare nella direzione inversa per spingerle di nuovo fuori. - Trasferire 22 mL (5,5 mL per provetta per ultracentrifuga) della frazione di iodixanolo al 25% nella provetta conica da 50 mL. Fare attenzione che tutti i capillari tocchino il fondo del tubo e avviare la pompa. Quando l'ultima frazione del 25% sta per essere immessa nei capillari di ingresso, arrestare la pompa.
- Ripetere il passaggio 2.24 con 20 mL (5 mL per provetta per ultracentrifuga) della frazione di iodixanolo al 40% e poi con 24 mL (6 mL per provetta per ultracentrifuga) della frazione al 60%.
- Se nella provetta per l'ultracentrifuga è rimasto ancora del volume vuoto, continuare ad aggiungere altra frazione del 60% fino a quando le provette dell'ultracentrifuga non sono completamente riempite di liquido.
- Riempi ogni tubo fino a quando il lisato non forma una cupola sopra la parte superiore ma non trabocca dal tubo. Arrestare la pompa e rimuovere con cautela ogni capillare di uscita, facendo attenzione a non disturbare il gradiente di iodixanolo.
- Tappare ogni provetta per ultracentrifuga con un distanziatore e caricarla in un rotore Type 70 Ti (vedere la tabella dei materiali).
NOTA: Assicurarsi che il rotore sia adeguatamente bilanciato. Non tentare di utilizzare l'ultracentrifuga senza un'adeguata formazione. - Caricare il rotore Type 70 Ti nell'ultracentrifuga e centrifugare a 489.000 x g per 1 ora a 18 °C.
- Dopo la centrifugazione, scaricare il rotore dall'ultracentrifuga. Utilizzare una pinza ad ago per rimuovere con cautela ciascuna provetta di ultracentrifuga dal rotore, facendo attenzione a non disturbare il gradiente di iodixanolo.
- Utilizzare un supporto ad anello di supporto con morsetto per fissare una provetta per ultracentrifuga.
- Collegare un ago da 20 GA a una siringa da 5 ml e mettere da parte. Utilizzare una salvietta priva di lanugine per rimuovere il tappo dalla provetta per ultracentrifuga.
- Penetrare la parete della provetta per ultracentrifuga con l'ago circa 3 mm al di sotto dell'interfaccia iodixanolo al 40%-60% (Figura 2).
- Aspirare lentamente l'interfaccia e parte della frazione del 40%. Evitare di aspirare la parte superiore della frazione al 40%, per un prelievo totale di circa 4 mL.
- Tenendo un dito sulla parte superiore aperta della provetta per ultracentrifuga, estrarre la siringa e trasferire la frazione AAV aspirata in una provetta conica da 50 mL. Gettare la siringa in un contenitore per oggetti taglienti. Scartare la provetta per ultracentrifuga.
- Ripetere i passaggi 2.31-2.35 per ciascuna provetta per ultracentrifuga.
- Diluire la frazione di AAV aspirata circa due volte nel tampone di lisi AAV fino a un volume di 40 mL.
- Sciacquare il tubo peristaltico come descritto nei passaggi 2.16-2.17.
NOTA: A questo punto l'esperimento può essere messo in pausa. Conservare la frazione di AAV diluita a 0 °C per una notte. - Caricare 20 mL ciascuno della frazione AAV diluita in due nuove provette per ultracentrifuga.
- Per il secondo ciclo di centrifugazione in gradiente di densità dello iodio, la frazione AAV diluita viene sottoposta come descritto sopra utilizzando solo 10 mL (5 mL per provetta per ultracentrifuga) della frazione al 40% e 14 mL (7 mL per provetta per ultracentrifuga) della frazione al 60%. Ripetere i passaggi 2.29-2.36 per l'ultracentrifugazione e l'aspirazione.
NOTA: Prima di riporre la pompa peristaltica e il tubo, sciacquare il tubo con acqua deionizzata come descritto nei passaggi 2.16-2.17.
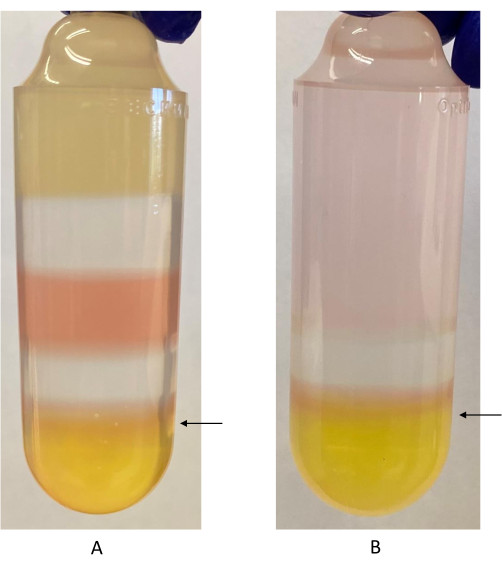
Figura 2: Gradiente di iodixanolo con interfaccia di iodixanolo al 40%-60% etichettata. (A) Primo gradiente di iodixanolo. Il rosso fenolo è utilizzato nelle frazioni di iodixanolo al 40% e al 60% di iodixanolo. Appare come un colore diverso a causa della differenza di pH tra le due frazioni. La freccia indica dove deve essere inserita la siringa per raccogliere la frazione rAAV, appena sotto l'interfaccia iodixanolo 40%-60%. (B) Secondo gradiente di iodixanolo. In questa fase vengono utilizzate solo le frazioni di iodixanolo al 40% e al 60%. La freccia indica il punto in cui la siringa deve essere inserita per raccogliere la frazione rAAV. Fare clic qui per visualizzare una versione più grande di questa figura.
3. Scambio tampone e concentrazione del virus
- Dopo aver ottenuto la frazione AAV dal secondo ciclo di centrifugazione a gradiente di densità dello iodixanolo, diluirla due volte con una soluzione tampone AAV.
- Equilibrare il filtro centrifugo aggiungendo 20 mL di soluzione tampone AAV sulla parte superiore del filtro. Centrifugare l'apparecchio a 3000 x g a 18 °C per 5 minuti ed eliminare il flusso.
- Aggiungere la frazione AAV diluita al filtro centrifugo. Centrifugare l'apparecchio a 3000 x g a 18 °C per 5 minuti ed eliminare il flusso.
- Aggiungere 20 mL di soluzione tampone AAV nella parte superiore del filtro. Centrifugare l'apparecchio a 3000 x g a 18 °C per 5 minuti ed eliminare il flusso.
NOTA: Se la membrana del filtro centrifugo si ostruisce, causando un flusso inefficiente della soluzione, utilizzare con cautela una micropipetta P200 per aspirare su e giù la soluzione nella parte superiore del filtro. Prestare la massima attenzione a non toccare il filtro con il puntale della micropipetta. - Ripetere il passaggio 3.4 altre due volte.
NOTA: Assicurarsi che l'AAV non sia eccessivamente concentrato mantenendo almeno 1 mL di volume nella parte superiore del filtro. Potrebbe essere necessario regolare i tempi di centrifugazione per evitare un'eccessiva concentrazione. Se l'AAV è troppo concentrato, possono verificarsi precipitazioni. - Per un titolo finale compreso tra 10e 12 vg/mL, ridurre il virus a un volume finale di circa 1 mL.
NOTA: A seconda della spina dorsale del sierotipo utilizzato e dell'efficienza di confezionamento del virus, potrebbe essere necessario concentrare il virus in un volume inferiore. - Utilizzare una micropipetta p1000 per trasferire l'AAV concentrato dalla parte superiore del filtro centrifugo in una provetta per microcentrifuga. Utilizzare una micropipetta p200 per lavare il filtro centrifugo con 50 μL di tampone AAV per raccogliere eventuali virus rimanenti e raggrupparli con il resto dell'AAV concentrato.
- Mettere da parte un'aliquota di 2 μL dell'AAV concentrato per la titolazione. Vedere la Tabella supplementare 1 per visualizzare i primer ITR che possono essere utilizzati per la qPCR.
- Passare l'AAV concentrato attraverso un filtro per siringa da 0,2 μm per sterilizzarlo.
NOTA: Quando si sterilizza un piccolo volume, fare attenzione a selezionare un filtro per siringa con un diametro ridotto per ridurre al minimo la perdita di virus. L'uso di un filtro a basso legame proteico di piccolo diametro si tradurrà in una perdita virale minima (dati non mostrati). - L'AAV sterilizzato può essere utilizzato immediatamente per trasdurre le cellule o conservato a 4 °C per l'uso entro quattro settimane. Deve essere conservato a -70 °C per una conservazione a lungo termine.
Risultati
Questo metodo può essere utilizzato per ottenere titoli di almeno10-12 particelle virali per ml. Un titolo può essere ottenuto (Figura 3) mediante qPCR utilizzando i primer ITR forniti nella Tabella supplementare 1, mediante ddPCR o con qualsiasi altro metodo di titolazione. Titoli non ottimali potrebbero derivare dall'uso di un gene cap che codifica per un capside con scarsa efficienza di confezionamento.
Un'altra possibile ...
Discussione
Il protocollo di purificazione a gradiente di densità a doppio iodixanolo è il metodo universale perché è applicabile a qualsiasi variante mutante AAV, indipendentemente dalla loro specificità recettoriale. I primi metodi di purificazione dell'AAV si basavano sulla densità delle particelle e includevano la centrifugazione isopicnica in CsCl e la centrifugazione continua a gradiente di densità del saccarosio19. Successivamente, sono stati sviluppati approcci sierotipo-specifici, che hanno fa...
Divulgazioni
Gli autori non hanno divulgazioni da segnalare.
Riconoscimenti
Nessuno.
Materiali
| Name | Company | Catalog Number | Comments |
| 5810 R benchtop centrifuge | Eppendorf | 22625501 | |
| 8-channel peristaltic pump | Watson-Marlow | 020.3708.00A | |
| Automated cell counter | NanoEntek | EVE-MC | |
| Avanti J-E high-speed centrifuge | Beckman Coulter | 369001 | |
| Benzonase | Thermo Scientific | 88701 | |
| Biological safety cabinet | Labconco | 322491101 | |
| CO2 incubator with shaker | Set at 8% CO2 and 37 °C | ||
| Conical centrifuge tubes | Thermo Scientific | 339652 | 50 mL |
| Conical centrifuge tubes | Thermo Scientific | 339650 | 15 mL |
| Disposable micro-pipets | Fisherbrand | 21-164-2G | Capillaries |
| Dulbecco's phosphate buffered saline without CaCl2 and MgCl2 (DPBS) (10x) | Sigma-Aldrich | D1408 | |
| ECLIPSE Ts2R-FL inverted microscope | Nikon | ||
| Expi293 Expression Medium | Gibco | A1435101 | |
| Expi293F cells | Gibco | A14527 | |
| Filter tips | USA Scientific | 1126-7810 | 1000 µL |
| Filter tips | USA Scientific | 1120-8810 | 200 µL |
| Filter tips | USA Scientific | 1120-1810 | 20 µL |
| Filter tips | USA Scientific | 1121-3810 | 10 µL |
| Hypodermic needles | Tyco Healthcare | 820112 | 20 GA x 1-1/2 A |
| Ice bucket with lid | VWR | 10146-184 | |
| JS-5.3 rotor | Beckman Coulter | 368690 | |
| Magnesium chloride solution (1 M) | Millipore Sigma | M1028-100ML | |
| Metal stand and clamp | Fisherbrand | 05-769-6Q | |
| Microcentrifuge tubes | Eppendorf | 22600028 | 1.5 mL |
| Needle nose pliers | |||
| Optima XE-90 ultracentrifuge | Beckman Coulter | A94471 | |
| Opti-MEM I Reduced-Serum Medium | Gibco | 31985062 | |
| OptiPrep density gradient media (iodixanol) | Serumwerk | AXS-1114542 | 60% iodixanol solution |
| P1000 Pipet | Gilson | F144059M | |
| P2 Pipet | Gilson | F144054M | |
| P20 Pipet | Gilson | F144056M | |
| P200 Pipet | Gilson | F144058M | |
| Phenol red solution | Sigma-Aldrich | P0290 | |
| Phosphate buffered saline (PBS) | Sigma-Aldrich | P4474 | |
| Pipet-Aid XP pipette controller | Drummond Scientific | 4-000-101 | |
| Plasmid pCapsid | De novo or Addgene, etc. | N/A | We used pACGrh74. |
| Plasmid pHelper | Addgene | 112867 | |
| Plasmid pTransgene | De novo or Addgene, etc. | N/A | We used pdsAAV-GFP. |
| Pluronic F-68 polyol solution (10%) | Mp Biomedicals | 92750049 | |
| Polyethylene glycol 8000 | Research Products International | P48080-500.0 | |
| Polyethylenimine HCl Max (PEI-Max) | Polysciences | NC1038561 | Dilute in water to 40 μM |
| Polypropylene centrifuge tubes, sterile | Corning | 431123 | 500 mL |
| Polypropylene centrifuge tubes, sterile | Corning | 430776 | 250 mL |
| Polypropylene Optiseal tubes | Beckman Coulter | 361625 | |
| Serological pipettes | Alkali Scientific | SP250-B | 50 mL |
| Serological pipettes | Alkali Scientific | SP225-B | 25 mL |
| Serological pipettes | Alkali Scientific | SP210-B | 10 mL |
| Serological pipettes | Alkali Scientific | SP205-B | 5 mL |
| Shaker flasks | Fisherbrand | PBV1000 | 1 L |
| Shaker flasks | Fisherbrand | PBV50-0 | 500 mL |
| Shaker flasks | Fisherbrand | PBV250 | 250 mL |
| Shaker flasks | Fisherbrand | PBV12-5 | 125 mL |
| Sodium chloride solution (5 M) | Fisher Scientific | NC1752640 | |
| Sterile syringes | Fisherbrand | 14-955-458 | 5 mL |
| Syringe filter | Millipore | SLGV013SL | 0.22 micron |
| Tris-HCl pH 8.5 (1 M) | Kd Medical | RGE3363 | |
| Trypan blue solution | Gibco | 15250061 | |
| Tube rack assembly | Beckman Coulter | 361646 | |
| Tube spacers (x4) | Beckman Coulter | 361669 | |
| Tubing for peristaltic pump | Fisher Scientific | 14190516 | |
| Type 70 Ti fixed-angle titanium rotor | Beckman Coulter | 337922 | |
| Ultra low temperature freezer | Set at -70 °C | ||
| Vivaspin 20 centrifugal concentrator | Sartorius | VS2041 | |
| Water bath | Set at 37 °C |
Riferimenti
- Strauss, K. A., et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: the Phase III SPR1NT trial. Nat Med. 28 (7), 1390-1397 (2022).
- Fuller-Carter, P. I., Basiri, H., Harvey, A. R., Carvalho, L. S. Focused update on AAV-based gene therapy clinical trials for inherited retinal degeneration. BioDrugs. 34 (6), 763-781 (2020).
- George, L. A., et al. Multiyear factor VIII expression after AAV gene transfer for hemophilia A. N Engl J Med. 385 (21), 1961-1973 (2021).
- Naso, M. F., Tomkowicz, B., Perry, W. L., Strohl, W. R. Adeno-Associated Virus (AAV) as a vector for gene therapy. Biodrugs. 31 (4), 317-334 (2017).
- Atchison, R. W., Casto, B. C., Hammon, W. M. c. D. Adenovirus-associated defective virus particles. Science. 149 (3685), 754-756 (1965).
- Wu, Z., Yang, H., Colosi, P. Effect of genome size on AAV vector packaging. Mol Ther. 18 (1), 80-86 (2010).
- Samulski, R. J., Muzyczka, N. AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol. 1 (1), 427-451 (2014).
- Zolotukhin, S. Production of recombinant adeno-associated virus vectors. Hum Gene Ther. 16 (5), 551-557 (2005).
- Penaud-Budloo, M., François, A., Clément, N., Ayuso, E. Pharmacology of recombinant adeno-associated virus production. Mol Ther - Methods Clin Dev. 8, 166-180 (2018).
- Costa-Verdera, H., et al. Understanding and Tackling immune responses to adeno-associated viral vectors. Hum Gene Ther. 34 (17-18), 836-852 (2023).
- Ertl, H. C. J. Mitigating serious adverse events in gene therapy with AAV Vectors: Vector dose and immunosuppression. Drugs. 83 (4), 287-298 (2023).
- Pupo, A., et al. AAV vectors: The Rubik's cube of human gene therapy. Mol Ther. 30 (12), 3515-3541 (2022).
- Marsic, D., et al. Vector design tour de force: Integrating combinatorial and rational approaches to derive novel adeno-associated virus variants. Mol Ther. 22 (11), 1900-1909 (2014).
- Grimm, D., Zolotukhin, S. E Pluribus Unum: 50 Years of research, millions of viruses, and one goal-tailored acceleration of AAV evolution. Mol Ther. 23 (12), 1819-1831 (2015).
- Biswas, M., et al. Engineering and in vitro selection of a novel AAV3B variant with high hepatocyte tropism and reduced seroreactivity. Mol Ther - Methods Clin Dev. 19, 347-361 (2020).
- Perabo, L., et al. In vitro selection of viral vectors with modified tropism: the adeno-associated virus display. Mol Ther. 8 (1), 151-157 (2003).
- Crosson, S. M., Dib, P., Smith, J. K., Zolotukhin, S. Helper-free production of laboratory grade AAV and purification by iodixanol density gradient centrifugation. Mol Ther - Methods Clin Dev. 10, 1-7 (2018).
- Chan, C., Harris, K. K., Zolotukhin, S., Keeler, G. D. Rational design of AAV-rh74, AAV3B, and AAV8 with limited liver targeting. Viruses. 15 (11), 2168 (2023).
- Schmidt, O. W., Cooney, M. K., Foy, H. M. Adeno-associated virus in adenovirus type 3 conjunctivitis. Infect Immun. 11 (6), 1362-1370 (1975).
- Grimm, D., Kern, A., Rittner, K., Kleinschmidt, J. A. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 9 (18), 2745-2760 (1998).
- Zolotukhin, S., et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6 (6), 973-985 (1999).
- Clark, K. R., Liu, X., Mcgrath, J. P., Johnson, P. R. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther. 10 (6), 1031-1039 (1999).
- Debelak, D., et al. Cation-exchange high-performance liquid chromatography of recombinant adeno-associated virus type 2. J Chromatogr B Biomed Sci App. 740 (2), 195-202 (2000).
- Burova, E., Ioffe, E. Chromatographic purification of recombinant adenoviral and adeno-associated viral vectors: methods and implications. Gene Ther. 12 (1), S5-S17 (2005).
- Adams, B., Bak, H., Tustian, A. D. Moving from the bench towards a large scale, industrial platform process for adeno-associated viral vector purification. Biotechnol Bioeng. 117 (10), 3199-3211 (2020).
- Grieger, J. C., Choi, V. W., Samulski, R. J. Production and characterization of adeno-associated viral vectors. Nat Protoc. 1 (3), 1412-1428 (2006).
- Florea, M., et al. High-efficiency purification of divergent AAV serotypes using AAVX affinity chromatography. Mol Ther Methods Clin Dev. 28, 146-159 (2022).
- Chamberlain, K., Riyad, J. M., Weber, T. Expressing transgenes that exceed the packaging capacity of adeno-associated virus capsids. Hum Gene Ther Methods. 27 (1), 1-12 (2016).
- Green, E. A., Hamaker, N. K., Lee, K. H. Comparison of vector elements and process conditions in transient and stable suspension HEK293 platforms using SARS-CoV-2 receptor binding domain as a model protein. BMC Biotechnol. 23 (1), 7 (2023).
- Erbacher, P., Zou, S., Bettinger, T., Steffan, A. M., Remy, J. S. Chitosan-based vector/DNA complexes for gene delivery: Biophysical characteristics and transfection ability. Pharm Res. 15 (9), 1332-1339 (1998).
- Vandenberghe, L. H., et al. Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum Gene Ther. 21 (10), 1251-1257 (2010).
- Summerford, C., Samulski, R. J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 72 (2), 1438-1445 (1998).
- Wright, J. F., et al. Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation. Mol Ther. 12 (1), 171-178 (2005).
- Gruntman, A. M., et al. Stability and compatibility of recombinant adeno-associated virus under conditions commonly encountered in human gene therapy trials. Hum Gene Ther Methods. 26 (2), 71-76 (2015).
- Srivastava, A. Rationale and strategies for the development of safe and effective optimized AAV vectors for human gene therapy. Mol Ther Nucleic Acids. 32, 949-959 (2023).
- Mullard, A. FDA approves first gene therapy for Duchenne muscular dystrophy, despite internal objections. Nat Rev Drug Discov. 22 (8), 610-610 (2023).
- Center for Biologics Evaluation and Research. Approved Cellular and Gene Therapy Products. US Food Drug Adm. , (2023).
- Kang, L., et al. AAV vectors applied to the treatment of CNS disorders: Clinical status and challenges. J Control Release Off J Control Release Soc. 355, 458-473 (2023).
- De Wolf, D., Singh, K., Chuah, M. K., VandenDriessche, T. Hemophilia gene therapy: The end of the beginning. Hum Gene Ther. 34 (17-18), 782-792 (2023).
- Simons, E. J., Trapani, I. The opportunities and challenges of gene therapy for treatment of inherited forms of vision and hearing loss. Hum Gene Ther. 34 (17-18), 808-820 (2023).
Ristampe e Autorizzazioni
Richiedi autorizzazione per utilizzare il testo o le figure di questo articolo JoVE
Richiedi AutorizzazioneEsplora altri articoli
This article has been published
Video Coming Soon