Method Article
Dual CRISPR-Interference Strategy for Targeting Synthetic Lethal Interactions Between Non-Coding RNAs in Cancer Cells
In This Article
Summary
This study presents a dual CRISPRi system targeting long non-coding RNAs in melanoma cells. It enables combinatorial gene knockdown and synthetic lethal screening, identifying cancer-specific lncRNA interactions for potential therapeutic strategies.
Abstract
Long non-coding RNAs (lncRNAs) represent a vast and functionally diverse class of RNA molecules, with over 100,000 predicted in the human genome. Although lncRNAs are less conserved across species compared to protein-coding genes, they play critical roles in gene regulation, chromatin interactions, and cancer progression. Their involvement in cancer makes them promising therapeutic targets. CRISPR interference (CRISPRi), utilizing catalytically inactive Cas9 fused with a transcriptional repressor such as KRAB-MeCP2, offers a precise method for targeting nuclear lncRNAs and assessing their functions. This study introduces a dual CRISPRi system using orthogonal CRISPRi technologies from Staphylococcus aureus and Streptococcus pyogenes based on dCas9-KRAB-MeCP2, optimized for combinatorial targeting of lncRNAs in human melanoma cells. The protocol facilitates combinatorial gene knockdown or synthetic lethal screening of lncRNA pairs, providing a novel tool for cancer research. By exploring synthetic lethality between lncRNAs, this approach can help identify lncRNA interactions critical for cancer cell survival, offering new therapeutic strategies. The dual system's functionality is demonstrated, highlighting its potential in identifying critical cancer-specific lncRNA interactions.
Introduction
Although less than 3% of the human genome encodes proteins, approximately 80% of the genome is transcribed1,2. Among the non-coding transcriptional units, tens of thousands are classified as long non-coding RNAs (lncRNAs) exceeding 200 nucleotides, and the total number of human lncRNAs is estimated to exceed 100,0003,4. In contrast to coding genes, lncRNAs are less conserved across species. Given that humans share 99% of their genome with primates like chimpanzees, lncRNAs are hypothesized to have a much greater influence on phenotypic evolution5,6. These findings indicate important cellular functions of lncRNAs. Although the regulation of lncRNAs and their interactions with RNA-binding proteins and other RNAs remain incompletely understood, and many lncRNAs are yet to be fully annotated, it is clear that lncRNAs exhibit cell- and tissue-specific expression patterns in health and disease, such as cancer7,8,9,10. They are implicated in diverse functions, including gene transcription regulation, involvement in chromatin interactions11, RNA processing12, RNA stabilization13, and regulation of translation14.
In cancer, highly diverse cell type-specific lncRNAs influence tumor development and metastasis by regulating gene expression, highlighting their potential as valuable therapeutic targets15. Beyond the detection of lncRNAs as biomarkers in tumor samples16, targeting tumor-specific lncRNAs to disrupt their downstream functions holds significant potential in both clinical applications and basic research. RNA-based approaches to elucidate the roles of lncRNAs include antisense oligonucleotides (ASOs), short hairpin RNAs, and small interfering RNAs (siRNAs)17,18. While siRNA is commonly utilized for gene silencing screens, the siRNA-based knockdown is restricted to the cytoplasm19. However, lncRNA frequently operates within the nucleus.
Alternatively, Clustered Regularly Interspaced Short Palindromic Repeats interference (CRISPRi) can be used to inhibit lncRNAs in human cancers20. Moreover, genome-wide CRISPRi screens can be easily programmed and target a wide range of coding and non-coding genes to examine their functional impact21,22. In CRISPRi a catalytic deficient Cas9 (dCas9) is fused to a transcriptional repressor domain, such as the Krüppel-associated box (KRAB) domain23. Gene repression by dCas9-KRAB is guided by a guide RNA (gRNA) to the region of interest. CRISPRi controls genes at the DNA levels, leading to higher efficiency and desired loss-off-function phenotypes in contrast to RNA interference, which is active at the post-transcriptional level24. In response to the limited efficacy of KRAB in target silencing, a fusion of KRAB and MeCP2 with dCas9 was introduced as a more effective repressing strategy25.
Although single gene silencing may affect cancer viability, synthetic dual or multiple lethal interactions can rescue cancer cells from cell death26. Synthetic lethality involves two or more genes, each of which can compensate for the function of the other one. To overcome issues with single-gene knockdown screens, dual CRISPR strategies targeting lethal protein-coding gene pairs offer a promising approach27.
Here, we present a protocol for the combinatorial use of orthogonal CRISPRi-based targeting of lncRNA or other non-coding RNA pairs using Staphylococcus aureus and Streptococcus pyogenes dCas9 fused to KRAB-MeCP2 in human melanoma cells (see Figure 1). The protocol can be utilized for combinatorial classical CRISPR knockdown or as CRISPRi-based screening of synthetic lethal pairs in cancer. The use of two different dCas9 species, SpCas9 and SaCas9, in the CRISPRi approach allows for independent targeting of distinct genomic loci with minimal cross-reactivity, enhancing specificity and flexibility while ensuring a high on-target selectivity. Different protospacer adjacent motifs (PAMs) are utilized: NGG for SpCas9 and NNGRRT for SaCas9. The dual dCas9 system addresses challenges such as limited sgRNA compatibility by enabling precise, simultaneous modulation and reducing sgRNA-RNP complex competition when using a single type of dCas9. This innovation improves the robustness and versatility of dual sgRNA library screening. In conclusion, we provide a fully functional dual CRISPRi system in melanoma cells as a cancer model.
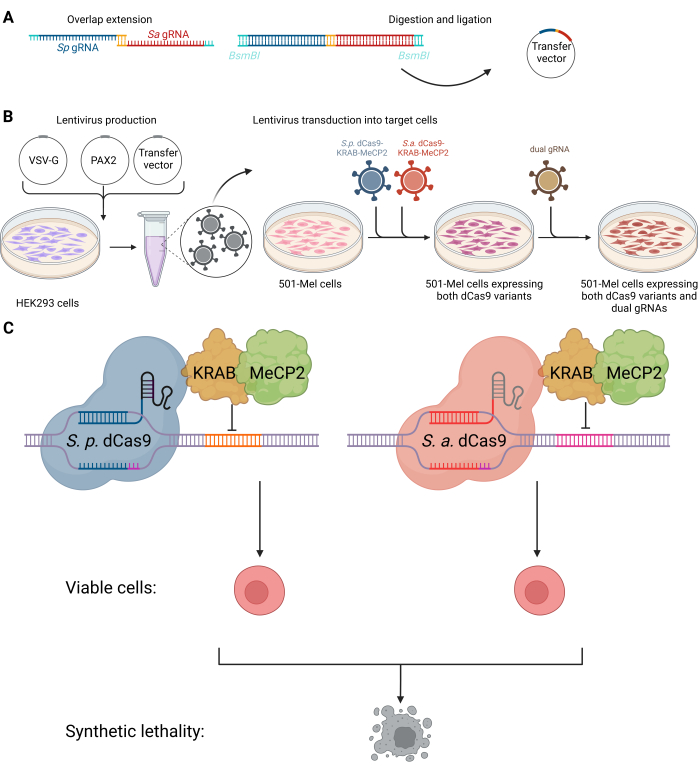
Figure 1: Scheme of the dual CRISPRi system to target synthetic lethal interactions of non-coding RNAs. (A) Cloning procedure of the dual gRNA vector. (B) HEK293 cells were transfected with envelope plasmid VSV-G, packaging plasmid PAX2, and transfer vector to produce lentiviruses for subsequent transduction into 501-mel cells. Lentiviruses containing the genetic information for SpdCas9-KRAB-MeCP2 (blue) and SadCas9-KRAB-MeCP2 (red) were integrated into 501-mel cells simultaneously. Following antibiotic selection, a second lentiviral transduction was performed to integrate the desired dual gRNAs (olive green). (C) 501-mel cells expressing the dCas9 variants interact with their corresponding gRNAs to silence target gene expression, resulting in cancer cell death. Created with BioRender.com. Please click here to view a larger version of this figure.
Protocol
The human melanoma cell line 501-mel (RRID: CVCL_4633) was kindly provided by the Aifantis Lab (New York University). The Lenti-X 293T HEK cell line was purchased from Takara Bio. These cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM) high glucose supplemented with 10% fetal bovine serum (FBS) at 37 °C in a 5% CO2 atmosphere under sterile conditions. Cells were passaged until they reached 80%-90% confluency. Cell lines were regularly tested for mycoplasma contamination.
1. Designing gRNAs using an online gRNA design platform
- Design one gRNA specific to SpCas9 and one gRNA specific to SaCas9 for each target lncRNA to evaluate which variant yields superior knockdown results.
- Target the desired lncRNAs, here RP11-120D5.1 (RP11) and XLOC_030781 (XLOC), using the most efficient gRNA for each S. pyogenes (gRNA-sp) Cas9 and S. aureus (gRNA-sa) Cas9.
NOTE: The S. pyogenes gRNAs can be extracted from Petroulia et al.28. - Follow site-specific recommendations to prioritize the highest on-target activity, expressed by high on-target scores, while minimizing off-target matches as suggested by gRNA design tools.
- Choose the genome GRCh38 (hg, Homo sapiens). Select the PAM site for the specific SpCas9 (NGG) and SaCas9 (NNGRRT).
- Use the off-target scoring method from Hsu et al.29. Position the gRNAs within a window of -150 bp to +50 bp around the transcriptional start site for optimal performance.
- Choose gRNAs with the highest on-target and off-target score (see Supplementary Table 1).
- Combine a non-targeting or scrambled control gRNA, here against Rosa26, with each target gRNA to create a dual gRNA vector, targeting one lncRNA. The combination of target gRNAs in one plasmid enables site-specific modification of both target sites.
NOTE: The dual gRNA vector harboring control gRNA and target gRNA enables knockdown of one target lncRNA, while dual gRNA vectors containing gRNAs targeting to sites allow simultaneous knockdown of targeted lncRNAs.
2. gRNA cloning
- Perform an overlap extension PCR using two DNA fragments containing the gRNA sequence. Use the U6 forward primer and H1 reverse primer (see Supplementary Table 2) for amplification as described by Najm et al.27.
- Set up a 50 µL PCR reaction with 25 µL of 2x High-fidelity PCR master mix with HF buffer and 1 µL of each DNA fragment containing either the S. pyogenes or S. aureus gRNA. Run the reaction under the following cycling conditions: initial denaturation at 98 °C for 30 s, followed by 15 cycles of denaturation at 98 °C for 5 s, annealing at 55 °C for 10 s, and elongation at 72 °C for 15 s.
- Add 2.5 µL of U6 forward primer and 2.5 µL of H1 reverse primer after completing the initial 15 cycles, and subject the reaction to an additional 20 cycles under the same conditions, with a final extension for 5 min at 72 °C.
- Purify the PCR products using a PCR purification kit according to the manufacturer's instructions.
- Digest 1 µg of the modified plasmid using 1 µL of BsmB1 restriction enzyme and 1 µL of 10x buffer for 30 min at 37 °C in a 10 µL reaction.
- Clone the PCR products into the digested dual-gRNA-Zeo-GFP plasmid (see Supplementary Figure 1 and Supplementary File 1) using a HiFi DNA assembly reaction protocol as described by others30.
- Transform chemically competent E. coli cells by mixing 50 µL of bacteria culture with 4 µL of assembly product according to the manufacturer's instructions.
3. Lentivirus production
CAUTION: Handle active lentiviruses at all steps while following appropriate safety guidelines. Always perform work with viruses in a Class 2 safety cabinet to prevent hazards from unavoidable aerosol formation. Conduct all work outside the safety cabinet, such as centrifugation, in aerosol-tight containers and rotors approved for use with Risk Group 2 organisms. Wear appropriate protective clothing, including a lab coat, disposable gloves, and safety goggles, at all times in the work area. The work surfaces have to be treated with a disinfectant solution, and other waste must also be treated with a disinfectant solution, allowing them to remain in the sealed cabinet under UV light for at least 240 min. Utilized serological pipettes and pipette tips must be rinsed in a disinfectant solution before disposal in autoclavable bottles. Afterward, the waste should be disposed of in the S2 waste container. The S2 waste will be inactivated through autoclaving. Transduced cell lines can be handled under S1 conditions no earlier than 2 days after transduction and only after at least two complete changes of the culture medium with a virus-free medium.
- Count Lenti-X 293T HEK cells by mixing 10 µL of cells with 10 µL of Trypan blue solution in a hemocytometer. Plate 4 x 106 Lenti-X 293T HEK cells per 10 cm plate to achieve ~80% confluency the next day.
- Work in a Class 2 laboratory from this step onward. Mix 500 µL of a suitable transfection medium with 11.25 µg transfer vector SpdCas9-KRAB-MeCP2 (see Supplementary File 2), 5.5 µg envelope plasmid VSV-G, and 16.5 µg pPAX2 vector. Incubate for 5 min at room temperature. For the second dCas9 variant, use the transfer vector SadCas9-KRAB-MeCP2 (see Supplementary File 3).
- In a separate tube, mix 500 µL of reduced-serum medium with 36 µL of PEI reagent (stock: 1 mg/mL). Incubate for 5 min at room temperature.
- Combine the mixtures and incubate for 15 min at room temperature. Add the mixture carefully dropwise to the cell medium (6 mL) and incubate overnight at 37 °C and 5% CO2. Ensure cells remain attached to avoid reduced lentiviral yield.
- Discard the medium into an autoclavable waste bottle using a serological pipette after 12-15 h. From now on, rinse consumables, including serological pipettes and pipette tips, with the disinfectant solution before disposing of them in the autoclave waste. Add 5 mL of fresh DMEM medium to the transfected cells using a new pipette.
- Harvest lentiviruses by transferring the supernatant using a serological pipette to a 50 mL canonical tube 48 h post-transfection. Store the canonical tube in the fridge. Add 5 mL of fresh medium to the cells and incubate the cells for a further 24 h.
- Repeat the collection of lentiviruses by transferring the supernatant to the same 50 mL canonical tube containing the first harvest. Again, add 5 mL of fresh medium, incubate for 24 h, and transfer the supernatant afterward to the 50 mL canonical tube. Store combined supernatants of first, second, and third harvests in the 50 mL canonical tube at 4 °C until filtration and centrifugation.
- Centrifuge the combined supernatant for 5 min at 500 x g and 4 °C in aerosol-tight containers and rotors approved for use with Risk Group 2 organisms to pellet detached cells and debris. Filter the supernatant through 0.2 µm sterile cell culture-grade syringe filters.
- Concentrate the filtered supernatant at 1,000 x g and 4 °C using a centrifugal filter unit with an appropriate molecular weight cutoff of 100 kDa to approximately 500 µL. Store virus aliquots of 50-100 µL at -80 °C until further use.
4. Lentivirus transduction and selection of stable transfected cells
CAUTION: Always perform work with viruses in a Class 2 safety cabinet.
- Plate 501-mel cells in a 6-well plate at 2 x 105 cells/well with a final culture volume of 2 mL, 1 day before transduction.
- Replace the culture medium with 2 mL of prewarmed DMEM medium supplemented with polybrene at a final concentration of 6 µg/mL.
- From now on, work in Class 2 lab is required. Add 50 µL of lentiviruses containing SpdCas9-KRAB-MeCP2 and SadCas9-KRAB-MeCP2 simultaneously to the cells. Rinse consumables, including serological pipettes and pipette tips, with the disinfectant solution before disposing of them in the autoclave waste. Shake the plate gently and incubate at 37 °C in a 5% CO2 atmosphere.
- Discard the medium into an autoclavable bottle using a serological pipette and add 2 mL of fresh medium containing blasticidin (10 µg/mL) and puromycin (2 µg/mL) 16 h post-transduction.
- Continue selection by replacing the medium with fresh 2 mL medium containing blasticidin (10 µg/mL) and puromycin (2 µg/mL) after 2 days. Handle transduced cells under S1 conditions after 7 days post-transduction.
NOTE: Antibiotic selection must be performed until all non-transduced control cells have died. Alternative selection strategies may be used depending on the resistance cassette in the transfer vector. If required, optimize antibiotic concentrations using a killing curve. Elaborate single-cell clone selection through flow cytometry or serial dilution and plating, followed by protein quantification, may be required to obtain cell populations that express dCas9 fusions at sufficient levels.
5. Protein quantification and Western blot analysis
- Count stable transfected 501-mel cells by mixing 10 µL of cells with 10 µL of Trypan Blue solution in a hemocytometer. Centrifuge 1 x 106 of the cells for 5 min at 500 x g and 4 °C. Discard the supernatant and resuspend the cell pellet in 1 mL of ice-cold PBS.
- Repeat centrifugation and resuspend the cell pellet in 50 µL of RIPA buffer containing protease inhibitors. Incubate on ice for 15 min and remove cell debris by centrifugation at 13,000 x g for 10 min at 4 °C. Store the supernatant at -20 °C or use immediately for protein quantification as described by others31.
- Mix 10 µg protein with 4 x LDS buffer, 50 mM DTT and fill up to 20 µL with distilled water. Separate the proteins on a 10% separation gel by using Tris/glycine-based SDS-PAGE at 135 V for 1 h as previously described32. Transfer proteins to a PVDF membrane at 90 V for 90 min with a wet tank transfer.
- Incubate the membrane in blocking buffer (5% non-fat milk in TBS-T) for 1 h at 180 rpm at room temperature.
- Add 1:10,000 anti-GAPDH mouse monoclonal antibody in the blocking buffer as internal control. Add the primary antibody anti-Cas9 (S. pyogenes) or anti-Cas9 (S. aureus) as 1:1,000 dilution in the blocking buffer and incubate overnight at 4 °C with gentle shaking.
- Wash the membrane 3x with TBST buffer for 10 min each. Incubate with secondary anti-mouse HRP antibody (1:10,000) for 1 h at room temperature.
- Wash 3x with TBST buffer for 10 min each. Detect signals using ECL Substrate and an imaging system.
6. Dual LncRNA repression viability assay
CAUTION: Always perform work with viruses in a Class 2 safety cabinet.
- Seed 0.1 x 105 cells containing stable transfected SadCas9-KRAB and SpdCas9-KRAB 7 days post-transduction in a 96 well plate in 100 µL of prewarmed DMEM medium 1 day before transduction.
- On the day of transduction, replace the culture medium with 100 µL of prewarmed DMEM medium supplemented with polybrene at a final concentration of 6 µg/mL. From now on, work in Class 2 lab is required. Add the appropriate volume of lentiviruses to the cells.
NOTE: The volume of lentiviruses containing the dual gRNA vector have to be examined using GFP to generate a linear curve by titrating lentiviruses. - Replace the medium with fresh medium containing 500 µg/mL Zeocin, 5 µg/mL blasticidin, and 1 µg/mL puromycin 16 h post-transduction.
- Perform luminescence detection 5 days post-transduction using a cell viability assay according to the manufacturer's instructions.
Results
The expression cassettes of SadCas9-KRAB-MeCP2 and SpdCas9-KRAB-MeCP2 were integrated into 501-mel cells simultaneously using transduction with equal amounts of lentiviruses. Since this CRISPRi protocol relies on the expression of dCas9-KRAB-MeCP2 variants, Western blot analysis is ideal for confirming their presence at the protein level. Following the enrichment of positively transfected cells, samples were collected for Western blot analysis to confirm the presence of the dCas9-KRAB-MeCP2 orthologs. Upon successful transduction, a single band corresponding to the SpdCas9-KRAB-MeCP2 fusion protein (202 kDa) and the SadCas9-KRAB-MeCP2 fusion protein (170 kDa) is expected and was confirmed, as shown in Figure 2A, using antibodies specific to the respective Cas9 orthologs. However, if Western blot is not feasible, qPCR can be used to verify the fusion proteins at the mRNA level.
Once 501-mel cells stably expressing the functional repressors were generated, second lentiviral transduction was performed using a dual gRNA vector. RP11 and XLOC were previously shown to be upregulated in short-term cultures of metastatic melanoma, and individual CRISPRi targeting demonstrated their impact on proliferation and cell survival28. Thus, we selected this lncRNA pair for evaluation as a proof-of-concept synthetic lethal non-coding RNA combination, anticipating a cumulative, easily observable growth phenotype in the modified cells.
To investigate the effects of dual CRISPRi in cancer cells using viability assays, both targeted lncRNAs must be effectively repressed. qPCR is the most suitable method to confirm this repression by measuring their transcript levels. We evaluated the functionality of the developed dual CRISPRi protocol by determining RNA levels and examining the cell viability when targeting the potentially lethal RP11 and XLOC pair. Therefore, different gRNAs targeting RP11 and XLOC were introduced to reduce the target RNA levels. The repression of RNA was investigated using target-specific gRNAs for either SadCas9-KRAB-MeCP2 or SpdCas9-KRAB-MeCP2, tested individually and in combination alongside a control gRNA targeting Rosa26 (see Figure 2B). Although gRNAs targeting XLOC or RP11 individually effectively suppressed their respective targets, simultaneous knockdown of both XLOC and RP11 was achieved only with the XLOC-sa/RP11-sp pair.
As both XLOC and RP11 RNA levels were successfully reduced in the 501-mel cell line with the selected XLOC-sa/RP11-sp gRNA pair, a dual lncRNA repression viability assay was performed (see Figure 2C). After 5 days, cell viability was 81% and 89% of the Rosa26 control when the target gRNAs were used individually. Combining RP11-sp and XLOC-sa resulted in a further significant reduction compared to RP11-sp alone, with cell viability at 59% of the Rosa26 control.
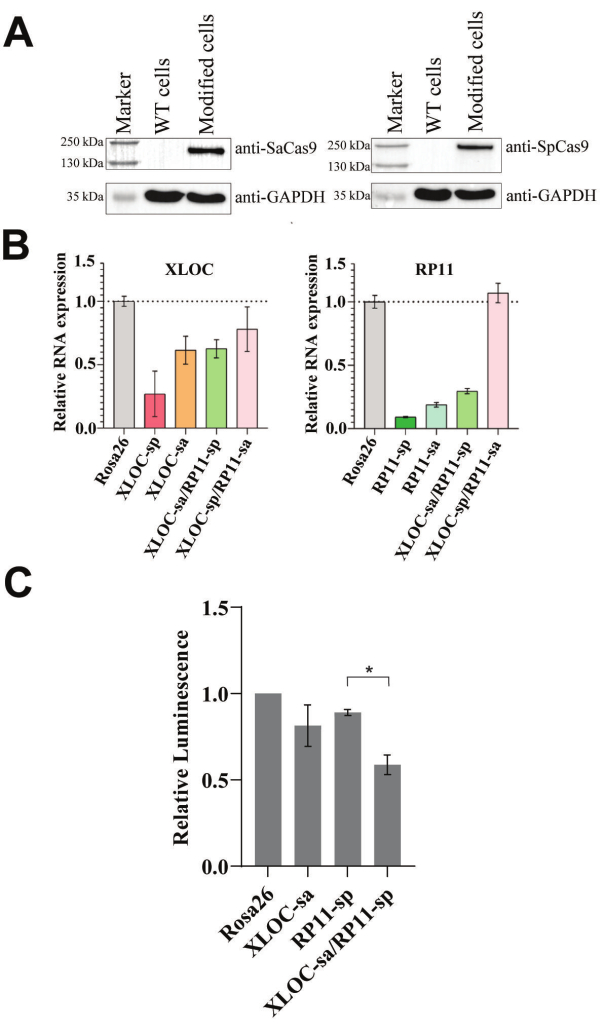
Figure 2: Functionality of the dual lncRNA repression system. (A) Western blot analysis was performed to detect the dCas9-KRAB-MeCP2 variants in modified 501-mel cells using anti-Cas9 antibodies. 501-mel wild-type (WT) cells without transduction were used as control (see Supplementary Figure 3, Supplementary Figure 4, Supplementary Figure 5, Supplementary Figure 6 for the raw blot). (B) qPCR-based analysis of RP11 and XLOC RNA levels relative to Rosa26 control cells after the transduction of dual gRNAs into modified 501-mel cells. GAPDH was used as the housekeeping gene. Data is expressed as mean ± SD. (C) The dual lncRNA repression viability assay was performed after integrating dual gRNAs into modified 501-mel cells in three independent transductions. Luminescence values were normalized to the Rosa26 control. Viability results are expressed as the mean (n = 3) ± SEM. An unpaired t-test was performed. Asterisk (*) indicates a significant difference at p < 0.05. Please click here to view a larger version of this figure.
Supplementary Figure 1: Plasmid map. The map was created with Benchling (https://benchling.com/). The utilized plasmids in the protocol were modified from Addgene #96921 and #110824. Please click here to download this File.
Supplementary Figure 2: Supplementary Figure 2: Western blot analysis of
dCas9-KRAB expression. (A) SadCas9-KRAB expression in 501-mel cells 6 days post-transfection. (B) Sequential transduction of SadCas9-KRAB and SpdCas9-KRAB in 501-mel cells led to reduced SadCas9-KRAB expression 3 weeks post-transfection. (C) Simultaneous transduction of SadCas9-KRAB and SpdCas9-KRAB in 501-mel cells resulted in reduced SadCas9-KRAB expression 3 weeks post-transfection. (D) SpdCas9-KRAB expression could be observed after simultaneous transduction of SadCas9-KRAB and SpdCas9-KRAB. LV. The volume of lentiviruses used in µL. Please click here to download this File.
Supplementary Figure 3: Western blot SaCas9-KRAB raw data. Please click here to download this File.
Supplementary Figure 4: Western blot SpCas9-KRAB raw data. Please click here to download this File.
Supplementary Figure 5: GAPDH_SpCas9-KRAB raw data. Please click here to download this File.
Supplementary Figure 6: GAPDH_SaCas9-KRAB raw data. Please click here to download this File.
Supplementary Table 1: Utilized guide RNA sequences. Please click here to download this File.
Supplementary Table 2: Utilized DNA sequences. Please click here to download this File.
Supplementary File 1: FASTA file of dual-grna-zeo-gfp. Please click here to download this File.
Supplementary File 2: FASTA file of dSpCas9-krab-mecp2. Please click here to download this File.
Supplementary File 3: FASTA file of dSaCas9-krab-mecp2. Please click here to download this File.
Discussion
In this study, we implemented a dual lncRNA targeting strategy in melanoma cells using CRISPRi based on dCas9-KRAB-MeCP2 from two orthogonal species. Utilizing this system, we successfully repressed a synthetic lethal pair of lncRNAs, RP11 and XLOC, leading to increased cell death, unlike when they were repressed individually. However, CRISPRi systems carry a risk of off-target gene repression, potentially affecting result interpretation. Although experimental validation was not conducted, we minimized potential off-target effects during gRNA design in silico.
Variability in transduction efficiency may impact reproducibility. Therefore, we optimized transduction by applying equal amounts of lentiviruses, determined through a standard curve calibration using varying lentivirus concentrations. However, the increased cargo size of the newly designed vector may impose limitations on lentiviral packaging efficiency, potentially reducing transduction efficiency, particularly in hard-to-transduce cell lines. As a result, achieving sufficient transduction and robust dCas9 expression may require high viral titers in certain instances. Additionally, insufficient knockdown can be circumvented by frequently monitoring dCas9-KRAB-MeCP2 protein levels and RNA expression of targeted lncRNAs. Alternatively, there are dCas9 versions available with only one repressor system to overcome this limitation, however, with the cost of decreased efficiency25.
Only the XLOC-sa-RP11-sp combination demonstrated successful activity, whereas the reverse XLOC-sp-RP11-sa configuration was ineffective. A plausible explanation for this difference in knockdown efficacy is the interplay between PAM specificity and chromatin accessibility33. Given that SaCas9 and SpCas9 recognize distinct PAM sequences, the target site for SpCas9 in the XLOC-sp-RP11-sa orientation may lack an optimal PAM, thereby reducing its binding affinity and subsequent activity. Furthermore, the spatial arrangement of the two dCas9 fusion protein variants could influence chromatin architecture and accessibility34,35, potentially restricting the recruitment of the transcriptional machinery or associated regulatory factors in the XLOC-sp-RP11-sa configuration. These findings suggest that the relative positioning of SaCas9 and SpCas9 within a given genomic context may critically impact their functional efficacy, an aspect that warrants further investigation for optimized CRISPR-based modulation strategies.
The developed protocol is adaptable for targeting or screening other synthetic lethal non-coding RNA pairs by modifying the dual gRNA vector. Once a cell line of interest is stably expressing both dCas9 variants, a variety of approaches become feasible. While RNAi, ASOs, and shRNAs can be employed for gene silencing, CRISPRi offers a more straightforward screening methodology, and its combination with small chemical compounds or inhibitors is also conceivable. By substituting the dual gRNA vector with a dual gRNA library, this protocol allows for the systematic screening of synthetic lethal pairs or other genetic dependencies in cancer cells, thereby enhancing the precision of cancer cell targeting.
Although we achieved integration of the dCas9-KRAB-MeCP2 variants through lentiviral transduction, alternative stable transfection methods can also be used to incorporate the desired enzymes into 501-mel cells. This is particularly relevant, as, in our experience, the silencing of large constructs such as dCas9-KRAB-MeCP2 can occur over time36,37. Alternatively, dCas9-KRAB was shown to be a smaller alternative23. In our experience, transduction with the smaller dCas9-KRAB fusion proteins derived from Cas9 orthologs led to reduced protein expression during prolonged cultivation (see Supplementary Figure 2). Therefore, consistent protein expression should be regularly monitored at recurring time points. Additionally, transducing two large fusion proteins along with selection markers and dual gRNA expression cassettes may induce cellular stress or may reach lentiviral packaging capacities.
Achieving 100% knockdown efficiency in dual CRISPRi can be challenging due to suboptimal gRNA design or insufficient dCas9 expression. To address this, multiple gRNAs per target should be tested using advanced design tools to identify the most effective sequences. Additionally, optimizing dCas9-KRAB-MeCP2 expression through improved vector design and characterization of clonal cell lines can enhance knockdown efficiency.
Alternative strategies for integrating the CRISPRi system include the use of transposase vectors or CRISPR-directed integration to achieve stable insertion into safe harbor genomic sites, which are known for maintaining stable expression over time38. However, many cell types, including melanoma cells, are challenging to transfect39, making lentiviral transduction the preferred method. Electroporation of transposase- or Cas-based enzymes may offer an alternative means to integrate the dCas9-KRAB-MeCP2 constructs into melanoma cells40. Implementing an inducible knockout system would be advantageous for activating dCas9-KRAB-MeCP2 expression as needed rather than constitutive expression to minimize cellular stress.
Finally, the developed approach is not limited to melanoma cells but can be extended to any cell type where synthetic lethality is being investigated. This approach is explicitly meant to be scaled up towards the gRNA library screening modality. However, due to the increased combinatorial complexity, e.g., with more than three gRNAs per gene pair, including controls and at least 300 x coverage, only a sub-transcriptomic fraction of expressed lncRNAs can be targeted practically. Therefore, users must carefully pre-select candidates by considering factors such as lncRNA expression levels and their known relevance in specific cellular contexts before proceeding with the library screening. Nevertheless, the combinatorial versatility of this approach makes it a valuable tool for investigating synthetic lethal interactions and other genetic dependencies of lncRNAs across various cancer types and potentially other diseases. This expands the currently available toolkit for studying these network interactions in cells.
Disclosures
Jochen Imig is currently CGCIII funded by Pfizer Inc. CGCIII is sponsored by Pfizer Inc., Merck KGaA, and AstraZeneca PLC. The sponsors had no role in the design, execution, interpretation, or writing of the study. All the authors have no conflicts of interest to disclose.
Acknowledgements
We acknowledge Stefan Raunser (Max Planck Institute of Molecular Physiology, Dortmund) for access to Biosafety Level 2 lab space. Special thanks to Eric Wang for his intellectual contributions and to all past and present members of the Imig lab for their valuable discussions. Jochen Imig is currently CGCIII funded by Pfizer Inc. at CGC III.
Materials
| Name | Company | Catalog Number | Comments |
| Amicon Ultra Centrifugal Filter, 100 kDa MWCO | Millipore | UFC910024 | |
| anti-Cas9 (S. aureus) (6H4) mouse monoclonal antibody | Cell Signaling Technology | 48989 | |
| anti-Cas9 (S. pyogenes) (7A9-3A3) monoclonal antibody | Cell Signaling Technology | 14697 | |
| anti-GAPDH mouse monoclonal antibody | Sigma Aldrich | G8795 | |
| Anti-Mouse IgG (whole molecule)–Peroxidase antibody produced in rabbit | Sigma Aldrich | A9044 | |
| Bio-Rad ChemiDoc MP Imaging System | Bio-Rad | ||
| Blasticidine S hydrochloride | Sigma Aldrich | 15205-25MG | |
| CellTiter-Glo Luminescent Cell Viability Assay | Promega | G7570 | |
| Centrifuge | Eppendorf | 5804R | |
| Centrifuge | Eppendorf | 5415R | |
| CFX Connect Real-Time PCR Detection System | Bio-Rad | ||
| Clarity Western ECL Substrate, 500 mL | Bio-Rad | 1705061 | |
| CO2 Incubator Model CB 170 | Binder | ||
| cOmplete Protease Inhibitor Cocktail | Roche | 11697498001 | |
| Esp3I (BsmB1) restriction enzyme | Thermo Scientific | ER0451 | |
| Falcon 10 mL Serological Pipet | Corning | 356551 | |
| Falcon 25 mL Serological Pipet | Corning | 357525 | |
| Falcon 5 mL Serological Pipet | Corning | 356543 | |
| Fetal Bovine Serum | Sigma Aldrich | F9665-500ML | |
| Gibco Dulbecco’s modified Eagle’s medium (DMEM), high glucose, pyruvate | Gibco | 41966029 | |
| Human melanoma cell line 501-mel | was kindly provided by the Aifantis Lab (New York University) | RRID: CVCL_4633 | |
| Immobilon -P PVDF Membrane | Millipore | IPVH00010 | |
| Lenti-X 293T HEK cell line | Takara Bio | 632180 | |
| Mini Trans-Blot Cell system | Bio-Rad | ||
| Mini-PROTEAN Tetra Handcast System | Bio-Rad | ||
| NEBuilder HiFi DNA Assembly Reaction | New England Biolabs | E2621 | |
| Non-fat milk powder | Biomol | 54650 | |
| NuPAGE LDS Sample Buffer (4x) | Invitrogen | NP0008 | |
| One Shot Stbl Chemically Competent E. coli | Invitrogen | C737303 | |
| Opti-MEM I Reduced Serum Medium | Gibco | 31985062 | |
| PCR Tubes 0.5 ml (Flat Cap) | VWR International | 732-3207 | |
| Phosphate-Buffered Saline (PBS) | Corning | 45000-446 | |
| Phusion High-Fidelity PCR Master Mix with HF Buffer | Thermo Scientific | F531L | |
| Pierce BCA Protein Assay Kit | Thermo Scientific | 23225 | |
| Polybrene | Sigma Aldrich | TR-1003-G | |
| Polyethylenimine, branched | Sigma Aldrich | 408727 | |
| Puromycin dihydrochloride | Santa Cruz Biotechnology | sc-108071A | |
| QIAquick PCR Purification Kit | Qiagen | 28104 | |
| SafeSeal Microcentrifuge Tube 1.5 mL | Sarstedt | 72,706 | |
| Sodium chloride, 5 M Aqua Solution, RNase Free | Alfa Aesar | J60434.AE | |
| Sodium Dodecyl Sulfate (SDS) | Sigma Aldrich | L3771 | |
| Syringe Filter PES 33mm 0.2 μM | Fisher Scientific | 15206869 | |
| TC Dish 100, Standard | Sarstedt | 8,33,902 | |
| TC Plate 6 Well, Standard, F | Sarstedt | 83,39,20,005 | |
| Tris base | Roche | 10708976001 | |
| TWEEN 20 | Sigma Aldrich | P9416-50ML |
References
- Lander, E. S., et al. Initial sequencing and analysis of the human genome. Nature. 409 (6822), 860-921 (2001).
- ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 489 (7414), 57-74 (2012).
- Szcześniak, M. W., Wanowska, E., Mukherjee, N., Ohler, U., Makałowska, I. Towards a deeper annotation of human lncRNAs. Biochim Biophys Acta Gene Regul Mech. 1863 (4), 194385 (2020).
- Fang, S., et al. NONCODEV5: a comprehensive annotation database for long non-coding RNAs. Nucl Acids Res. 46 (D1), D308-D314 (2018).
- Lin, J., et al. Human-specific lncRNAs contributed critically to human evolution by distinctly regulating gene expression. eLife. 12, RP89001.2 (2023).
- Sarropoulos, I., Marin, R., Cardoso-Moreira, M., Kaessmann, H. Developmental dynamics of lncRNAs across mammalian organs and species. Nature. 571 (7766), 510-514 (2019).
- Melé, M., et al. Human genomics. The human transcriptome across tissues and individuals. Science. 348 (6235), 660-665 (2015).
- Cabili, M. N., et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 25 (18), 1915-1927 (2011).
- Liu, S. J., Dang, H. X., Lim, D. A., Feng, F. Y., Maher, C. A. Long noncoding RNAs in cancer metastasis. Nat Rev Cancer. 21 (7), 446-460 (2021).
- Ahmad, M., Weiswald, L. B., Poulain, L., Denoyelle, C., Meryet-Figuiere, M. Involvement of lncRNAs in cancer cells migration, invasion and metastasis: cytoskeleton and ECM crosstalk. J Exp Clin Cancer Res. 42 (1), 173 (2023).
- Jain, A. K., et al. LncPRESS1 Is a p53-Regulated LncRNA that Safeguards Pluripotency by Disrupting SIRT6-Mediated De-acetylation of Histone H3K56. Mol Cell. 64 (5), 967-981 (2016).
- Bergmann, J. H., et al. Regulation of the ESC transcriptome by nuclear long noncoding RNAs. Genome Res. 25 (9), 1336-1346 (2015).
- Kretz, M., et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature. 493 (7431), 231-235 (2013).
- Yoon, J. H., et al. LincRNA-p21 suppresses target mRNA translation. Mol Cell. 47 (4), 648-655 (2012).
- Huarte, M. The emerging role of lncRNAs in cancer. Nat Med. 21 (11), 1253-1261 (2015).
- Badowski, C., He, B., Garmire, L. X. Blood-derived lncRNAs as biomarkers for cancer diagnosis: the Good, the Bad and the Beauty. NPJ Precis Oncol. 6 (1), 40 (2022).
- Zong, X., et al. Knockdown of nuclear-retained long noncoding RNAs using modified DNA antisense oligonucleotides. Meth Mol Biol. 1262, 321-331 (2015).
- Gagnon, K. T., Li, L., Chu, Y., Janowski, B. A., Corey, D. R. RNAi factors are present and active in human cell nuclei. Cell Rep. 6 (1), 211-221 (2014).
- Zeng, Y., Cullen, B. R. RNA interference in human cells is restricted to the cytoplasm. RNA. 8 (7), 855-860 (2002).
- Yang, J., et al. CRISPR/Cas9-mediated noncoding RNA editing in human cancers. RNA Biol. 15 (1), 35-43 (2018).
- Liu, S. J., et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science. 355 (6320), aah7111 (2017).
- Tsung, K., et al. CRISPRi screen of long non-coding RNAs identifies LINC03045 regulating glioblastoma invasion. PLoS Genet. 20 (6), e1011314 (2024).
- Gilbert, L. A., et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 154 (2), 442-451 (2013).
- Boettcher, M., McManus, M. T. Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol Cell. 58 (4), 575-585 (2015).
- Yeo, N. C., et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat Meth. 15 (8), 611-616 (2018).
- Parrish, P. C. R., et al. Discovery of synthetic lethal and tumor suppressor paralog pairs in the human genome. Cell Rep. 36 (9), 109597 (2021).
- Najm, F. J., et al. Orthologous CRISPR-Cas9 enzymes for combinatorial genetic screens. Nat Biotechnol. 36 (2), 179-189 (2018).
- Petroulia, S., et al. CRISPR-inhibition screen for lncRNAs linked to melanoma growth and metastasis. bioRxiv. , (2024).
- Hsu, P. D., et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 31 (9), 827-832 (2013).
- New England Biolabs. . NEBuilder HiFi DNA Assembly Reaction (E2621) v2. , (2020).
- Cortés-Ríos, J., et al. Protein quantification by bicinchoninic acid (BCA) assay follows complex kinetics and can be performed at short incubation times. Anal Biochem. 608, 113904 (2020).
- Junior, N. . Polyacrylamide Gel Electrophoresis (SDS-PAGE) v1. , (2019).
- Chen, X., Liu, J., Janssen, J. M., Gonçalves, M. A. F. V. The chromatin structure differentially impacts high-specificity CRISPR-Cas9 Nuclease strategies. Mol Ther Nucl Acids. 8, 558-563 (2017).
- Schep, R., et al. Impact of chromatin context on Cas9-induced DNA double-strand break repair pathway balance. Mol Cell. 81 (10), 2216-2230.e10 (2021).
- Daer, R. M., Cutts, J. P., Brafman, D. A., Haynes, K. A. The impact of chromatin dynamics on Cas9-Mediated genome editing in human cells. ACS Syn Biol. 6 (3), 428-438 (2017).
- Chavez, M., Rane, D. A., Chen, X., Qi, L. S. Stable expression of large transgenes via the knock-in of an integrase-deficient lentivirus. Nat Biomed Eng. 7 (5), 661-671 (2023).
- Ellis, J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Human Gene Ther. 16 (11), 1241-1246 (2005).
- Shrestha, D., et al. Genomics and epigenetics guided identification of tissue-specific genomic safe harbors. Genome Biol. 23 (1), 199 (2022).
- Chu, Z., et al. Enhanced gene transfection and induction of apoptosis in melanoma cells by branched poly(β-amino ester)s with uniformly distributed branching units. J Control Release. 367, 197-208 (2024).
- Han, S. Y., et al. Nucleofection is a highly effective gene transfer techfnique for human melanoma cell lines. Exp Dermatol. 17 (5), 405-411 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved