Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Whole Mount Preparation of the Adult Drosophila Ventral Nerve Cord for Giant Fiber Dye Injection
W tym Artykule
Podsumowanie
An in vivo dissection of the adult Drosophila ventral nerve cord (VNC) is demonstrated. This particular dissection method causes little damage to the VNC allowing the subsequent labeling of the giant fiber neurons with fluorescent dye for high resolution imaging.
Streszczenie
To analyze the axonal and dendritic morphology of neurons, it is essential to obtain accurate labeling of neuronal structures. Preparing well labeled samples with little to no tissue damage enables us to analyze cell morphology and to compare individual samples to each other, hence allowing the identification of mutant anomalies.
In the demonstrated dissection method the nervous system remains mostly inside the adult fly. Through a dorsal incision, the abdomen and thorax are opened and most of the internal organs are removed. Only the dorsal side of the ventral nerve cord (VNC) and the cervical connective (CvC) containing the big axons of the giant fibers (GFs)1 are exposed, while the brain containing the GF cell body and dendrites remains2 in the intact head. In this preparation most nerves of the VNC should remain attached to their muscles.
Following the dissection, the intracellular filling of the giant fiber (GF) with a fluorescent dye is demonstrated. In the CvC the GF axons are located at the dorsal surface and thus can be easily visualized under a microscope with differential interference contrast (DIC) optics. This allows the injection of the GF axons with dye at this site to label the entire GF including the axons and their terminals in the VNC. This method results in reliable and strong staining of the GFs allowing the neurons to be imaged immediately after filling with an epifluorescent microscope. Alternatively, the fluorescent signal can be enhanced using standard immunohistochemistry procedures3 suitable for high resolution confocal microscopy.
Protokół
1. Dissection of the ventral nerve cord
Dissection and dye filling of the GF can be performed at room temperature. For all dissection steps cold (4°C) saline should be used to slow down cell metabolism and keep the neurons alive longer.
- Anesthetize adult flies (4 days or older) with CO2 or chill them on ice until they are immobilized. Anesthetizing with ice will require more time (up to 30 min) but will keep the flies immobilized for longer.
- Place one fly in a Sylgard coated Petri dish. This dissection requires the use of a high magnification (80-100X) dissection stereomicroscope.
- With Vannas scissors, remove legs, proboscis, and wings.
- Use coarse forceps and fine insect pins (minutien pins) to orient the fly dorsal side up in the Sylgard dish. Pin the abdomen first and then elongate the animal slightly by placing two pins on each side of the neck, behind the head, so that the neck is slightly stretched and accessible (see Figure 2).
- Submerge the fly in fresh, cold Drosophila saline. Most commonly used Drosophila salines are sufficient for the dissection, however here we used a saline described in Gu and O'Dowd, 20064. An air bubble will surround the fly. Remove the air with a syringe.
- Make a short, lateral incision into the dorsal end of the abdomen. Starting at the incision, make a shallow, straight cut along the dorsal midline of the abdomen towards the thorax. Then continue the shallow, longitudinal cut in the thorax as precisely as possible along the midline toward the connective. It is critical to make the cuts shallow as to not damage the VNC (Figure 1).
- Use two fine insect pins to carefully open the thorax. Spread the body halves apart using the pins and place the pins through the flight muscles (Figure 2). The thorax should not be opened further as depicted in the Figure 2 to avoid ripping of the cuticle. To ensure a straight orientation of the VNC the pins should be placed in the same anterior posterior position on each side.
- Access to the VNC for dye injection and proper imaging may be obstructed by internal organs. Therefore, use forceps and scissors to remove the stomach, gut, heart, and reproductive organs. Pull out the salivary glands lying on both sides of the VNC. Rinse the dissection carefully with fresh saline to remove floating tissue and fat.
- Remove the fat body from underneath the cervical connective (CvC). The fat body is best visible at a high magnification. Use fine forceps to carefully grasp the tissue from the side and pull it out.
The dissection can be performed within 5 to 10 minutes. By using fresh, cold saline the dissected, undamaged nervous system will remain alive for at least one hour.
2. Intracellular GF fill
It is recommended to perform the dye filling as soon as possible after the dissection.
- Place the Sylgard dish with the dissected fly under an upright microscope with a fixed stage (Figure 3) and a 10x air objective. Center the sample with the posterior end of the fly positioned toward the dye fill electrode holder. Use the differential interference contrast (DIC) mode and focus on the CvC. The big axons of the GFs should be clearly visible on the dorsal surface of the CvC.
- Dilute Lucifer Yellow CH dilithium salt in deionized H2O to a concentration of 1%. Pull a Borosilicate glass electrode with filament to a resistance of approx. 60-80 MΩ. The tip of the glass electrode should be long and sharp. Fill the tip of the electrode with 1% Lucifer Yellow solution by placing the electrode with the end opposite the electrode tip into the Lucifer Yellow solution for approx. 30-60 seconds. Then backfill the electrode with 3M LiCl using a Hamilton syringe leaving an air bubble between the dye and LiCl. Connect the dye fill electrode to the head stage.
- Insert a chloride coated silver wire ground electrode into the saline and place the dye fill electrode above the fly parallel to the VNC using a 3D hydraulic micromanipulator. The dye fill electrode should be connected to an amplifier that allows passing current in order to release negatively or positively charged dyes from the electrode by passing hyper- or depolarizing current, respectively.
- Switch to a 40x water immersion lens and lower the tip of the dye fill electrode onto one of the GFs. The GF axons lay close to the dorsal surface of the CvC. Use a forward (toward the head) movement to insert the electrode into the GF.
- Change your light source to epifluorescent light and switch to a Lucifer Yellow filter. Apply a hyperpolarizing current to the dye fill electrode with the amplifier to inject the Lucifer Yellow into the GF. The quantity of dye injected can be controlled by the amount of current passed as well as the duration the current is passed. To prevent the dye from spilling out of the injection site or damage the GF, it is recommended to inject using a low current (approx 3-5 nA) over 1-5 minutes.
- Apply current until the GF terminal in the VNC can be seen clearly (Figure 4A). For trans-synaptic staining of postsynaptic neurons, continue to pass current on until dye can also be seen in postsynaptic neurons. Switch off the current and remove the dye fill electrode.
3. Representative Results:
A representative dye fill for one GF with Lucifer Yellow is shown in Figure 4A. The image was acquired as an image stack in Nikon Elements software using a spot camera mounted on the microscope. In this sample current was passed through the electrode for about 2 minutes. The GF is thoroughly filled and the large GF terminal in the thorax is well labeled (Figure 4A, ellipse). No postsynaptic neurons are visible in this case, although Lucifer Yellow is small enough to pass through gap junctions. If the dye is not injected long enough into the GF then trans-synaptic fills of the postsynaptic neurons may not been seen because the signal is too weak to be detected. Neurons postsynaptic to the GFs (Figure 4B, arrow heads) can be visualized more reliably using even smaller neuronal tracers, such as Neurobiotin. Injecting a 7% Neurobiotin in 2 M potassium acetate solution with a depolarizing current for 2 minutes is sufficient to label postsynaptic neurons strongly. Neurobiotin can be visualized for confocal microscopy using Streptavidin coupled to dyes of different wavelengths. Because Neurobiotin is not a fluorescent dye, co-injection of Rhodamin-Dextran or Alexa hydrazides in the same solution, can be used to monitor a successful injection into the GF (Figure 4C, from Boerner and Godenschwege, 20105).
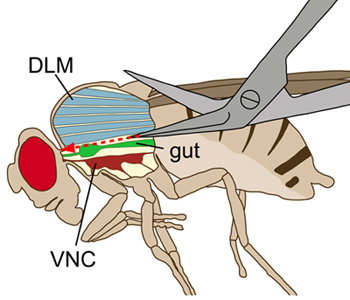
Figure 1. Schematic drawing of a fly viewed laterally. The thorax is displayed with a longitudinal incision to visualize the internal structures. During the dissection, the dorsal longitudinal flight muscles (DLMs, blue) were cut without damaging the ventral nerve cord (VNC, deep red), which lies ventral to the flight muscles. The gut (green) lies above the VNC.
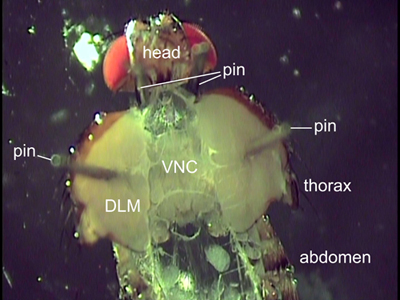
Figure 2. Dorsal view of a dissected fly. Two pins are placed behind the head to position the fly. The pins affixed through the flight muscles (DLM) hold the thorax open. Internal organs were removed to expose the ventral nerve cord (VNC).
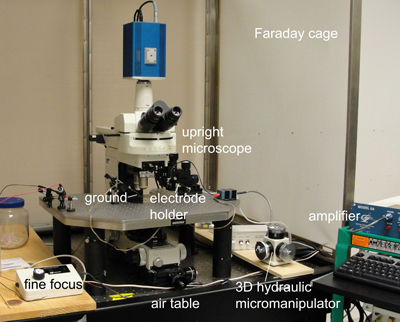
Figure 3. Dye fill setup.
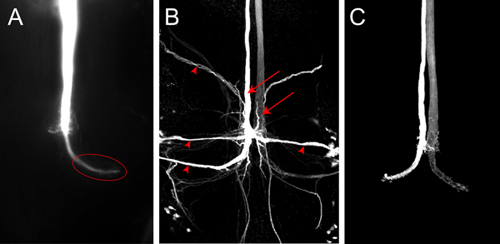
Figure 4. A) Z-projection of the posterior part of the VNC showing one GF filled with Lucifer Yellow. The image was acquired immediately after dye filling with a spot camera. In wild-type animals the terminal (ellipse) in the thorax is clearly visible. B) Confocal image of a GF dye fill with Neurobiotin in a wild-type fly. Both GFs (arrows) and neurons postsynaptic to the GFs (arrow heads) are labeled. Neurobiotin was visualized with Streptavidin coupled to Cy3. C) Confocal image of wild-type GFs filled with Alexa555.
Dyskusje
The nervous system can be dissected without extracting it from the fly body. This has two advantages, first, the dissection causes little damage to the nervous system, and second, most nerves stay attached to the muscles and sensory organs. Performing the dissection, as described, prepares the sample for straight forward dye-labeling of the GFs. Conveniently, the motoneurons postsynaptic to the GFs stay attached to the muscles. Axons, therefore, are undamaged, keeping the neurons alive longer. In addition, damage to the ...
Ujawnienia
No conflicts of interest declared.
Podziękowania
The project described was supported by Grant Number R01HD050725 from the National Institute of Child Health and Human Development to T.A.G. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health. We thank members of the Godenschwege lab as well as Barbara Schreader for their input to the manuscript and video.
Materiały
| Name | Company | Catalog Number | Comments |
| Sylgard 184 Silicone Elastomer Kit | Dow Corning | ||
| Dissection Microscope | AmScope | SM-2TZ | |
| Vannas Scissors Superfine | Academic Instruments | VS1023 | |
| Dumont Forceps Dumontstar 55 | Fine Science Tools | 11295-51 | |
| Austerlitz Insect pins | Fine Science Tools | 26002-10 | 0.1mm |
| Borosilicate Glass Electrodes | World Precision Instruments, Inc. | 1B100F-4 | |
| Vertical Pipette Puller 700c | David Kopf Instruments | Model 700C | |
| Lucifer Yellow CH dilithium salt | Sigma-Aldrich | L0259 | 1% in H2O |
| Fixed Stage Upright Microscope & Camera | Nikon Instruments | FN-1 & DS-U2 Camera | |
| Plan Fluor 10X Air Objective | Nikon Instruments | CFI Plan Fluor 10X NA 0.3 WD 16mm | |
| Fluor 40X water dipping Objective | Nikon Instruments | CFI Fluor 40X W NA 0.80 WD 2.0mm | |
| 3D hydraulic Micromanipulator | Narishige International | Model MW0-3 | |
| Amplifier | Getting Instruments, Inc. | Model 5A |
Odniesienia
- Power, M. E. The thoracico-abdominal nervous system of an adult insect Drosophila melanogaster. J. Comp. Neurol. 88, 347-409 (1948).
- Allen, M. J., Godenschwege, T. A., Tanouye, T. A., Phelan, P. Making an escape: development and function of the Drosophila giant fibre system. Semin. Cell Dev. Biol. 17, 31-41 (2006).
- Boerner, J., Duch, C. Average shape standard atlas for the adult Drosophila ventral nerve cord. J. Comp. Neurol. 518, 2437-2455 (2010).
- Gu, H., O'Dowd, D. K. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in sity. J. Neurosci. 26, 265-272 (2006).
- Boerner, J., Godenschwege, T. A. Application for the Drosophila ventral nerve cord standard in neuronal circuit reconstruction and in-depth analysis of mutant morphology. J. Neurogent. 24, 158-1567 (2010).
- Godenschwege, T. A., Kristiansen, L. V., Uthaman, S. B., Hortsch, M., Murphey, R. K. A conderved role for Drosophila Neuroglian and human L1-CAM in central-synapse formation. Curr. Biol. 16, 12-23 (2006).
- Uthaman, S. B., Godenschwege, T. A., Murphey, R. K. A mechanism distinct from highwire for the Drosophila ubiquitin conjugase bendless in synaptic growth and maturation. J. Neurosci. 28, 8615-8623 (2008).
- Storkebaum, E. Dominant mutations in the tyrosyl-tRNA synthetase gene recapitulate in Drosophila features of human Charcot-Marie-Tooth neuropathy. Proc. Natl. Acad. Sci. USA. 106, 11782-11787 (2009).
- Allen, M. J., Godenschwege, T. A. Electrophysiological recordings from the Drosophila giant fiber system (GFS). Cold Spring Harb. Protoc. 2010, pdb.prot5453-pdb.prot5453 (2010).
- Augustin, H., Allen, M. J., Partridge, L. Electrophysiological Recordings from the Giant Fiber Pathway of D. melanogaster. J. Vis. Exp. , (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone