Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Live-cell Imaging of Migrating Cells Expressing Fluorescently-tagged Proteins in a Three-dimensional Matrix
W tym Artykule
Podsumowanie
Cellular processes such as cell migration have traditionally been studied on two-dimensional, stiff plastic surfaces. This report describes a technique for directly visualizing protein localization and analyzing protein dynamics in cells migrating in a more physiologically relevant, three-dimensional matrix.
Streszczenie
Traditionally, cell migration has been studied on two-dimensional, stiff plastic surfaces. However, during important biological processes such as wound healing, tissue regeneration, and cancer metastasis, cells must navigate through complex, three-dimensional extracellular tissue. To better understand the mechanisms behind these biological processes, it is important to examine the roles of the proteins responsible for driving cell migration. Here, we outline a protocol to study the mechanisms of cell migration using the epithelial cell line (MDCK), and a three-dimensional, fibrous, self-polymerizing matrix as a model system. This optically clear extracellular matrix is easily amenable to live-cell imaging studies and better mimics the physiological, soft tissue environment. This report demonstrates a technique for directly visualizing protein localization and dynamics, and deformation of the surrounding three-dimensional matrix.
Examination of protein localization and dynamics during cellular processes provides key insight into protein functions. Genetically encoded fluorescent tags provide a unique method for observing protein localization and dynamics. Using this technique, we can analyze the subcellular accumulation of key, force-generating cytoskeletal components in real-time as the cell maneuvers through the matrix. In addition, using multiple fluorescent tags with different wavelengths, we can examine the localization of multiple proteins simultaneously, thus allowing us to test, for example, whether different proteins have similar or divergent roles. Furthermore, the dynamics of fluorescently tagged proteins can be quantified using Fluorescent Recovery After Photobleaching (FRAP) analysis. This measurement assays the protein mobility and how stably bound the proteins are to the cytoskeletal network.
By combining live-cell imaging with the treatment of protein function inhibitors, we can examine in real-time the changes in the distribution of proteins and morphology of migrating cells. Furthermore, we also combine live-cell imaging with the use of fluorescent tracer particles embedded within the matrix to visualize the matrix deformation during cell migration. Thus, we can visualize how a migrating cell distributes force-generating proteins, and where the traction forces are exerted to the surrounding matrix. Through these techniques, we can gain valuable insight into the roles of specific proteins and their contributions to the mechanisms of cell migration.
Protokół
1. Generation of stable cell line (e.g. MDCK cells)
- Plate cells at 80-90% confluency in a p35 dish. Do not let cells form 100% confluent monolayer, which will reduce transfection efficiency.
- Transfect the cells with the plasmid of interest using Lipofectamine 2000. Optimize transfection conditions using manufacturer's protocol.
- Next day, passage the cells into two p150 petri dishes. The large dish is recommended to allow enough spacing between the stable colonies.
- Next day, change the media and add 500 μg/ml of G418 to each dish. The G418 concentration should be optimized for individual cell lines.
- Change media every other day for approximately 2 weeks. After 2 weeks, G418-resistant colonies should start to form, and will be visible by the naked eye.
- Using an inverted fluorescent microscope, identify GFP positive colonies. Mark these colonies on the plate using a sharpie pen.
- To selectively trypsinize the colonies from the tissue culture plate, aspirate out the media, and wash the cells twice with PBS or trypsin solution. On the second wash, do not aspirate out all the solution. Leave a thin layer of liquid on the bottom of the plate to prevent cells from drying.
- For each marked colony: use a sterilized cotton swab to wipe as close as possible around the edge of the colony. This will create an island of wet area containing the cell colony. Pipette 10 μl of trypsin onto the colony. Repeat for every colony, and proceed quickly to avoid drying. An experienced researcher can usually pick ~12 colonies per p150 plate.
- Incubate plate at 37 °C for 5-10 minutes until cells detach from the plate and appear round.
- For each colony: Pipette 10 μl of trypsin onto the colony, and pipette up and down a couple times to detach cells from the plate. Then pipette all the cells from the colony into a single well in a 24 well dish.
- After stable colonies have grown, protein expression of each colony is analyzed using standard western blot and immunofluorescence. Expand these cell lines for further analysis.
2. Surface modification of glass bottom dish for optimal collagen binding (Optional)
- To silanize the glass, pipette 300 μl of 2% 3-Aminopropyltrimethoxysilane solution onto the glass portion of each p35 dish with a 10 mm opening. See Figure 1 for the silanization and cross-linking schematic. 3-Aminopropyltrimethoxysilane is diluted in filtered water.
- Incubate for 1 hour at room temperature.
- Aspirate out the 3-Aminopropyltrimethoxysilane solution and wash with filtered water three times for 10 minutes each.
- Aspirate out the water and place dishes on hot plate set to 50 °C for 1.5 hours. Place tops of dishes slightly off the dish so that moisture in the dish can escape.
- Remove dishes from heat and allow to cool.
- Pipette 300 μl of 2% glutaraldehyde solution onto the glass portion of each dish. The glutaraldehyde is diluted in PBS.
- Incubate for 1 hour.
- Aspirate out the glutaraldehyde solution and wash dishes with PBS three times for 10 minutes each.
- Sterilize dishes by exposure to UV light for 1 hour. The silanized dishes may be stored at room temperature.
3. Preparation of 3D collagen gel with tracer particles
- Wash fluorescent tracer particles by spinning down 100 μl of stock tracer particles (1010 particles/ml) in a centrifuge at 15,000 rpm for 5 minutes, aspirate out liquid, and add 500 μl of media. Repeat 5 times. After last wash, resuspend particles in 30 μl of media.
- Trypsinize GFP expressing cells as usual.
- Resuspend cell pellet to approximately 2 x 106 cells/ml.
- Pipette 240 μl of growth media into an eppendorf tube.
- Add 12.6 μl of 1M Hepes, 20 μl of filtered water, 50 μl of cell solution, then 10 μl of fluorescent particles.
- Lastly, add 167 μl of bovine dermis Collagen I solution (to obtain a working concentration of 1 mg/ml).
- Mix solution thoroughly, then pipette 80 μl of the solution onto the glass portion of the silanized glass bottom dish.
- Place dish into incubator and allow the gel to polymerize at 37 °C for 30 minutes. See Figure 1A for typical collagen gel in a p35 glass bottom dish.
- Add 2 ml of growth media carefully to each dish.
4. Procedure for time-lapse image acquisition
- Turn on the temperature control of the microscope enclosure and let the scope chamber equilibrate to a steady state temperature to 37 °C. See Figure 2 for an example of live-cell imaging system.
- Change media to the new media supplemented with 25 mM Hepes to maintain a neutral pH. For DIC imaging, exchange the top of the dish with one that has a glass top. Using a thin strip of parafilm, cover the side of the dish to prevent media evaporation.
- For an oil immersion objective, place one drop of immersion fluid on the objective.
- Place the collagen gel containing dish on microscope stage, and make sure the dish makes contact with the immersion liquid.
- Focus the sample and search for cells of interest to image. To minimize stage drift, allow dish to settle for about 45 minutes before starting a long capture.
- Specify the parameters of image acquisition. Minimize laser exposure, which could damage the cells, by modulating the laser power, exposure time, frequency and duration of capture time. The actual parameters will vary with the microscope system and cell type.
- During the time-lapse acquisition, an inhibitor may be added. For the inhibitor addition experiment, do not seal the dish with parafilm.
- Prepare Hepes supplemented media with drug at a desired working concentration.
- When ready to add drug to media, pause the image capture, and carefully remove the dish top without disturbing the dish.
- Aspirate out the media in the dish, and pipette the drug containing media into the dish. Then, carefully replace the dish top.
- The sample may be out-of-focus due to the addition of new media, readjust the focus.
- Restart the capture, then monitor the capture for the next 30 minutes, and readjust the focus as necessary. See Figure 4 for an example of drug addition experiment.
5. FRAP procedure and analysis
- The FRAP setup varies between systems, follow manufacturer's instruction.
- Set up parameters for live-cell imaging.
- Set up parameters for photobleaching. Use sufficient laser power to photobleach the fluorescent signal without damaging the cell. Test these parameters on practice cells.
- Start image capture, and allow at least 5 frames of image capture before photobleaching the region of interest.
- Continue capture, and recovery time should be sufficient to capture the full fluorescence recovery. See Figure 5 for an example of FRAP experiment.
- Analyze the fluorescence recovery by measuring the average fluorescent intensity of the photobleached area (before and after photobleaching) over time. Using a statistical analysis software (e.g. Excel), fit the recovery curve to the exponential equation: I=If(1-e-kt), where I is the intensity, If is the final intensity, t is time, and τ½ is the time it takes for intensity to reach half the final value: τ½=ln(2)/k. The half-time is a measure of the rate of mobility of the protein.
- Obtain the parameters, half-time and final intensity from the exponential-fit curve. Calculate the mobile fraction by taking the ratio of the final to initial fluorescence intensities.
6. Representative Results
An example of the live-cell imaging of healthy epithelial cells within the matrix is shown in Figure 3. Healthy cells exhibit a smooth, continuous membrane, and distinct nucleus, whereas unhealthy cells often have a disrupted membrane, and an excessive number of vacuoles. In a 3D matrix, single epithelial cells migrate1, and over the course of several days, epithelial cells form three-dimensional, spherical, multi-cellular cysts within the matrix2. The cells are also highly dynamic, and migrate within the cyst (Figure 3). The matrix deformation as a result of the traction forces exerted by migrating cells is analyzed through the displacement of tracer particles embedded in the surrounding matrix (Figure 4). The tracer particle movements are displayed as a maximum projection image of different time points, and each time point is pseudo-colored to indicate the time (Figure 4B). Alternatively, the images of an individual tracer particle are displayed as a montage to show the movement of the tracer particle over time (Figure 4C). All image analysis was done using ImageJ. These qualitative assessments of matrix deformation, and therefore the forces exerted by migrating cells, are useful first approximation of traction force distribution. The quantitative estimation of traction forces exerted by migrating cells in 3D is beyond the scope of this protocol and is described elsewhere3,4.
A typical fluorescence recovery after photobleaching experiment is shown in Figure 5. The region of interest must be photobleached using optimized laser settings so that the fluorescence intensity is visibly diminished compared to background levels (to maximize signal-to-noise ratio), while maintaining healthy and undamaged cells. The optimal setting must be determined empirically as each FRAP setup is different (e.g., FRAP using laser-scanning confocal system).
The exposure and time-interval of post-FRAP image acquisition must be carefully controlled to avoid background photobleaching. The use of lower laser power, short exposure time, and the use of sensitive camera and high efficient optics are essential for high quality FRAP imaging. If the fluorescence bleaching during the image acquisition is significant, this background fading must be quantified and normalized from the observed fluorescence intensity recovery. Due to 3D nature of environment, the z-component of fluorescence intensity recovery may become significant. Therefore, it is important to define a photobleached volume by taking pre and post 3D-stack images of photobleached region. Further analysis and modeling of fluorescence recovery is discussed elsewhere5,6.
The unique combination of live-cell imaging techniques: GFP to follow the dynamics of cytoskeletal proteins, red fluorescence tracer particles to monitor the matrix deformation, and the addition of inhibitors, can be used simultaneously for the analysis of protein dynamics, traction force and molecular pathways.
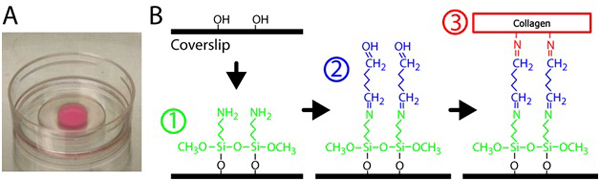
Figure 1. Preparation of collagen gel. A) Polymerized collagen matrix on a glass-bottom dish. The pink color of the gel is due to embedded fluorescent particles. B) Procedure to treat the glass bottom dishes to crosslink the collagen gel to the glass surface. First, the glass bottom dish is treated with 3-Aminopropyltrimethoxysilane solution, then glutaraldehyde solution that crosslinks the collagen matrix to the glass.
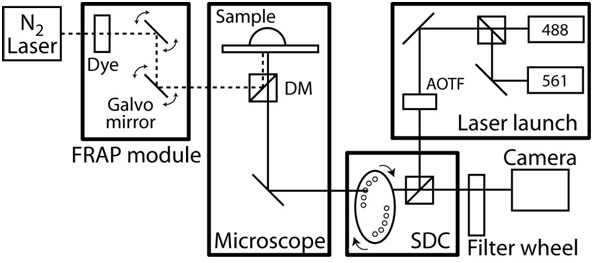
Figure 2. Schematics of confocal/FRAP microscope setup. The confocal microscope is based on a Zeiss AxioObserver with a CoolSnap HQ II CCD camera and completely automated by Slidebook software (Intelligent Imaging Innovations). The confocal unit is custom designed and based on Yokogawa spinning disk unit CSU10 and two solid-state lasers (488 nm with 50 mW and 561 nm with 40 mW) with Acousto-Optical Tunable Filter (AOTF) to allow milliseconds switching between two lasers. The emission filters are 525/50 nm and 620/60 nm (#118661 and #118085, Chroma Technology) and dichroic mirror is 488-568 BrightLine Dual band (Semrock). The objectives are a long working distance 40x C-Apochromat objective with NA 1.1 and working distance of 0.62 mm, and a 63x Plan-Apochromat objective with NA 1.4 and a working distance of 0.19 mm. The microscope also includes a FRAP photoablation system that consists of a fiber optically pumped dye laser, a computer controlled beam position and intensity, and a diffraction limited spot size. Furthermore, the microscope is equipped with an x-y motorized stage that includes 0.1 micron linear encoders on each axis. During the time-lapse imaging, the environmental temperature is maintained by a custom designed microscope chamber and a heater with a feedback temperature control. To isolate any noise and vibration, the entire microscope system is on a vibration-free table.
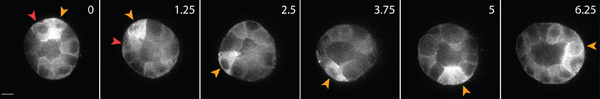
Figure 3. Live cell imaging of epithelial cells expressing GFP-actin. These cells formed a cyst after 4 days of culture in a 3D collagen matrix. Some cells move along the surface of cyst (yellow arrowhead), while others migrate within the interior of cyst (red arrowhead). Scale bar 10 μm, time in hours.
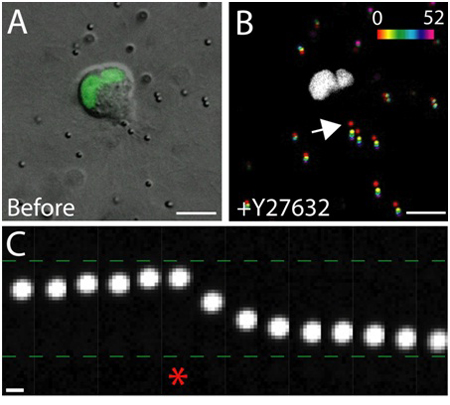
Figure 4. The effect of Rho-kinase inhibition on traction force. A) DIC image of a migrating MDCK cell expressing GFP tagged nuclear marker. The image was taken immediately before the treatment of Rho-kinase inhibitor Y-27632. Scale bar 10 μm. B) Particle displacement resulting from the addition of Y-27632. The particle positions at various time points (0 - 52 min) were pseudo-colored according to the intensity scale, then projected onto a single image. The white region is the GFP positive nucleus in the migrating cells. Scale bar 10 μm. Time in minutes. C) The particle movement at the trailing edge of the cell (see arrow in B). Asterisk denotes the last frame captured before Y-27632 addition. The tracer particle moved toward and away from the trailing edge of the migrating cell before and after the addition of Y-27632, respectively. Scale bar 1 μm.
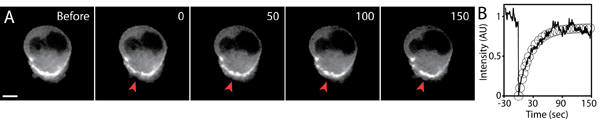
Figure 5. FRAP analysis of GFP-actin expressing cell migrating in a three-dimensional matrix. A) Timelapse images of the GFP-actin expressing cell of before and after the photobleaching. A small region of the GFP-actin accumulated at the cell rear was photobleached (red arrowhead) at timepoint 0. Time in seconds, scale bar 5 μm. B) The average fluorescence intensity of the photobleached region (solid line), and the exponential fit of the fluorescence recovery (circles) plotted over time. The fluorescence intensity was normalized to the initial value before photobleaching.
Dyskusje
Here we describe a method for using live-cell imaging to study the mechanisms of cell migration in a three-dimensional matrix. The success of this technique depends on obtaining "good" clones stably expressing GFP-tagged proteins. The low level of GFP proteins will require an excess excitation exposure that compromises cell health, while too high GFP level will have undesirable side effects on the cell. Thus, the vector choice to transfect genes into cells is important (e.g., the promoter, fluorescence tag, etc). There a...
Ujawnienia
No conflicts of interest declared.
Podziękowania
We thank Dr. Grant Sumida for critical reading of the manuscript. This work was supported by a Beckman Young Investigator Award (SY), a Hellman Family New Faculty Award (SY), a NIH EUREKA, the University of California Cancer Research Coordinating Committee.
Materiały
| Name | Company | Catalog Number | Comments |
| Collagen, bovine, Type I | BD Biosciences | 354231 | Stock is about 3 mg/ml |
| 3-aminopropyltrimethoxysilane | Sigma-Aldrich | 281778 | Dilute in water |
| glutaraldehyde | Sigma-Aldrich | 340855 | Dilute in PBS |
| 1M Hepes | Invitrogen | 15630-080 | |
| Fluospheres polystyrene microspheres 1 μm, red fluorescence (580/605) | Invitrogen | F13083 | |
| Geneticin (G418) | Invitrogen | 11811-031 | |
| DMEM | Invitrogen | 31600-034 | |
| Fetal Bovine Serum | Atlanta Biologicals | S115500 | |
| Penicillin/Streptomycin | Invitrogen | 15140-122 | |
| Kanamycin | Invitrogen | 15160-054 |
Odniesienia
- Shih, W., Yamada, S., Myosin, . A dependent retrograde flow drives 3D cell migration. Biophys. J. 98 (8), L29-L31 (2010).
- O'Brien, L. E., Yu, W., Tang, K., Jou, T. S., Zegers, M. M., Mostov, K. E. Morphological and biochemical analysis of Rac1 in three-dimensional epithelial cell cultures. Methods Enzymol. 406, 676-691 (2006).
- Legant, W. R., Miller, J. S., Blakely, B. L., Cohen, D. M., Genin, G. M., Chen, C. S. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat. Methods. 7 (12), 969-971 (2010).
- Del Alamo, J. C., Meili, R., Alonso-Latorre, B., Rodriguez-Rodriguez, J., Aliseda, A., Firtel, R. A., Lasheras, J. C. Spatio-temporal analysis of eurkaryotic cell motility by improved force cytometry. Proc. Natl. Acad. Sci. U.S.A. 104 (33), 13343-13348 (2007).
- Lippincott-Schwartz, J., Snapp, E., Kenworthy, A. Studying protein dynamics in living cells. Nat. Rev. Mol. Cell Biol. 2 (6), 444-456 (2001).
- Phair, R. D., Misteli, T. Kinetic modeling approaches to in vivo imaging. Nat. Rev. Mol. Cell Biol. 2 (12), 898-907 (2001).
- Giepmans, B. N., Adams, S. R., Ellisman, M. H., Tsien, R. Y. Fluorescent toolbox for assessing protein location and function. Science. 312 (5771), 217-224 (2006).
- Shaner, N. C., Steinbach, P. A., Tsien, R. Y. A guide to choosing fluorescent proteins. Nat. Methods. 2, 905-909 (2005).
- Cukierman, E., Pankov, R., Stevens, D. R., Yamada, K. M. Taking cell-matrix adhesions to the third dimension. Science. 294 (5547), 1708-1712 (2001).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone