Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Quantifying Single Microvessel Permeability in Isolated Blood-perfused Rat Lung Preparation
W tym Artykule
Podsumowanie
The isolated blood-perfused lung preparation makes it feasible to visualize microvessel networks on the lung surface. Here we describe an approach to quantify permeability of single microvessels in isolated lungs using real time fluorescence imaging.
Streszczenie
The isolated blood-perfused lung preparation is widely used to visualize and define signaling in single microvessels. By coupling this preparation with real time imaging, it becomes feasible to determine permeability changes in individual pulmonary microvessels. Herein we describe steps to isolate rat lungs and perfuse them with autologous blood. Then, we outline steps to infuse fluorophores or agents via a microcatheter into a small lung region. Using these procedures described, we determined permeability increases in rat lung microvessels in response to infusions of bacterial lipopolysaccharide. The data revealed that lipopolysaccharide increased fluid leak across both venular and capillary microvessel segments. Thus, this method makes it possible to compare permeability responses among vascular segments and thus, define any heterogeneity in the response. While commonly used methods to define lung permeability require postprocessing of lung tissue samples, the use of real time imaging obviates this requirement as evident from the present method. Thus, the isolated lung preparation combined with real time imaging offers several advantages over traditional methods to determine lung microvascular permeability, yet is a straightforward method to develop and implement.
Wprowadzenie
Increased microvascular permeability in lungs leads to development of alveolar edema and compromised gas exchange and is a major characteristic of acute lung injury (ALI)1-3. Thus, estimates of vascular permeability are important in defining the extent of lung injury and efficacy of proposed therapeutic interventions. Gravimetric analysis such as blood free lung wet-to-dry ratio and microvascular filtration coefficient are widely used methods to estimate permeability4,5. Other methods include quantifying the retention of radioactive or fluorescent probes in lung tissue6-8. However, the above methods require postexperiment processing of lung tissue samples toward elucidating the permeability data. Moreover, since one animal can be only used for a single treatment protocol, large animal numbers may be needed for a complete study. A common characteristic of the above methods is that they determine the mean vascular permeability for all blood vessels within the tissue sample. However, it is well established that pulmonary micro- and macro-vessels are phenotypically different9. Hence, permeability responses may be heterogeneous among the various vessel segments as well9,10. Thus, quantifying mean permeability of all pulmonary vessels in a tissue sample may not adequately reflect this heterogeneity.
In the isolated blood-perfused lung preparation, blood vessels on the lung surface can be visualized by an upright microscope4,11,12. This enables characterizing responses in single vessels and thus, addressing any heterogeneity in the responses13. In addition, by utilizing fluorescence imaging of microvessels, fluorescence based assays can be incorporated. Further, a left atrial microcatheter can be used to deliver agents and fluorescence probes into blood vessels11,14. The microcatheter limits the delivery to a small lung region, thus exposing only the blood vessels within the region to the infused agents and fluorophores. This allows multiple small regions within the same lung to be used for separate experiments, leading to an overall reduction in animals needed for a study.
Real time imaging enables capture of dynamic changes in vascular and extravascular fluorescence of single microvessels of the isolated lung preparation. Thus, for each microvessel within an image field, changes in fluorescence during infusion of fluorophores and washoff can be recorded, and quantified offline14. Using values of maximum and residual vascular fluorescence, a permeability index for each microvessel within the imaging field can be determined. To determine permeability changes in response to inflammatory or injurious agents, the desired agent can be administered first and then the permeability index determined. In addition, the image field can be set anywhere within the lung region infused by the microcatheter, thus enabling a high degree of flexibility in selecting the desired vascular network. Thus, the isolated blood-perfused lung preparation in tandem with real time imaging provides an attractive experimental model to quantify permeability in single lung microvessels.
Protokół
All experiments performed on animals were as approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center.
1. Tubing for Perfusing Rat Lung Preparations
- Prepare a tubing system with Tygon tubing (#18) for blood perfusion as shown in Figure 1. Connect pressure transducers (P23XL) and place the tubing on the microscope stage.
- Set water bath temperature to 37 °C.
- Connect the lung inlet and outlet temporarily with Tygon tubing.
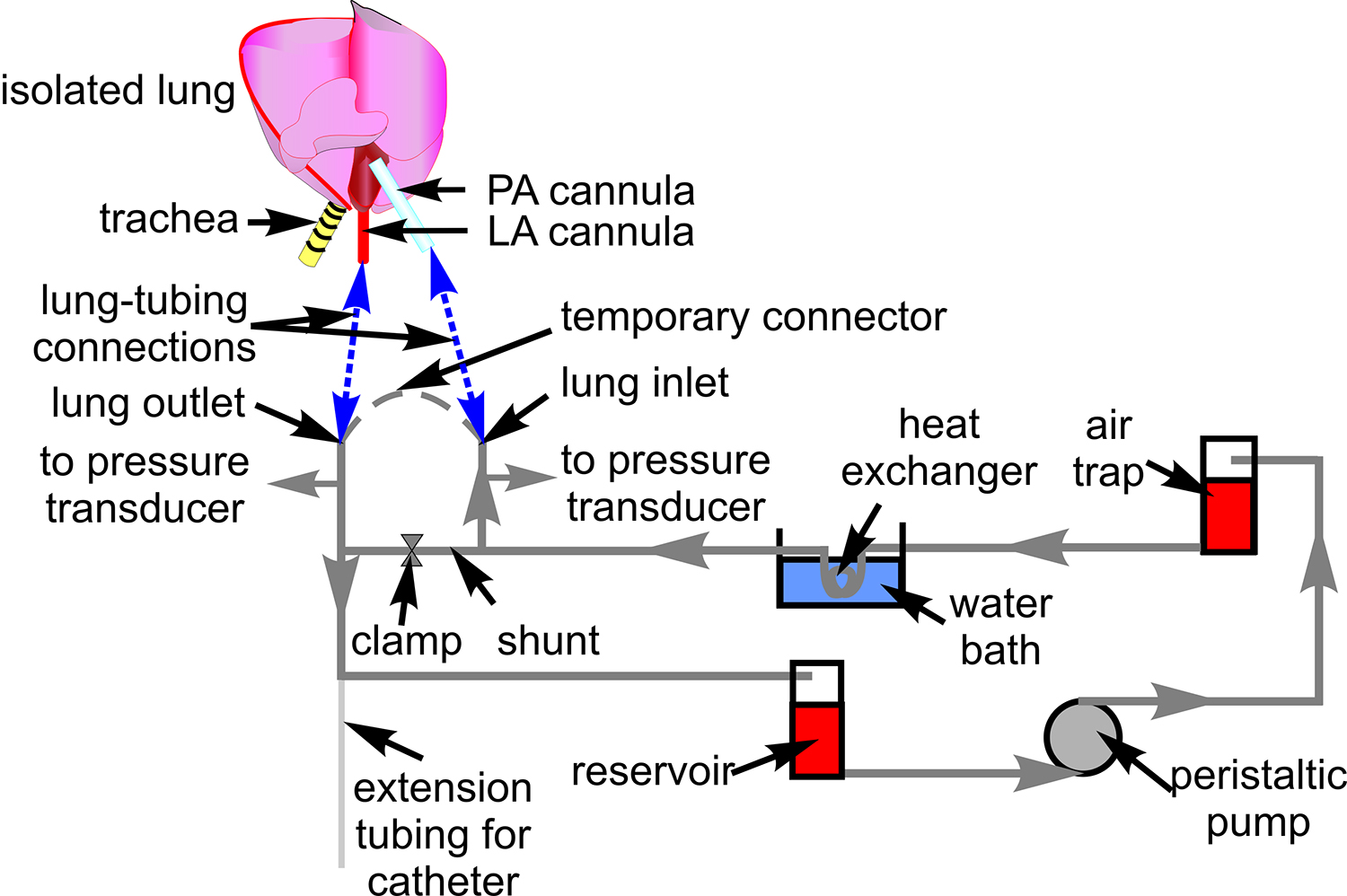
Figure 1. Blood perfusion tubing. The schematic shows the tubing setup used for circulating blood through the isolated lung preparation. Also indicated are associated components through which the tubing is routed. A schematic of the heart and lung is included to show sites of connection between the tubing and pulmonary artery (PA), and left atrial (LA) cannula (blue dashed lines).
2. Preparation of Isolated Rat Lungs
- Anesthetize rats (male Sprague-Dawley Rats; 250-300 g) with ketamine (80-100 mg/kg) and with xylazine (5-10 mg/kg). After ensuring a surgical plane of anesthesia, place the animal in a supine position.
- Perform tracheostomy, insert a tracheal cannula (PE-90) and secure with surgical suture.
- Infuse heparin (100-200 U) into the heart by cardiac puncture (21 G butterfly needle), wait for 60 sec and exsanguinate blood (~12-15 ml blood).
- Prepare two cannulas (Tygon tubing, 3 mm diameter; 4 cm long; flared at one end) and fill with saline.
- Perform a thoracotomy. Make an incision (3 mm) on the right ventricle and slide the flared end of a cannula into the incision toward the pulmonary artery. Secure cannula to pulmonary artery with surgical sutures.
- Incise (3 mm) the apex of the left ventricle and slide the flared end of the second cannula into the incision. Guide the cannula into the left atrium. Secure cannula to the left ventricle with an umbilical tape (2 mm width).
- Dissect any connective tissue, and remove the lung and heart together with the attached cannulas. Place lung and heart on a petri dish. Reposition the lung so that the diaphragmatic surface is on top. The three cannulas should face the same direction.
- Place the lung preparation on a stage that can be manipulated in the X-Y directions. Any stage that comes with a microscope can be modified to hold the tubing or a custom-made stage built, if needed.
3. Blood Perfusion of Isolated Rat Lungs
- Mix the exsanguinated blood with an equal volume albumin (5%) solution and add to the reservoir. Start the peristaltic pump and set flow rate to 14 ml/min. Allow blood to fill the tubing.
- With the shunt open, remove the temporary lung inlet-outlet connector. Attach the pulmonary artery and left atrial cannula to the lung inlet and outlet, respectively. Ensure that there are no air bubbles in the tubing.
- Connect the tracheal cannula to a third pressure transducer (P23XL). Inflate lungs with 30% oxygen via the tracheal cannula and maintain pressure at 5 cm H2O.
- Close the shunt with a clamp to begin lung perfusion. Maintain pulmonary artery and left atrial pressures at ~10 and 3 cm H2O respectively.
4. Preparing Lung for Microvascular Infusion
- Raise the rear end of the extension tubing for the catheter above the level of the lung and secure.
- Prepare an infusion catheter by inserting about 30 cm of a 40 cm long PE10 tubing into a ~30 cm long PE90 tubing. Connect the exposed end of the PE10 tubing to a needle (30 G) attached to a syringe (1 cc).
- Insert the catheter combination into the raised rear end of the extension tubing. Guide the catheter combination through the left atrium and into the lung until it meets resistance. Then, push the PE10 catheter alone further into the lung until it meets resistance. Note that applying excessive force while inserting the catheter will injure the lung.
- Fill the 1 cc syringe with Ringer’s solution and attach to a syringe pump. Set infusion rate to 10 µl/min.
- Infusion site will become pale compared to rest of the lung.
- Moisten the lung with saline and cover the lung with plastic wrap with a hole large enough to leave the infusion site uncovered.
- Swab some stopcock grease on the bottom of an O-ring (custom-made) and place a glass coverslip (#1.5, 22 mm diameter) on the bottom of the O-ring. Gently position the O-ring with the coverslip on the infusion site. Secure the O-ring with a standard test-tube holder. The coverslip/O-ring combination does not distort the alveolar structures underneath it, as recently reported15.
- Position an objective (20X) of a fluorescence microscope above the O-ring and focus on the lung surface.
5. Imaging Fluorescent Dextran Transit Through Microvessels
- Prepare the microscope for imaging FITC florescence.
- Select a region to be imaged and acquire images at the rate of 1/minute using image acquisition software.
- Begin infusion of FITC-dextran 20 kD (0.5 mg/ml) using the syringe pump. Fluorescence in blood vessels will gradually increase and reach a maximum. Continue infusion for 60 min.
- Then switch to Ringer’s infusion to wash off luminal fluorescence. Maintain infusion for over 10 min. Continue image acquisition at 1/min during wash-off.
6. Image Analysis
- Open the image acquisition file.
- Place regions of interest over microvessels within the image frame.
- Play the image file frame-by-frame and record the fluorescence intensity at each region of interest for all frames.
- Plot the change in fluorescence intensity for each region of interest as a function of time.
- Quantify the maximum fluorescence intensity and the residual fluorescence intensity after 10 min of washoff.
- Calculate the ratio of maximum to residual fluorescence intensity at each region of interest to get the permeability index for microvessels associated with that region of interest.
Wyniki
An isolated blood-perfused lung preparation connected to the perfusion tubing and related equipment is shown in Figure 2. For demonstration purposes, we used a Sprague Dawley rat, though the procedures described herein can be used with any rat species. Infusions through a left atrial microcatheter reach only a small region of the lung. The infused region can be identified by the infusion-induced discoloration (Figure 3). The lung preparation as positioned for real-time imaging is shown i...
Dyskusje
The isolated blood-perfused lung preparation coupled with real time imaging provides a simple tool for determination of permeability changes in single lung microvessels. We applied this method to define permeability changes in response to infusions of LPS. Our data clearly suggest that LPS infusion caused an increase in microvascular permeability. Further, the data also indicate that permeability changes induced by LPS were similar in both venules and capillaries. Thus, a major advantage of this technique is the ability ...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The studies were supported by NIH HL75503 to KP.
Materiały
| Name | Company | Catalog Number | Comments |
| Tygon Tubing | Fisher Scientific | #18 | |
| Pressure Transducer | Data Sciences International | P23XL | Need quantity 3 |
| Butterfly Needle | Greiner Bio-One | 450081 | 21 G |
| Peristaltic pump | Cole Parmer | Masterflex L/S | |
| PE-90 tubing | Becton Dickinson | 427421 | 30 cm needed |
| PE-10 tubing | Becton Dickinson | 427401 | 40 cm needed |
| Syringe Pump | Braintree Scientific | BS8000 | |
| O-ring | Custom made with a 20 mm diamter hole and a handle to secure O-ring to holder | ||
| Upright fluorescence microscope | Olympus America | BX61WI | |
| Image Acquisition Software | Molecular Devices | Metamorph | |
| FITC Dextran 20KD | Sigma Aldrich | 0.5 mg/ml (A dextran of different molecular size can be selected, if trial experiments indicate its suitability based on the calculated permeability index values) | |
| Lipopolysaccharide | Sigma Aldrich | Serotype 0111:B4 |
Odniesienia
- Ware, L. B., Matthay, M. A. The acute respiratory distress syndrome. N Engl J Med. 342, 1334-1349 (2000).
- Matthay, M. A., et al. The acute respiratory distress syndrome. J Clin Invest. 122, 2731-2740 (2012).
- Bhattacharya, J., Matthay, M. A. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 75, 593-615 (2013).
- Parthasarathi, K., et al. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest. 116, 2193-2200 (2006).
- Parthasarathi, K., Bhattacharya, J. Localized Acid instillation by a wedged-catheter method reveals a role for vascular gap junctions in spatial expansion of Acid injury. Anat Rec (Hoboken). 294, 1585-1591 (2011).
- Gorin, A. B., Stewart, P. A. Differential permeability of endothelial and epithelial barriers to albumin flux. J Appl Physiol Respir Environ Exerc Physiol. 47, 1315-1324 (1979).
- Boutoille, D., et al. FITC-albumin as a marker for assessment of endothelial permeability in mice: comparison with 125I-albumin. Exp Lung Res. 35, 263-271 (2009).
- Thorball, N. FITC-dextran tracers in microcirculatory and permeability studies using combined fluorescence stereo microscopy, fluorescence light microscopy and electron microscopy. Histochemistry. 71, 209-233 (1981).
- Stevens, T. Functional and molecular heterogeneity of pulmonary endothelial cells. Proc Am Thorac Soc. 8, 453-457 (2011).
- Ofori-Acquah, S. F., et al. Heterogeneity of barrier function in the lung reflects diversity in endothelial cell junctions. Microvasc Res. 75, 391-402 (2008).
- Kandasamy, K., et al. Real-time imaging reveals endothelium-mediated leukocyte retention in LPS-treated lung microvessels. Microvasc Res. 83, 323-331 (2012).
- Kandasamy, K., et al. Lipopolysaccharide induces endoplasmic store Ca2+-dependent inflammatory responses in lung microvessels. PloS One. 8, (2013).
- Qiao, R. L., Bhattacharya, J. Segmental barrier properties of the pulmonary microvascular bed. J Appl Physiol. 71, 2152-2159 (1991).
- Parthasarathi, K. Endothelial connexin43 mediates acid-induced increases in pulmonary microvascular permeability. Am J Physiol Lung Cell Mol Physiol. 303, (2012).
- Wu, Y., Perlman, C. E. In situ methods for assessing alveolar mechanics. J Appl Physiol 1985. 112, 519-526 (2012).
- Kuebler, W. M., et al. A novel signaling mechanism between gas and blood compartments of the lung. Journal Clin Invest. 105, 905-913 (2000).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone