Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Mammalian Cell Encapsulation in Alginate Beads Using a Simple Stirred Vessel
W tym Artykule
Podsumowanie
This video and manuscript describe an emulsion-based method to encapsulate mammalian cells in 0.5% to 10% alginate beads which can be produced in large batches using a simple stirred vessel. The encapsulated cells can be cultured in vitro or transplanted for cellular therapy applications.
Streszczenie
Cell encapsulation in alginate beads has been used for immobilized cell culture in vitro as well as for immunoisolation in vivo. Pancreatic islet encapsulation has been studied extensively as a means to increase islet survival in allogeneic or xenogeneic transplants. Alginate encapsulation is commonly achieved by nozzle extrusion and external gelation. Using this method, cell-containing alginate droplets formed at the tip of nozzles fall into a solution containing divalent cations that cause ionotropic alginate gelation as they diffuse into the droplets. The requirement for droplet formation at the nozzle tip limits the volumetric throughput and alginate concentration that can be achieved. This video describes a scalable emulsification method to encapsulate mammalian cells in 0.5% to 10% alginate with 70% to 90% cell survival. By this alternative method, alginate droplets containing cells and calcium carbonate are emulsified in mineral oil, followed by a decrease in pH leading to internal calcium release and ionotropic alginate gelation. The current method allows the production of alginate beads within 20 min of emulsification. The equipment required for the encapsulation step consists in simple stirred vessels available to most laboratories.
Wprowadzenie
Mammalian cell encapsulation has been broadly studied as a means to protect transplanted cells from immune rejection1 or to provide a three-dimensional support for immobilized cell culture2,3,4. Pancreatic islet encapsulation in alginate beads has been used to reverse diabetes in allogeneic5,6 or xenogeneic7,8,9,10,11,12 rodents. Preclinical and clinical trials of encapsulated pancreatic islet transplantation to treat type 1 diabetes are ongoing13,14,15. For transplantation applications or larger-scale in vitro immobilized cell production, nozzle-based bead generators are generally used. Typically, a mixture of alginate and cells is pumped through a nozzle to form droplets that fall into an agitated solution containing divalent cations, resulting in the external gelation of the droplets. Coaxial gas flow16,17, nozzle vibration18, electrostatic repulsion19 or rotating wires20 facilitate droplet formation at the nozzle tip.
The main drawbacks of conventional bead generators are their limited throughput and the limited range of solution viscosities that will result in adequate bead formation21. At high flow rates, the fluid exiting the nozzle breaks up into droplets smaller than the nozzle diameter, decreasing size control. Multi-nozzle bead generators can be used to increase the throughput, but the uniform distribution of flow among the nozzles and the use of solutions >0.2 Pas is problematic22. Lastly, all of the nozzle-based devices are expected to impart some damage to islets, since the diameter of the nozzles used is between 100 µm and 500 µm, while ~15% of human islets can be larger than 200 µm23.
In this video, we describe an alternative method to encapsulate mammalian cells by forming droplets in a single emulsification step instead of drop-by-drop. Since bead production is performed in a simple stirred vessel, the method is suitable for small (~1 mL) to large-scale (103 L range) bead production with low equipment costs24. This method allows the production of beads with high sphericity using a broad range of alginate viscosities with short (e.g. 20 min) bead generation times. This method was originally developed by Poncelet et al.25,26 and used to immobilize DNA27, proteins28 including insulin29, and bacteria30. We have recently adapted these methods to the encapsulation of mammalian cells using pancreatic beta cell lines31,32 and primary pancreatic tissue32.
The principle of the method is to generate a water-in-oil emulsion consisting of alginate droplets in mineral oil, followed by internal gelation of the alginate droplets (Figure 1). First the encapsulant (e.g., cells) is dispersed in an alginate solution containing a fine grain calcium salt with low solubility at the initial process pH. The alginate mixture is then added to an agitated organic phase to create an emulsion, usually in the presence of a surfactant. In the case of mammalian cell encapsulation, components present in serum can act as surfactants. Next, the pH is reduced in order to solubilize the calcium salt by adding an oil-soluble acid that partitions into the aqueous phase. Acetic acid, with a mineral oil/water partition coefficient <0.00533, should be pre-dissolved in oil, then added to the emulsion where it is mixed in the oil phase and rapidly partitions into the aqueous phase34. Figure 2 illustrates the chemical reactions and diffusion that take place during the acidification and internal gelation step. Finally, the encapsulated cells are recovered by phase inversion, phase separation accelerated by centrifugation, repeated washing steps and filtration. These steps can then be followed by bead and cell sampling for quality control analyses, in vitro cell culture and/or transplantation of the encapsulated cells.
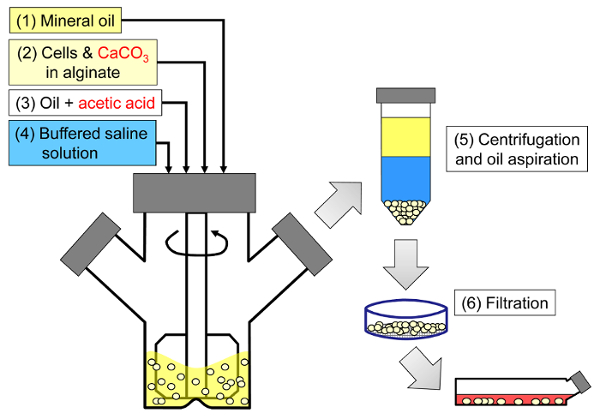
Figure 1: Schematic of the emulsification-based process to encapsulate mammalian cells. Beads are first produced by emulsifying an alginate, cell and CaCO3 mixture in mineral oil (steps 1 and 2 in the schematic), triggering internal gelation by adding acetic acid (step 3). The gelled beads are then separated from the oil by adding an aqueous buffer to trigger phase inversion (step 4), followed by centrifugation and oil aspiration (step 5), and then filtration (step 6). Finally, the beads collected on the filter are transferred into cell culture medium for in vitro culture or for transplantation. Please click here to view a larger version of this figure.
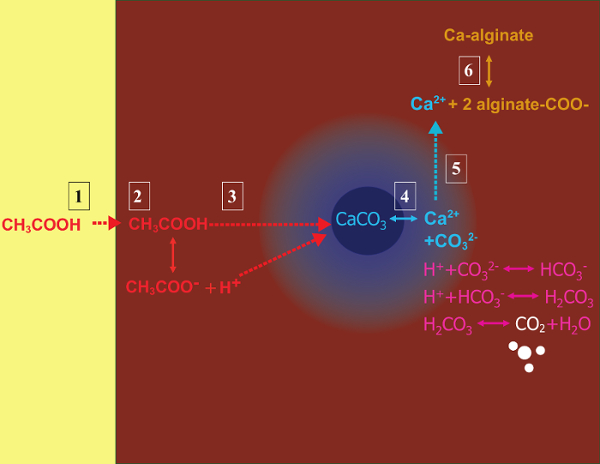
Figure 2: Reactions and diffusion steps occurring during internal gelation. (1) Acetic acid is added to the organic phase and is transported to the alginate droplets by convection. (2) The acetic acid partitions into the aqueous phase. (3) In the presence of water, the acid dissociates and diffuses to reach the CaCO3 grains depicted in dark blue. (4) The H+ ions are exchanged with the Ca2+ ions in CaCO3, releasing Ca2+ ions. (5) The calcium ions diffuse until they encounter unreacted alginate, leading to the ionotropic cross-linking of the alginate chains. Please click here to view a larger version of this figure.
Contrary to conventional nozzle-based cell encapsulators, a broad bead size distribution is expected from this process due to the mechanism of droplet formation in stirred emulsification. For a subset of applications, this bead size distribution may be problematic. For example, a larger fraction of cells may be exposed at the bead surface in smaller beads. If nutrient (e.g. oxygen) limitations are a concern, these limitations may be exacerbated in larger beads. An advantage of the stirred emulsification method is that the average bead size can readily be adjusted by changing the agitation rate during the emulsification step. The broad bead size distribution can also be exploited to study the effect of bead size on encapsulated cell performance.
Mammalian cell encapsulation by emulsification and internal gelation is an interesting alternative for laboratories that are not equipped with a bead generator. Furthermore, this method gives users the option of reducing the processing time, or generating beads at very low or very high alginate concentrations.
The protocol outlined below describes how to encapsulate cells in 10.5 mL of 5% alginate solution prepared in 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer. The alginate consists of a 50:50 mixture of transplantation-grade LVM (low viscosity high mannuronic acid content) and MVG (medium viscosity high guluronic acid content) alginate. Calcium carbonate at a final concentration of 24 mM is used as the physical cross-linking agent. Light mineral oil constitutes the organic phase, while acetic acid is used to acidify the emulsion and trigger internal gelation. However, the alginate type and composition, as well as the process buffer selected depend on the desired application32. A variety of alginate types (see Table of materials) have been used to produce beads with this protocol.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Prepare the Alginate Solution, the CaCO3 Suspension and the Acidified Oil
- Prepare the process buffer and medium.
- Prepare the typical process buffer used to generate high-concentration alginate beads using 10 mM HEPES, 170 mM NaCl. Adjust the pH to 7.4.
- Prepare the typical culture medium using Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), 6 mM glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin.
- Prepare the alginate stock solution at 1.17 fold the final desired concentration in the beads.
- First, weigh the appropriate amount of alginate powder. For example, to obtain a final concentration of 5% alginate with 50:50 LVM and MVG alginate, weigh out a total of 1.17 g sodium alginate by combining 0.583 g LVM alginate powder and 0.583 g MVG alginate powder.
- Place 20 mL process buffer into an autoclavable glass jar on a magnetic stir plate and agitate at ~200 rpm. Progressively add the sodium alginate powder to the solution.
- Leave the solution stirring overnight at a low speed. If necessary, fasten the flask to the stir plate.
- If the solution is incompletely dissolved, fasten the bottle onto a rotary mixer and continue mixing at 37 °C for an additional 24 h.
- Sterilize the alginate solution by autoclaving the solution for 30 min in a vessel that is less than half full. Allow the temperature to decrease below 60 °C before retrieving the solution.
- If necessary, remove particles by filtering the alginate solution shortly after autoclaving before it reaches room temperature, while the viscosity remains sufficiently low.
- Prepare the calcium carbonate suspension at 21-fold the final desired concentration for internal gelation.
- Weigh out the CaCO3 powder. For example, add 1 g CaCO3 to 20 mL process buffer to obtain 24 mM CaCO3 as the final gelling agent concentration.
- Autoclave the CaCO3 suspension for 30 min.
NOTE: The CaCO3 concentration will change over time due to the bicarbonate - CO2 equilibrium. Avoid using the same stock CaCO3 suspension for more than 10 encapsulation procedures.
- Autoclave the stirred vessel used for the emulsification process. Prior to use, remove any traces of condensed water remaining in the spinner flask.
- Immediately before the emulsification process, prepare the acidified oil. Dissolve 44 µL acetic acid per 11 mL mineral oil placed in a 50 mL conical tube.
NOTE: A common mistake is incomplete dissolution of the acetic acid. Avoid pipetting small amounts of acetic acid and ensure that the acetic acid is completely dissolved in oil by repeated vortexing.
CAUTION: acetic acid is a toxic and volatile acid. Handle this reagent under a fume hood and keep the acetic acid/oil solution in a closed container until the emulsification step. Refer to the MSDS information on this reagent for further information. - Allow all the solutions to reach room temperature before proceeding to cell encapsulation.
2. Alginate Bead Generation by Emulsification and Internal Gelation
- Place 10 mL light mineral oil in the spinner flask and start agitation at a low rotation speed (e.g. 250 rpm for the spinner flask used in this video).
- If the cells used for the process are from adherent cultures, apply trypsin to suspend the cells. End the reaction by adding FBS-containing medium or trypsin inhibitor and collect a sample for cell enumeration.
- Determine the cell concentration and viability after Trypan blue staining using a hemocytometer or an automated cell counter, as previously described32.
- Centrifuge the cells for 7 min at 300 x g and wash them once in the desired medium for cell immobilization. Ensure that this medium contains emulsifiers such as either FBS or bovine serum albumin (BSA). For example, use DMEM containing 10% FBS.
- Re-suspend the cell pellet in the same medium to obtain 10.5-fold the desired final concentration in the beads.
- Add 1.1 mL of the 10.5-fold concentrated cell stock to 9.9 mL of the alginate solution. Then, add 550 µL of CaCO3 suspension and mix by stirring with a sterile spatula to ensure an even distribution of the CaCO3.
- Immediately transfer 10.5 mL of the alginate, cell and CaCO3 mixture into the agitating oil using a syringe.
NOTE: For highly viscous solutions, aspirate and eject the mixture very slowly to avoid air bubbles. In some cases, it is preferable to stop the agitation while adding the mixture to the flask to avoid alginate entrainment onto the impeller shaft. - Immediately after adding the alginate, cell and CaCO3 mixture to the oil, increase the agitation rate.
NOTE: For the 5% LVM:MVG alginate used here, a rotation speed of 1025 rpm was applied to generate beads of approximately 900 µm diameter suitable for in vitro culture35. Higher rotation speeds and lower alginate concentrations will lead to lower average bead diameters, as described in previous publications25,32. Slight changes in impeller and vessel geometry, as well as alginate properties can greatly affect the average bead diameter. For each change in vessel configuration or alginate lot, a standard curve relating the surface area moment average bead diameter to the rotation speed should be generated32. - Start the timer and emulsify the alginate for 12 min.
- Add 10 mL of the oil and acetic acid solution to acidify the emulsion, dissolve the CaCO3 and hence physically cross-link the alginate into gelled beads. Allow 8 min for this acidification step.
3. Bead Recovery
- Reduce the agitation rate to 400 rpm. Add 40 mL of process buffer mixed with 10% medium to increase the pH and to cause phase inversion.
- Stop the agitation 1 min later and transfer the mixture to 50 mL centrifuge tubes. Rinse the spinner flask with an additional 20 mL medium and add this to the tube. Aspirate as much aqueous phase as possible from the spinner flask before aspirating the oil phase to minimize bead contact with the oil phase.
NOTE: Use a large-bore pipette (e.g. 25 mL pipette) at this step and from this point on to avoid damaging the beads. For smaller volumes, the bead suspension can also be manipulated with cut pipette tips. To obtain such tips, cut the end of the pipette tip with scissors while wearing eye protection. - Centrifuge the tubes for 3 min at 630 x g to accelerate bead settling and phase separation.
- Remove the oil and excess aqueous solution by aspirating with a Pasteur pipette.
- Wash the beads at least once with medium. Filter the bead suspension on 40 µm nylon cell strainers, and aspirate excess liquid associated with the beads from below the strainer. Transfer the beads into a known volume of medium using a sterile spatula.
NOTE: Smaller or larger pore size filters can be used at this step, depending on the target minimum bead diameter. However, gravity filtration is recommended rather than pressure-driven filtration through sub-micron pore size filters to avoid damaging the beads. - Measure the bead volume by volume displacement (volume after adding the beads, minus the volume prior to adding the beads) and top up the medium to obtain the desired bead:total volume ratio, which is typically 1:5, or 1 mL beads in 4 mL medium. From this point on, always use large bore pipettes to avoid damaging the beads.
- Transfer the encapsulated cells into T-flasks for in vitro culture or transplantation experiments.
4. Quality Control and Applications
NOTE: In order to ensure encapsulated cell and bead quality, the bead size distribution and cell survival after the process should be quantified. Reversing the gel to recover the cells from within the beads for further analysis is commonly performed.
- To assess the bead size distribution, stain the beads with toluidine blue-O.
- Place 0.5 mL beads into 4 mL process buffer containing 10% complete medium.
- Add 500 µL of a 1 g/L toluidine blue solution prepared in process buffer.
- Incubate for 60 min in a conical tube on a rotary shaker at 50 rpm.
- Add 5 mL process buffer containing 10% complete medium and immediately transfer the beads and solution into a Petri dish and acquire images on a low-magnification microscope or using a hand-held digital camera. If needed, place the Petri dish on a light box before acquiring images with a hand-held camera to enhance the contrast and avoid shadows.
- Perform image analysis to quantify the bead size distribution as previously described32, for example using image analysis freeware36.
- To assess cell survival qualitatively, stain the cells using ethidium homodimer to identify dead cells and calcein AM to identify live cells.
- Add 1 volume of beads to 4 volumes of process buffer containing 10% complete medium.
- Add the appropriate amount of calcein AM and ethidium homodimer stock solutions to obtain 4 µM and 2 µM concentrations, respectively. Generate single stain controls by adding only one of the reagents to some bead samples.
- Incubate the beads on ice for 20 min.
- Place the solution between a slide and coverslip using a spacer such as an o-ring to avoid compressing the beads.
- Proceed to fluorescence microscopy. Use the single stain controls to assess fluorescence bleed-through. Select appropriate fluorescence filters based on the excitation/emission wavelengths associated with calcein AM (494/517 nm) and ethidium homodimer bound to DNA (528/617 nm).
- To recover the cells from the beads for further analysis, degel the alginate using citrate or another chelating solution.
- Prepare a degelling solution containing 55 mM citrate, 10 mM HEPES and 95 mM NaCl at pH 7.4.
- Mix this solution with 10% medium, and then add 1 mL alginate beads to 9 mL of the mixture.
- Degel the beads on ice with 75 rpm agitation for 20 min.
NOTE: The cells can now be used for analysis or removed from the degelled alginate solution by centrifugation. For example, cell viability after degelling can be quantified by Trypan Blue staining. Alternatively, the cells can be centrifuged and washed, followed by mRNA, DNA and/or protein sampling and analysis.
Access restricted. Please log in or start a trial to view this content.
Wyniki
At the end of the emulsification and internal gelation process, a bead volume similar to the initial alginate and cell mixture volume should be recovered. The beads should be highly spherical with few defects (Figure 3). The beads should be sufficiently strong to withstand pipetting through large-bore pipettes. At high alginate concentrations, encapsulated oil or air droplets may be observed in the beads. This likely occurs due to the entrapment of oil in alg...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
Various steps (depicted in Figure 2) during the internal gelation reaction can limit the overall kinetics. For calcium carbonate grains larger than ~2.5 µm, the rate of carbonate dissolution has been shown to be rate-limiting26,44. The acidification step that leads to internal calcium release has also been shown to be the critical process variable affecting cell survival32. The conditions that lead to internal gelation ar...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We thank Jill Osborne for her ground-laying work on the emulsification process and Lauren Wilkinson for technical support. We thank Dr. Igor Laçik, Dr. Timothy J. Kieffer and Dr. James D. Johnson for their input and collaboration. We thank Diabète Québec, JDRF, ThéCell, the Centre québécois sur les matériaux fonctionnels (CQMF), the Natural Sciences and Engineering Research Council (NSERC), the Centre for Human Islet Transplantation and Beta-cell Regeneration, the Canadian Stem Cell Network, the Michael Smith Foundation for Health Research, le Fonds québécois de la recherche sur la nature et les technologies and COST 865 for financial support.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Reagents and consumables | |||
| LVM alginate (transplantation-grade) | Novamatrix | Non-applicable | Referred to as "alginate #1" in the results. |
| MVG alginate (transplantation-grade) | Novamatrix | Non-applicable | Referred to as "alginate #2" in the results. |
| Alginate (cell culture-grade) | Sigma | A0682 (low viscosity) or A2033 (medium viscosity) | A2033 is referred to as "alginate #3" in the results. |
| DMEM | Life Technologies | 11995-065 | |
| Fetal bovine serum, characterized, Canadian origin | Thermo Fisher Scientific | SH3039603 | |
| Glutamine | Life Technologies | 25030 | |
| Penicillin and streptomycin | Sigma | P4333-100ML | |
| HEPES, cell culture tested | Sigma | H4034-100G | |
| NaCl | Thermo Fisher Scientific | S271-1 | |
| Fine-grain CaCO3 | Avantor Materials | 1301-01 | After preparing the CaCO3 suspension, sonicate and use within one month. |
| Light mineral oil | Thermo Fisher Scientific | O121-4 | Sterile filter through a 0.22 μm pore size membrane prior to use. |
| Glacial acetic acid | Thermo Fisher Scientific | A38-500 | Handle with caution: refer to MSDS. |
| Sterile spatulas | Sigma | CLS3004-100EA | |
| Sterile nylon cell strainers, 40 µm | Thermo Fisher Scientific | 08-771-1 | |
| Serological pipettes (2 mL, 5 mL, 10 mL, 25 mL) | Sarstedt | 86.1252.001, 86.1253.001, 86.1254.001 and 86.1685.001 | |
| Pasteur pipettes | VWR | 14673-043 | |
| Toluidine Blue-O | Sigma | T3260 | |
| Equipment | |||
| 100 mL microcarrier spinner flasks | Bellco | 1965-00100 | The impeller configuration with recent models may not be suitable for adequate emulsification. A blade able to sweep the oil down to 0.5 cm from the bottom of the flask can be custom-made from a Teflon sheet. |
| Magnetic stir plate with adjustable speed | Bellco | 7760-06005 | The rotation speed should be calibrated (e.g. using a tachometer) prior to use. |
| Cell counter | Innovatis | Cedex AS20 | This system is now sold by Roche. This automated cell counter can also be replaced by manual cell enumeration after Trypan blue staining using a hemocytometer. |
| LED light box | Artograph | LightPad® PRO | This item can be replaced by other types of illuminators. |
| Handheld camera | Canon | PowerShot A590 IS | A variety of handheld cameras can be used to capture toluidine blue-o stained bead images. A ruler should be placed next to the Petri dish containing the beads prior to acquiring images. |
| Fluorescence microscope with phase contrast and adequate fluorescence filters | Olympus | IX81 | Several microscopy systems were used to image the beads. The results shown here were obtained with an IX81 microscope equipped with GFP and TRITC fluorescence filters. To capture entire beads, 4X to 20X objectives were used depending on the agitation rate. Live/dead staining images were typically captured with 20X to 40X objectives. |
| Image aquisition software | Molecular Devices | Metamorph | A variety of image acquisition software can be used to acquire phase contrast and fluorescence images. |
| Image analysis freeware | CellProfiler | Non-applicable | A variety of image analysis software can be used to identify beads as objects and analyze bead size (e.g. ImageJ). |
Odniesienia
- Scharp, D. W., Marchetti, P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv Drug Deliv Rev. 67-68, 35-73 (2014).
- Chayosumrit, M., Tuch, B., Sidhu, K. Alginate microcapsule for propagation and directed differentiation of hESCs to definitive endoderm. Biomaterials. 31 (3), 505-514 (2010).
- Sidhu, K., Kim, J., Chayosumrit, M., Dean, S., Sachdev, P. Alginate microcapsule as a 3D platform for propagation and differentiation of human embryonic stem cells (hESC) to different lineages. J Vis Exp. (61), (2012).
- Tostoes, R. M., et al. Perfusion of 3D encapsulated hepatocytes--a synergistic effect enhancing long-term functionality in bioreactors. Biotechnol Bioeng. 108 (1), 41-49 (2011).
- Duvivier-Kali, V. F., Omer, A., Parent, R. J., O'Neil, J. J., Weir, G. C. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes. 50 (8), 1698-1705 (2001).
- Omer, A., et al. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation. 79 (1), 52-58 (2005).
- Rayat, G. R., Rajotte, R. V., Ao, Z., Korbutt, G. S. Microencapsulation of neonatal porcine islets: protection from human antibody/complement-mediated cytolysis in vitro and long-term reversal of diabetes in nude mice. Transplantation. 69 (6), 1084-1090 (2000).
- Korbutt, G. S., Mallett, A. G., Ao, Z., Flashner, M., Rajotte, R. V. Improved survival of microencapsulated islets during in vitro culture and enhanced metabolic function following transplantation. Diabetologia. 47 (10), 1810-1818 (2004).
- Luca, G., et al. Improved function of rat islets upon co-microencapsulation with Sertoli's cells in alginate/poly-L-ornithine. AAPS PharmSciTech. 2 (3), E15(2001).
- Omer, A., et al. Survival and maturation of microencapsulated porcine neonatal pancreatic cell clusters transplanted into immunocompetent diabetic mice. Diabetes. 52 (1), 69-75 (2003).
- Schneider, S., et al. Long-term graft function of adult rat and human islets encapsulated in novel alginate-based microcapsules after transplantation in immunocompetent diabetic mice. Diabetes. 54 (3), 687-693 (2005).
- Cui, H., et al. Long-term metabolic control of autoimmune diabetes in spontaneously diabetic nonobese diabetic mice by nonvascularized microencapsulated adult porcine islets. Transplantation. 88 (2), 160-169 (2009).
- Krishnan, R., Alexander, M., Robles, L., Foster, C. E. 3rd, Lakey, J. R. Islet and stem cell encapsulation for clinical transplantation. Rev Diabet Stud. 11 (1), 84-101 (2014).
- Robles, L., Storrs, R., Lamb, M., Alexander, M., Lakey, J. R. Current status of islet encapsulation. Cell Transplant. 23 (11), 1321-1348 (2014).
- Desai, T., Shea, L. D. Advances in islet encapsulation technologies. Nat Rev Drug Discov. , (2016).
- Anilkumar, A. V., Lacik, I., Wang, T. G. A novel reactor for making uniform capsules. Biotechnol Bioeng. 75 (5), 581-589 (2001).
- Wolters, G. H., Fritschy, W. M., Gerrits, D., van Schilfgaarde, R. A versatile alginate droplet generator applicable for microencapsulation of pancreatic islets. J Appl Biomater. 3 (4), 281-286 (1991).
- Heinzen, C., Marison, I., Berger, A., von Stockar, U. Use of vibration technology for jet break-up for encapsulation of cells, microbes and liquids in monodisperse microcapsules. Practical Aspects of Encapsulation Technologies. , 19-25 (2002).
- Poncelet, D., et al. A Parallel plate electrostatic droplet generator: Parameters affecting microbead size. Applied Microbiology and Biotechnology. 42 (2-3), 251-255 (1994).
- Prüße, U., Dalluhn, J., Breford, J., Vorlop, K. D. Production of Spherical Beads by JetCutting. Chemical Engineering & Technology. 23 (12), 1105-1110 (2000).
- Hoesli, C. A. Bioprocess development for the cell-based treatment of diabetes (PhD thesis). , University of British Columbia. (2010).
- Brandenberger, H., Widmer, F. A new multinozzle encapsulation/immobilisation system to produce uniform beads of alginate. J Biotechnol. 63 (1), 73-80 (1998).
- Merani, S., Toso, C., Emamaullee, J., Shapiro, A. M. Optimal implantation site for pancreatic islet transplantation. Br J Surg. 95 (12), 1449-1461 (2008).
- Reis, C. P., Neufeld, R. J., Vilela, S., Ribeiro, A. J., Veiga, F. Review and current status of emulsion/dispersion technology using an internal gelation process for the design of alginate particles. J Microencapsul. 23 (3), 245-257 (2006).
- Poncelet, D., et al. Production of alginate beads by emulsification/internal gelation. I. Methodology. Appl Microbiol Biotechnol. 38 (1), 39-45 (1992).
- Poncelet, D., et al. Production of alginate beads by emulsification/internal gelation. II. Physicochemistry. Applied Microbiology and Biotechnology. 43 (4), 644-650 (1995).
- Alexakis, T., et al. Microencapsulation of DNA within alginate microspheres and crosslinked chitosan membranes for in vivo application. Appl Biochem Biotechnol. 50 (1), 93-106 (1995).
- Vandenberg, G. W., De La Noue, J. Evaluation of protein release from chitosan-alginate microcapsules produced using external or internal gelation. J Microencapsul. 18 (4), 433-441 (2001).
- Silva, C. M., Ribeiro, A. J., Figueiredo, I. V., Goncalves, A. R., Veiga, F. Alginate microspheres prepared by internal gelation: development and effect on insulin stability. Int J Pharm. 311 (1-2), 1-10 (2006).
- Larisch, B. C., Poncelet, D., Champagne, C. P., Neufeld, R. J. Microencapsulation of Lactococcus lactis subsp. cremoris. J Microencapsul. 11 (2), 189-195 (1994).
- Hoesli, C. A., et al. Reversal of diabetes by betaTC3 cells encapsulated in alginate beads generated by emulsion and internal gelation. J Biomed Mater Res B Appl Biomater. 100 (4), 1017-1028 (2012).
- Hoesli, C. A., et al. Pancreatic cell immobilization in alginate beads produced by emulsion and internal gelation. Biotechnol Bioeng. 108 (2), 424-434 (2011).
- Reinsel, M. A., Borkowski, J. J., Sears, J. T. Partition Coefficients for Acetic, Propionic, and Butyric Acids in a Crude Oil/Water System. Journal of Chemical & Engineering Data. 39 (3), 513-516 (1994).
- Xiu-Dong, L., Wei-Ting, Y., Jun-Zhang, L., Xiao-Jun, M., Quan, Y. Diffusion of acetic acid across oil/water interface in emulsification-internal gelation process for preparation of alginate gel beads. Chemical Research in Chinese Universities. 23 (5), 579-584 (2007).
- Fernandez, S. A., et al. Emulsion-based islet encapsulation: predicting and overcoming islet hypoxia. Bioencapsulation Innovations. (220), 14-15 (2014).
- Carpenter, A. E., et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7 (10), R100(2006).
- Hinze, J. O. Fundamentals of the hydrodynamic mechanism of splitting in dispersion processes. AIChE Journal. 1 (3), 289-295 (1955).
- Kolmogorov, A. N. On the breakage of drops in a turbulent flow (translated from Russian). Doklady Akademii Nauk. 66, 825-828 (1949).
- Davies, J. T. Drop Sizes of Emulsions Related to Turbulent Energy-Dissipation Rates. Chemical Engineering Science. 40 (5), 839-842 (1985).
- Pacek, A. W., Chamsart, S., Nienow, A. W., Bakker, A. The influence of impeller type on mean drop size and drop size distribution in an agitated vessel. Chemical Engineering Science. 54 (19), 4211-4222 (1999).
- Steiner, H., et al. Numerical simulation and experimental study of emulsification in a narrow-gap homogenizer. Chemical Engineering Science. 61 (17), 5841-5855 (2006).
- Tcholakova, S., Denkov, N. D., Lips, A. Comparison of solid particles, globular proteins and surfactants as emulsifiers. Phys Chem Chem Phys. 10 (12), 1608-1627 (2008).
- Lagisetty, J. S., Das, P. K., Kumar, R., Gandhi, K. S. Breakage of viscous and non-Newtonian drops in stirred dispersions. Chemical Engineering Science. 41 (1), 65-72 (1986).
- Draget, K. I., Ostgaard, K., Smidsrod, O. Homogeneous Alginate Gels - a Technical Approach. Carbohydrate Polymers. 14 (2), 159-178 (1990).
- Poncelet, D., Dulieu, C., Jacquot, M. Immobilized Cells. Wijffels, R. H. , Springer. Berlin Heidelberg. 15-30 (2001).
- Islam, A. W., Zavvadi, A., Kabadi, V. N. Analysis of Partition Coefficients of Ternary Liquid-Liquid Equilibrium Systems and Finding Consistency Using Uniquac Model. Chemical and Process Engineering-Inzynieria Chemiczna I Procesowa. 33 (2), 243-253 (2012).
- Quong, D., Neufeld, R. J., Skjak-Braek, G., Poncelet, D. External versus internal source of calcium during the gelation of alginate beads for DNA encapsulation. Biotechnol Bioeng. 57 (4), 438-446 (1998).
- De Vos, P., De Haan, B. J., Van Schilfgaarde, R. Upscaling the production of microencapsulated pancreatic islets. Biomaterials. 18 (16), 1085-1090 (1997).
- Gross, J. D., Constantinidis, I., Sambanis, A. Modeling of encapsulated cell systems. J Theor Biol. 244 (3), 500-510 (2007).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone