Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Extraction of Hemocytes from Drosophila melanogaster Larvae for Microbial Infection and Analysis
W tym Artykule
Podsumowanie
This method demonstrates how to visualize pathogen invasion into insect cells with three-dimensional (3D) models. Hemocytes from Drosophila larvae were infected with viral or bacterial pathogens, either ex vivo or in vivo. Infected hemocytes were then fixed and stained for imaging with a confocal microscope and subsequent 3D cellular reconstruction.
Streszczenie
During the pathogenic infection of Drosophila melanogaster, hemocytes play an important role in the immune response throughout the infection. Thus, the goal of this protocol is to develop a method to visualize the pathogen invasion in a specific immune compartment of flies, namely hemocytes. Using the method presented here, up to 3 × 106 live hemocytes can be obtained from 200 Drosophila 3rd instar larvae in 30 min for ex vivo infection. Alternatively, hemocytes can be infected in vivo through injection of 3rd instar larvae followed by hemocyte extraction up to 24 h post-infection. These infected primary cells were fixed, stained, and imaged using confocal microscopy. Then, 3D representations were generated from the images to definitively show pathogen invasion. Additionally, high-quality RNA for qRT-PCR can be obtained for the detection of pathogen mRNA following infection, and sufficient protein can be extracted from these cells for Western blot analysis. Taken together, we present a method for definite reconciliation of pathogen invasion and confirmation of infection using bacterial and viral pathogen types and an efficient method for hemocyte extraction to obtain enough live hemocytes from Drosophila larvae for ex vivo and in vivo infection experiments.
Wprowadzenie
Drosophila melanogaster is a well-established model organism for the study of innate immunity1. During the innate immune response, hemocytes play an important role in the response to pathogen challenge. Hemocytes are critical for encapsulating parasites, as well as having an important function in combating the pathogen through phagocytic action during fungal, viral, and bacterial infection2,3.
In order to best understand the host's innate immune response to pathogenic microbial infection, it is important to visualize how the pathogen invades host cells during infection. This visualization contributes to an understanding of the mechanism of invasion. Together with details of pathogen intracellular localization and the cellular response, these data can provide clues about the host response to infection and the cellular organelles with which the microbe interacts. Thus, 3D model reconstruction after imaging by microscopy can be helpful to determine the precise location of pathogens in host cells. In this study, we visualized the invasion of Coxiella burnetii (C. burnetii), the causative agent of Q fever, a zoonotic disease that poses a serious threat to both human and animal health, into primary Drosophila hemocytes. Recently, it was demonstrated that Drosophila are susceptible to the Biosafety level 2 Nine Mile phase II (NMII) clone 4 strain of C. burnetii and that this strain is able to replicate in Drosophila4, indicating that Drosophila can be used as a model organism to study C. burnetii pathogenesis.
Previous studies have used hemocytes to examine the host's innate immune response. Hemocytes have been used for morphological observations5,6,7, morphometric analysis2,8, phagocytosis analysis2,3, qRT-PCR2,9, immunoprecipitation10,11, immunofluorescent analysis10,12, immunostaining13, immunoblotting3,10,11 and immunohistochemistry9,14. Although Drosophila S2 cells are also available for various in vitro experiments, immortalization and potential pre-existing viral infection change their behavior15,16. The use of primary cells as opposed to an immortalized cell line, such as S2 cells, allows for the study of innate immune function in a system more representative of the whole organism. Additionally, the infection of hemocytes in vivo, prior to extraction, allows the cells to interact with other host proteins and tissue, an advantage over extraction of hemocytes prior to ex vivo infection. A number of different methods have been utilized to obtain a sufficient number of hemocytes in a short period of time to keep the hemocytes alive8,17,18,19.
In this study, we present a method to extract hemocytes from Drosophila 3rd instar larvae for pathogenic microbial infection with C. burnetii, Listeria monocytogenes (Listeria), or Invertebrate iridescent virus 6 (IIV6). We describe the methods for both in vivo and ex vivo hemocyte infections. In vivo- and ex vivo-infected hemocytes were visualized with confocal microscopy and used to build 3D models of C. burnetii invasion. Additionally, using the extraction protocol, ex vivo-infected hemocytes were used for gene and protein expression assays. Specifically, to examine the extent of infection with IIV6 and Listeria, total RNA or protein was isolated from the cells for qRT-PCR or Western blot analysis. Taken together, the protocol provides methods to rapidly collect high numbers of hemocytes from 3rd instar larvae and evidence that primary hemocytes, infected either in vivo or ex vivo, are a suitable platform for microbial pathogen infection studies and applicable downstream analyses such as microscopy, transcriptomics, and proteomics.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Ex vivo infection
- Medium and equipment
- Under sterile conditions, prepare fresh Drosophila Hemocyte Isolating Medium (DHIM) containing 75% Schneider's Drosophila medium with 25% Fetal Bovine Serum (FBS) and filter sterilize it.
- Layer 2-3 pieces of 10 cm x 10 cm paraffin film under a stereomicroscope.
- Prepare the glass capillary. Set the capillary puller heater to 55% of maximum. Pull the capillary tube to a sharp point of approximately 10 µm.
- Backfill the capillary with mineral oil.
- Assemble filled capillary tube onto the nanoinjector (Figure 1A), and open fused capillary tube tip by breaking off the tip with forceps (Figure 1B). The outer diameter of the tip should be 100 µm for easy uptake of the hemocytes.
- Eject as much oil as possible from the capillary tube tip, then fill with DHIM. For easy visualization of the border of up-taken hemolymph and the oil, include an air bubble between the oil and DHIM (Figure 1B').
- Hemolymph extraction
- Pick 3rd instar Drosophila larvae from the inside wall of food vials gently using forceps and place them into a 100 µm strainer (Figure 1C). 3rd instar larvae are found 3-6 days following fertile egg laying by an adult female.
NOTE: These experiments used the genotype, w1118;P{w+mC=Hml-GAL4.Δ}2,P{w+mC=UAS-2xEGFP}AH2, since hemocytes from these animals express enhanced green fluorescent protein (EGFP) to aid in identification of the cells by microscopy. - Pour 5 mL of sterile water over larvae and shake the strainer for 5 s. Place the strainer onto task wipe to remove the excess water (Figure 1C').
- Transfer the larvae into a 1.5 mL microcentrifuge tube. Anesthetize them with CO2 gas for 5 s (Figure 1D).
- Place the larvae onto paraffin film under the stereomicroscope, with dorsal-side facing up (Figure 2A).
- Place the glass capillary lightly onto the larval posterior body to hold it in place and disrupt the posterior cuticle open using fine pointed forceps (Figure 2B).
- Allow the hemolymph to flow onto the paraffin film (Figure 2C).
- Make a pool of hemolymph including hemocytes from 20-50 larvae at a time on the paraffin film.
- Take up pooled hemolymph using the glass capillary on the nanoinjector (Figure 2D).
NOTE: There should be approximately 10-20 µL of hemolymph. - Eject the hemolymph into a 1.5 mL microcentrifuge tube containing 500 µL of DHIM (Figure 2E).
- Repeat steps 1.2.4) - 1.2.9) for every batch of larvae.
- Pick 3rd instar Drosophila larvae from the inside wall of food vials gently using forceps and place them into a 100 µm strainer (Figure 1C). 3rd instar larvae are found 3-6 days following fertile egg laying by an adult female.
- Count the number of hemocytes.
- Pipette 5 µL of 0.4 % Trypan blue solution in a 0.6 mL microcentrifuge tube to stain the dead cells. Gently mix the DHIM and hemocytes in the 1.5 mL tube using a pipette, and transfer 5 µL of DHIM including hemocytes to the 0.6 mL microcentrifuge tube and mix gently.
- Pipette 10 µL of cells from the 1:1 Trypan blue:hemocyte mixture into the hemocytometer.
- Count the number of live hemocytes that are not stained with Trypan blue in each of the 4 corner fields of the hemocytomter and calculate the concentration of the hemocytes per milliliter using formula:
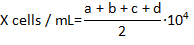
where X is the concentration of live hemocytes per milliliter; a, b, c, and d are the number of live cells (as determined by Trypan blue exclusion) in each 4 of the fields counted in the hemocytometer. Division by 2 of the total number of cells counted is due to the 1:1 dilution of the cells with Trypan blue. Cells stained with Trypan blue are considered dead.
- Ex vivo Infections
- Determine the number of wells to be seeded with cells based on timepoints and biological replicates needed for each experiment. 5.0×104 hemocytes are desirable for RNA and protein purification following infection.
- Calculate the volume of pathogen stock to be diluted with DHIM for infection using the following formula:
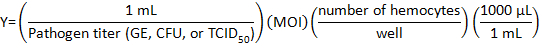
where Multiplicity of infection (MOI) is the number of viral or bacteria desired per cell.
NOTE: MOI used depends on the individual experiment and assays performed. Here, 10 genome equivalents (GE)/cell of C. burnetii, 10 CFU/cell of Listeria, or 1 TCID50/cell of IIV6 was used. - Prepare 500 µL of pathogen medium for each well of a 24-well plate by adding the proper volume of DHIM to the viral or bacterial volume in a tube.
- Place a 12 mm round cover glass (no. 1 thickness) in a well of a 24-well plate.
- Split the DHIM including the hemocytes into wells of a 24-well plate.
- Add 500 µL of pathogen medium to hemocytes in a well.
- Centrifuge the plate at 1,000 x g for 5 min.
- Incubate the plate for 1 h at 28 °C. Every 15 min, gently tilt the plate from back to front, then left to right for 5 s by hand.
- After the 1 h of invasion/attachment step 1.4.8), gently pipette off the pathogen medium and wash the hemocytes with fresh DHIM, and refill it with 500 µL of fresh DHIM.
- Incubate the infected hemocytes for the desired time. In these experiments, C. burnetii-or IIV6-infected hemocytes are incubated for 24 h, and Listeria-infected hemocytes are incubated for 1, 2, or 4 h.
2. In vivo infection
- Infection
- Warm the Drosophila fruit juice agar plate at room temperature for 15 min. Plates are made as previously described20.
- Add 30 g of agar to 700 mL of water and autoclave it for 40 min.
- Dissolve 0.5 g of methyl paraben in 10 mL of absolute ethanol.
- Add the methyl paraben solution to 300 mL of fruit juice concentrate.
- Quickly mix the juice concentrate into the autoclaved agar solution and dispense 5 mL into 10 × 35 mm Petri dishes.
- After the plates have cooled for 15 min, store them at 4 °C.
- Prepare 3rd instar larvae following steps 1.2.1) - 1.2.3).
- Place the yeast paste on an agar plate. Make a fine cut in the agar plate where larvae can migrate to avoid drying (Figure 3A). Assemble a 0.001 mm pointed tungsten needle with holding forceps using paraffin film (Figure 3B, C).
- Pipette 50 µL of high-titer mCherry expressing-C. burnetii (5.95×109 GE/mL) onto the paraffin film under the stereo microscope and place the larvae into the pool of bacteria.
- Place the larvae into the pool of bacteria. Prick the larvae with a tungsten needle (Figure 3D). Transfer the larvae onto an agar plate (Figure 3E).
- Transfer the remaining pathogen medium onto the agar plate and seal the plate with paraffin film.
- Keep the larvae on the plate in moist air until the desired time post-infection (Figure 3F). In this experiment, C. burnetii-infected larvae are on the plate for 24 h.
- Warm the Drosophila fruit juice agar plate at room temperature for 15 min. Plates are made as previously described20.
- Hemolymph extraction and plating of hemocytes
- Prepare the medium and equipment following step 1.1).
- Place a 12 mm round cover glass (no. 1 thickness) in a well of a 24-well plate. Pipette 500 µL of DHIM into the well.
- Extract the hemolymph from the infected larvae following steps 1.2.4) - 1.2.8).
- Eject the hemolymph into the well following step 2.2.2).
- Repeat 2.2.3) and 2.2.4) for multiple batches of larvae.
- Centrifuge the plate at 1,000 x g for 5 min.
3. Visualization
- Fixing and staining
- After allowing the hemocytes to settle on the round cover glass in the well, gently remove the medium from each well.
- Gently add 200 µL of 4% paraformaldehyde (PFA) to each well of settled hemocytes. Incubate the hemocytes for 20 min at room temperature.
- Remove the 4% PFA and gently add 200 µL of PBS containing 0.1% Triton X-100 and 1% Bovine Serum Albumin (BSA) to each well. Incubate the hemocytes for 10 min at room temperature.
- Remove the PBS and gently add 200 µL of 1× 4',6-diamidino-2-phenylindole (DAPI) to each well. Incubate the hemocytes for 10 min in the dark at room temperature.
- Remove the DAPI solution and gently add PBS to each well. Incubate the hemocytes for 5 min at room temperature.
- Drop 10 µL of the antifade mounting medium on a glass microscope slide.
- After removing the PBS from each well, remove the cover glass from the 24-well plate using fine-pointed forceps. Gently place the cover glass onto the antifade mounting medium on the glass slide, with the hemocytes facing down.
- Allow the slide to dry by placing it in the dark overnight.
- Confocal Imaging
- Configure the confocal microscope for three color imaging of DAPI, EGFP, and mCherry. Use the following setting: DAPI excitation (ex) 405 nm, emission (em) 415-480 nm; EGFP ex 488 nm, em 493-564 nm; mCherry ex 587 nm, em 597-700 nm.
- Place the sample on the microscope and focus on the sample using a 63X/1.4 numerical aperture (NA) objective. Locate desired hemocytes in the field of view for imaging.
- Adjust laser power and detector gains to achieve appropriate exposure of the sample. Check multiple z-planes to ensure the exposure level is appropriate for the entire sample thickness.
- Find the top and bottom position on the z-axis of a whole hemocyte. Set these positions as the start and end positions for z-sectioning.
- Only use scanning zoom to image the area containing the hemocyte. Zoom factors of 3X are often used.
- Collect the image series at appropriate resolution such as 1024 x 1024 pixels in the x-y plane, and 0.3 µm spacing in the z dimension.
- 3D model reconstruction
NOTE: Open source software exists that performs many of the functions described below for 3D model reconstruction.- Import the z-sectioned image series file into the software associated with the confocal microscope for 3D model reconstruction.
- Select a cell showing co-localization of nuclei stained with DAPI and mCherry expressing C. burnetii in an EGFP expressing hemocyte. Crop the image series to contain only the single cell.
- Select the 3D Viewer option which reconstructs the 3D model using the software's pre-packaged algorithm. Choose the desired type of 3D representation among Blend, Surface, and Mixed options. In this method, hemocytes, nuclei, and C. burnetii are shown using surface models.
- Observe the 3D reconstructed cell from various viewing positions by holding the mouse button and dragging the cursor around the screen. Adjust the cell orientation and position of the modeled light source to optimize the image. Other options for Opacity, Minimum and Maximum Threshold, Specular, Ambient, Shineness, and Gamma exist to optimize the image.
- Take cross-sections through the model using clipping and sectioning commands to visualize the interior contents of the hemocyte.
4. Application for gene and/or protein analysis
- Following the infection with IIV6 and Listeria, lyse cells for subsequent qRT-PCR or Western blot analysis as previously described21 and following manufacturer's instructions.
NOTE: Refer the Table of Materials for the primers for qRT-PCR and the antibodies for Western blot. - Analyze PCR products by agarose gel electrophoresis, as previously described22, to ensure the proper length of the amplified product.
Access restricted. Please log in or start a trial to view this content.
Wyniki
To collect live hemocytes for ex vivo infection, up to 3×106 hemocytes were extracted from 200 Drosophila 3rd instar larvae. To develop our method, a number of different techniques were attempted. Individual larval dissection would take up to 1.5 h, and an average of ~8000 cells were obtained using this method18, most of which were not alive by the end of collection. Next, we tried to extract hemolymph, which contained t...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
To better understand how host cells become infected, it is important to clarify the localization of pathogen in the cells, especially when experimenting on previously untested pathogen and cell type combinations4. While studying the cellular response cascade following infection can indicate productive pathogen invasion, the combination of imaging and cellular response data is essential to demonstrate pathogen invasion and infection. While reports showing 2D images of pathogen invasion into the hos...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors declare that they have no competing financial interests.
Podziękowania
We are grateful to Dr. Robert Heinzen for providing stocks of mCherry-expressing Coxiella burnetii. We thank Dr. Luis Teixeira for providing Invertebrate iridescent virus 6 and the Bloomington Stock Center for providing fly stocks. This project was funded in part by NIH grant R00 AI106963 (to A.G.G.) and Washington State University.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Schneider's Drosophila Medium | Thermo Fisher Scientific (Gibco) | 21720024 | 1.1.1), 2.1.2) |
| Fetal Bovine Serum | GE Healthcare Life Sciences (HyClone) | SH30070.03HI | 1.1.1), 2.1.2) |
| Filter (0.22 µL) | RESTEK | 26158 | 1.1.1) |
| Strainer (100 µm) | Greiner bio-one | 542000 | 1.2.1), 2) |
| Stereo microscope | Amscope | SM-1BSZ-L6W | 1.2), 2) |
| Glass capillary | Fisher Scientific | 21-171-4 | 1.1), 1.2), 2) |
| Capillary puller | Narishige International USA, Inc. | PC-10 | 1.1.3) |
| Mineral oil | Snow River Products | 1.1.4) | |
| Nanoinjector | Drummond Scientific Company | 3-000-204 | 1.1), 1.2), 2.2) |
| Forceps | VWR | 82027-402 | 1.1.5), 1.2), 2), 3.1.7) |
| CO2 delivery apparatus | Genesee Scientific | 59-122BC | 1.2), 2) |
| Trypan Blue | Thermo Fisher Scientific (Gibco) | 15250061 | 1.3) |
| Hemocytometer | Hausser Scientific | 3100 | 1.3) |
| 24 well plate | Greiner bio-one | 662160 | 1.4), 2.2) |
| Coxiella burnetii - mCherry | Dr. Heinzen, R. | 1.4), 2.2) | |
| Drosophila fruit juice plates | Cold Spring Harbor Protocols | 2.1) http://cshprotocols.cshlp.org/content/2007/9/pdb.rec11113.full | |
| Agar | Fisher Bioreagents | BP1423-500 | 2.1.1.1) |
| Methyl paraben | Amresco | 0572-500G | 2.1.1.2) |
| Absolute ethanol | Fisher Bioreagents | BP2818-500 | 2.1.1.2) |
| Welch's 100% Grape juice frozen concentrate, 340 mL | Amazon | B0025UJVGM | 2.1.1.3) |
| Petri dishes, 10 x 35 mm | Fisher Scientific | 08-757-100A | 2.1.1.4) |
| Microscope cover glass | Fisher Scientific | 12-545-80 | 1.4.4), 2.2.2) |
| Yeast, Bakers Dried Active | MP Biomedicals | 0210140001 | 2.1) Add 2 parts of water to 1 part of yeast (v/v) |
| Tungsten needle | Fine Science Tools | 10130-20 | 2.1) |
| Holding forceps | VWR | HS8313 | 2.1) |
| Paraformaldehyde | Fisher Scientific | FLO4042-500 | 3.1.3) |
| Triton X-100 | Fisher Scientific | BP151-500 | 3.1.3) |
| Bovine Serum Albumin | Fisher Scientific | BP9706-100 | 3.1.3) |
| 4',6-diamidino-2-phenylindole | Thermo Fisher Scientific | 62247 | 3.1.4) |
| Antifade mounting medium | Thermo Fisher Scientific | P36930 | 3.1.6) |
| Confocal microsope | Leica | TCS SP8-X White Light Confocal Laser Scanning Microscope | 3.2) |
| 3D imaging reconstruction software | Leica | LASX with 3D visualization module | 3.3) |
| Microscope slides | Fisher Scientific | 12-552-3 | 3.1.6) |
| Invertebrate iridescent virus 6 (IIV6) | Dr. Teixeria, L. | 4) PLoS Biol, 6 (12), 2753-2763, doi: 10.1371/journal.pbio.1000002, (2008) | |
| Listeria monocytogenes | ATCC | strain: 10403S | 4) Listeria monocytogenes strain 10403S (Bishop and Hinrichs, 1987) was grown in Difco Brain-heart infusion (BHI) broth (BD Biosciences) containing 50 µg/ml streptomycin at 30 °C. |
| DNase I | Thermo Fisher Scientific(Invitrogen) | 18068015 | gDNA degradation |
| cDNA Synthesis Kit | Bio-Rad | 1708891 | cDNA synthesis |
| IIV6_193R_F | IDT | qRT-PCR, 5'- TCT TGT TTT CAG AAC CCC ATT -3' | |
| IIV6_193R_R | IDT | qRT-PCR, 5'- CAC GAA GAA TGA CCA CAA GG -3' | |
| RpII_qRTPCR_fwd | SIGMA-ALDRICH | qRT-PCR, 5'- GAA GCG TTT CTC CAA ACG -AG | |
| RpII_qRTPCR_rev | SIGMA-ALDRICH | qRT-PCR, 5'- TTG AGC GTA AGC ATC ACC -TG | |
| SYBR Green qRT-PCR reagent | Thermo Fisher Scientific | K0251, K0252, K0253 | qRT-PCR |
| Real-Time PCR System | Thermo Fisher Scientific | 4351107, 7500 Software v2.0 | qRT-PCR |
| Anti-Listeria monocytogenes antibody | abcam | ab35132 | Western blot |
| Anti-Actin antibody produced in rabbit | SIGMA-ALDRICH | A2066 | Western blot |
| Anti-Rabbit IgG (H+L), HRP Conjugate | Promega | W4011 | Western blot |
Odniesienia
- Hoffmann, J. A. The immune response of Drosophila. Nature. 426 (6962), 33-38 (2003).
- Regan, J. C., et al. Steroid hormone signaling is essential to regulate innate immune cells and fight bacterial infection in Drosophila. PLoS Pathog. 9 (10), 1003720(2013).
- Yano, T., et al. Autophagic control of listeria through intracellular innate immune recognition in Drosophila. Nat Immunol. 9 (8), 908-916 (2008).
- Bastos, R. G., Howard, Z. P., Hiroyasu, A., Goodman, A. G. Host and Bacterial Factors Control Susceptibility of Drosophila melanogaster to Coxiella burnetii Infection. Infect Immun. 85 (7), (2017).
- Kacsoh, B. Z., Schlenke, T. A. High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoS One. 7 (4), 34721(2012).
- Tsuzuki, S., et al. Switching between humoral and cellular immune responses in Drosophila. is guided by the cytokine GBP. Nat Commun. 5, 4628(2014).
- Kurucz, E., et al. Definition of Drosophila. hemocyte subsets by cell-type specific antigens. Acta Biol Hung. 58, Suppl 95-111 (2007).
- Neyen, C., Bretscher, A. J., Binggeli, O., Lemaitre, B. Methods to study Drosophila immunity. Methods. 68 (1), 116-128 (2014).
- Arefin, B., et al. Apoptosis in Hemocytes Induces a Shift in Effector Mechanisms in the Drosophila. Immune System and Leads to a Pro-Inflammatory State. PLoS One. 10 (8), 0136593(2015).
- Rus, F., et al. Expression pattern of Filamin-240 in Drosophila blood cells. Gene Expr Patterns. 6 (8), 928-934 (2006).
- Kurucz, E., et al. Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. P Natl Acad Sci USA. 100 (5), 2622-2627 (2003).
- Markus, R., et al. Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. P Natl Acad Sci USA. 106 (12), 4805-4809 (2009).
- Bretscher, A. J., et al. The Nimrod transmembrane receptor Eater is required for hemocyte attachment to the sessile compartment in Drosophila melanogaster. Biol Open. 4 (3), 355-363 (2015).
- Zettervall, C. J., et al. A directed screen for genes involved in Drosophila blood cell activation. P Natl Acad Sci USA. 101 (39), 14192-14197 (2004).
- Flynt, A., Liu, N., Martin, R., Lai, E. C. Dicing of viral replication intermediates during silencing of latent Drosophila viruses. P Natl Acad Sci USA. 106 (13), 5270-5275 (2009).
- Jovel, J., Schneemann, A. Molecular characterization of Drosophila cells persistently infected with Flock House virus. Virology. 419 (1), 43-53 (2011).
- Stoepler, T. M., Castillo, J. C., Lill, J. T., Eleftherianos, I. A simple protocol for extracting hemocytes from wild caterpillars. J Vis Exp. (69), e4173(2012).
- Sampson, C. J., Williams, M. J. Protocol for ex vivo incubation of Drosophila primary post-embryonic haemocytes for real-time analyses. Methods Mol Biol. 827, 359-367 (2012).
- Nehme, N. T., et al. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 3 (11), 173(2007).
- Drosophila fruit juice egg plates. Cold Spring Harbor Protocols. (9), pdb.rec11113 (2007).
- Ahlers, L. R., Bastos, R. G., Hiroyasu, A., Goodman, A. G. Invertebrate Iridescent Virus 6, a DNA Virus, Stimulates a Mammalian Innate Immune Response through RIG-I-Like Receptors. PLoS One. 11 (11), 0166088(2016).
- Lee, P. Y., Costumbrado, J., Hsu, C. Y., Kim, Y. H. Agarose gel electrophoresis for the separation of DNA fragments. J Vis Exp. (62), (2012).
- Petraki, S., Alexander, B., Bruckner, K. Assaying Blood Cell Populations of the Drosophila melanogaster Larva. J Vis Exp. (105), (2015).
- Figliozzi, R. W., Chen, F., Chi, A., Hsia, S. C. Using the inverse Poisson distribution to calculate multiplicity of infection and viral replication by a high-throughput fluorescent imaging system. Virol Sin. 31 (2), 180-183 (2016).
- Rizki, T. M., Rizki, R. M. Lamellocyte differentiation in Drosophila. larvae parasitized by Leptopilina. Dev Comp Immunol. 16 (2-3), 103-110 (1992).
- Markus, R., Kurucz, E., Rus, F., Ando, I. Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster. Immunol Lett. 101 (1), 108-111 (2005).
- McCormack, R., et al. Perforin-2 Protects Host Cells and Mice by Restricting the Vacuole to Cytosol Transitioning of a Bacterial Pathogen. Infect Immun. 84 (4), 1083-1091 (2016).
- Ozgen, A., et al. Construction and characterization of a recombinant invertebrate iridovirus. Virus Res. 189, 286-292 (2014).
- Jakob, N. J., Muller, K., Bahr, U., Darai, G. Analysis of the first complete DNA sequence of an invertebrate iridovirus: coding strategy of the genome of Chilo iridescent virus. Virology. 286 (1), 182-196 (2001).
- Ghigo, E., Colombo, M. I., Heinzen, R. A. The Coxiella burnetii parasitophorous vacuole. Adv Exp Med Biol. 984, 141-169 (2012).
- Liu, F., et al. Drosophila melanogaster prophenoloxidases respond inconsistently to Cu2+ and have different activity in vitro. Dev Comp Immunol. 36 (3), 619-628 (2012).
- De Gregorio, E., et al. An immune-responsive Serpin regulates the melanization cascade in Drosophila. Dev Cell. 3 (4), 581-592 (2002).
- Kari, B., et al. The raspberry Gene Is Involved in the Regulation of the Cellular Immune Response in Drosophila melanogaster. PLoS One. 11 (3), 0150910(2016).
- Wu, A. R., et al. Quantitative assessment of single-cell RNA-sequencing methods. Nat Methods. 11 (1), 41-46 (2014).
- Buettner, F., et al. Computational analysis of cell-to-cell heterogeneity in single-cell RNA-sequencing data reveals hidden subpopulations of cells. Nat Biotechnol. 33 (2), 155-160 (2015).
- Nevil, M., Bondra, E. R., Schulz, K. N., Kaplan, T., Harrison, M. M. Stable Binding of the Conserved Transcription Factor Grainy Head to its Target Genes Throughout Drosophila melanogaster Development. Genetics. 205 (2), 605-620 (2017).
- Yang, C. P., et al. Transcriptomes of lineage-specific Drosophila neuroblasts profiled by genetic targeting and robotic sorting. Development. 143 (3), 411-421 (2016).
- Jaitin, D. A., et al. Dissecting Immune Circuits by Linking CRISPR-Pooled Screens with Single-Cell RNA-Seq. Cell. 167 (7), 1883-1896 (2016).
- Karaiskos, N., et al. The Drosophila embryo at single-cell transcriptome resolution. Science. 358 (6360), 194-199 (2017).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone