Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Mesenchymal Stem Cell Isolation from Pulp Tissue and Co-Culture with Cancer Cells to Study Their Interactions
W tym Artykule
Podsumowanie
We provide protocols for evaluation of mesenchymal stem cells isolated from dental pulp and prostate cancer cell interactions based on direct and indirect co-culture methods. Condition medium and trans-well membranes are suitable to analyze indirect paracrine activity. Seeding differentially stained cells together is an appropriate model for direct cell-cell interaction.
Streszczenie
Cancer as a multistep process and complicated disease is not only regulated by individual cell proliferation and growth but also controlled by tumor environment and cell-cell interactions. Identification of cancer and stem cell interactions, including changes in extracellular environment, physical interactions, and secreted factors, might enable the discovery of new therapy options. We combine known co-culture techniques to create a model system for mesenchymal stem cells (MSCs) and cancer cell interactions. In the current study, dental pulp stem cells (DPSCs) and PC-3 prostate cancer cell interactions were examined by direct and indirect co-culture techniques. Condition medium (CM) obtained from DPSCs and 0.4 µm pore sized trans-well membranes were used to study paracrine activity. Co-culture of different cell types together was performed to study direct cell-cell interaction. The results revealed that CM increased cell proliferation and decreased apoptosis in prostate cancer cell cultures. Both CM and trans-well system increased cell migration capacity of PC-3 cells. Cells stained with different membrane dyes were seeded into the same culture vessels, and DPSCs participated in a self-organized structure with PC-3 cells under this direct co-culture condition. Overall, the results indicated that co-culture techniques could be useful for cancer and MSC interactions as a model system.
Wprowadzenie
Mesenchymal stem cells (MSCs), with the ability of differentiation and contribution to regeneration of mesenchymal tissues such as bone, cartilage, muscle, ligament, tendon, and adipose, have been isolated from almost all tissues in the adult body1,2. Other than providing tissue homeostasis by producing resident cells in case of chronic inflammation or an injury, they produce vital cytokines and growth factors to orchestrate angiogenesis, immune system, and tissue remodeling3. The interaction of MSCs with cancer tissue is not well-understood, but accumulating evidence suggests that MSCs might promote tumor initiation, progression, and metastasis4.
The homing ability of MSCs to the injured or chronically inflamed area makes them a valuable candidate for stem cell-based therapies. However, cancer tissues, "never healing wounds", also release inflammatory cytokines, pro-angiogenic molecules, and vital growth factors, which attract MSCs to the cancerogenous area5. While there are limited reports showing inhibitory effects of MSCs on cancer growth6,7, their cancer progression and metastasis promoting effects have been extensively reported8. MSCs directly or indirectly affect carcinogenesis in different ways including suppressing immune cells, secreting growth factors/cytokines that support cancer cell proliferation and migration, enhancing angiogenic activity, and regulating epithelial-mesenchymal transition (EMT)9,10. Tumor environment consists of several cell types including cancer-associated fibroblasts (CAFs) and/or myofibroblasts, endothelial cells, adipocytes, and immune cells11. Of those, CAFs are the most abundant cell type in the tumor area that secrete various chemokines promoting cancer growth and metastasis8. It has been shown that bone marrow-derived MSCs can differentiate into CAFs in the tumor stroma12.
Dental pulp stem cells (DPSCs), characterized as the first dental tissue-derived MSCs by Gronthos et al.13 in 2000 and then widely investigated by others14,15, express pluripotency markers such as Oct4, Sox2, and Nanog16 and can differentiate into various cell linages17. Gene and protein expression analysis proved that DPSCs produce comparable levels of growth factors/cytokines with other MSCs such as vascular endothelial growth factor (VEGF), angiogenin, fibroblast growth factor 2 (FGF2), interleukin-4 (IL-4), IL-6, IL-10, and stem cell factor (SCF), as well as fms-like tyrosine kinase-3 ligand (Flt-3L) that might promote angiogenesis, modulate immune cells, and support cancer cell proliferation and migration18,19,20. While the interactions of MSCs with cancer environment have been well-documented in the literature, the relationship between DPSCs and cancer cells has not been evaluated yet. In the present study, we established co-culture and condition medium treatment strategies for a highly metastatic prostate cancer cell line, PC-3, and DPSCs to propose potential action of mechanism of dental MSCs in cancer progression and metastasis.
Protokół
Written informed consent of the patients was obtained after the approval from the Institutional Ethics Committee.
1. DPSC Isolation and Culture
- Transfer wisdom teeth obtained from young adults aged between 17 and 20 to 15 mL tubes containing complete Dulbecco's Modified Eagle Medium (DMEM) [low glucose DMEM media, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin/amphotericin (PSA) solution], within 8 h after resection. Keep the tissue material cold (4 °C) during transfer to avoid potential cell death.
- Remove the pulp tissue by sterile extraction forceps from the center of the tooth carefully, place the pulp tissue in the cold complete DMEM medium in 10 cm tissue culture dishes, and mince them into small pieces (2-3 mm) by scalpel.
NOTE: All experimental procedures should be carried out under sterile conditions in laminar flow hood. This non-enzymatic technique has been previously used21,22,23. - Place small pulp tissues inside the tissue culture treated 6-well plates and add 200 µL of complete DMEM media to cover each small pulp tissue pieces.
- Incubate the tissue culture wells at 37 °C in a humidified air atmosphere (80% humidity) with 5% CO2 for 2 h to provide tissue attachment.
NOTE: This step could be prolonged to 3-4 h by controlling the evaporation of media. - Add appropriate volume (2-2.5 mL) of complete DMEM medium to the wells and incubate at 37 °C in a humidified air atmosphere with 5% CO2 for cells to spread from the tissue.
NOTE: Cells become visible after approximately 4 days and reach confluency after 8-9 days. - When cells reach 80% confluency, remove media from 6-well plates, wash with 2 mL of phosphate buffered saline (PBS), and add 2 mL of trypsin. Incubate for 2 min in an incubator at 37 °C with humidified air atmosphere and 5% CO2. Then add 2 mL of complete DMEM medium followed by 2 min incubation to inhibit trypsin. Centrifuge cells at 300 x g for 5 min to pellet cells.
- Passage cells to the flasks in complete DMEM media and store for further experiments. Add 15 mL of complete DMEM media to the T-75 flasks and transfer cells from two wells of the 6-well plate to one T-75 flasks and incubate at 37 °C with humidified air atmosphere and 5% CO2.
2. Characterization of DPSCs
- Perform morphological analyses.
- Seed cells (step 1.6) in tissue culture coated flasks (or 6-well plates) in complete DMEM medium for at least 8 passages to observe cell morphology.
- Visualize cells by light microscope and define fibroblast-like cell morphology. Cells should attach to the culture dishes and have spindle-like cell morphology.
NOTE: Alternatively, cells can be cultured as single cells for up to 14 days in well-plates to observe colony formation capacity that is a specific characteristic of fibroblasts and MSCs.
- Perform surface marker analyses.
- Trypsinize the cells from step 1.7. Remove media from the T-75 tissue culture flask, wash with 2 mL of PBS, and add 2 mL of trypsin. Incubate for 2 min in an incubator at 37 °C with humidified air atmosphere and 5% CO2. Then add 2 mL of complete DMEM medium followed by 2 min incubation to inhibit trypsin. Centrifuge cells at 300 x g for 5 min to pellet cells.
- Fix the cells with 4% paraformaldehyde for 20 min at room temperature in 1.5 mL tubes and then wash them with 500 µL of PBS 3 times to remove paraformaldehyde.
- Incubate fixed cells with the antibodies against CD29, CD34, CD14, CD45, CD90, CD105, CD166, and CD73 for 1 h at 4 °C in 100 µL of PBS.
NOTE: The concentration of antibody used is 0.5 µg/mL. CD34, CD14, and CD45 are used as negative markers, while CD29, CD90, CD105, CD166, and CD73 are used as positive cell surface markers for MSCs. - Wash cells 3 times with PBS and use respective secondary antibodies such as fluorescein isothiocyanate (FITC), phycoerythrin (PE), etc. for labelling. Incubate cells with 1:500 diluted secondary antibodies in 100 µL of PBS for 30 min at 4 °C and wash 3 times with PBS.
- Keep samples in the dark for flow cytometry analysis and detect positive and negative staining by flow cytometry.
NOTE: Use unstained control cells to arrange forward and side scatter. Arrange gating of positively stained populations by excluding dead cells, debris, and un-stained population. Use 100 µm nozzle with 45 psi sheath pressure and collect 10,000 events to determine positive DPSCs by arranging channels.
- Perform differentiation of DPSCs.
- Seed 1 × 104 cells onto 24-well plates in complete DMEM media and incubate for 24 h at 37 °C in a humidified air atmosphere with 5% CO2.
- Formulate differentiation media using compete DMEM medium as base media. Prepare osteogenic media by mixing 100 nM dexamethasone,10 mM β-glycerophosphate, and 0.2 mM ascorbic acid. Prepare chondrogenic media by mixing 1× insulin-transferrin-selenium (ITS-G), 100 nM dexamethasone, 100 ng/mL transforming growth factor beta (TGF-β), 14 μg/mL ascorbic acid, and 1 mg/mL bovine serum albumin (BSA). Prepare adipogenic media by mixing 100 nM dexamethasone, 5 μg/mL insulin, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), and 60 μM indomethacin.
NOTE: Differentiation media can be kept at 4 °C for at least one week. - Change growth media to osteo-, chondro-, or adipo-genic differentiation media and refresh media twice a week for two weeks.
- Confirm differentiation by von Kossa and Alcian blue staining, enzyme activity (alkaline phosphatase activity), immunocytochemistry, and quantitative polymerase chain reaction (qPCR) analyses according to the protocols previously described21.
- Perform von Kossa and Alcian blue stainings on cells that are fixed with 4% paraformaldehyde at room temperature for 10 min. Wash the fixed cells with PBS and stain them with the vonKossa kit according to the manufacturer’s recommendations to observe calcium deposits.
- Prepare the Alcian blue staining solution by dissolving 1.00 g of Alcian blue dye in 100 mL of 3% (v/v) acetic acid for further staining. Wash the fixed cells with PBS and stain cells for 30 min with Alcian blue solution. Visualize the stained samples by a light microscope.
3. Preparation of Condition Medium (CM)
- Replace media of cells from step 1.7 with fresh complete DMEM 24 h before CM collection.
NOTE: Passage 2-4 is recommended. - Collect condition medium (CM) from cultured DPSCs when cells reach 80% confluency. Centrifuge collected media at 300 x g for 5 min to remove waste tissue material and cell debris.
NOTE: Alternatively, use 0.2 µm syringe filters to remove debris from the condition medium. - Collect supernatant and store at -20 °C for further experiments.
NOTE: Keep the supernatant at -80 °C for long-term storage.
4. Treatment of Cancer Cells with CM
- Perform cell viability analyses.
- Seed PC-3 cells (human prostate cancer cells) onto 96-well plates at a cell density of 5 × 103 cells/well in complete DMEM and incubate in a humidified chamber at 37 °C and 5% CO2 for 24 h.
- Treat cells with 10, 20, 30, 40, and 50% of CM (v/v) mixed with complete DMEM for 24 h.
- Measure cell viability by using 3-(4,5-dimethyl-thiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfo-phenyl)-2H-tetrazolium (MTS)-assay as described previously24.
- Perform terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analyses.
- Seed PC-3 cells onto 6-well cell culture plates at a cell density of 2 × 105 cells/well and incubate in a humidified chamber at 37 °C and 5% CO2 overnight.
- Mix 20% CM (v/v) with complete DMEM medium and apply to the cells for 24 h.
- Trypsinize cells and suspend in 50 μL of TUNEL reaction mixture (labeling solution + enzyme solution, supplied with the kit) and incubate at 37 °C for 60 min in a humidified and 5% CO2 atmosphere.
NOTE: For trypsinization, remove media from 6-well cell culture plates, wash with 1 mL of PBS and add 500 µL of trypsin. Incubate for 2 min in an incubator at 37 °C with humidified air atmosphere and 5% CO2. Add 1 mL of complete DMEM medium followed by 2 min incubation to inhibit trypsin. Centrifuge cells at 300 x g for 5 min to pellet cells. - Rinse with PBS and analyze cells in PBS by using flow cytometry.
- Perform qPCR analyses.
- Seed PC-3 cells onto 6-well cell culture plates at a cell density of 2 × 105 cells/well and incubate in a humidified incubator at 37 °C and 5% CO2 overnight.
- Mix 20% CM (v/v) with complete DMEM medium and apply to the cells for 24 h.
- Trypsinize cells and collect cell pellet for RNA isolation and cDNA synthesis.
NOTE: Remove media from 6-well cell culture plates, wash with 1 mL of PBS, and add 500 µL of trypsin. Incubate 2 min in an incubator at 37 °C with humidified air atmosphere and 5% CO2. Add 1 mL of complete DMEM medium followed by 2 min incubation to inhibit trypsin. Centrifuge cells at 300 x g for 5 min to pellet cells. - Perform qPCR experiments according to the previously described protocol25.
- Perform cell migration of cancer cells.
- Seed 1 × 105 PC-3 cells onto 12-well plates and incubate in a humidified incubator overnight at 37 °C and 5% CO2.
- Scratch cells with a sterile 200 μL tip and change medium immediately with fresh medium containing various concentrations of CM [e.g., 10, 20, 30, 40, and 50% of CM (v/v) mixed with complete DMEM].
- Observe scratches under an inverted microscope and take pictures at different time intervals (0 and 24 h).
- Measure the scratch closure by using Image J software using the formula:
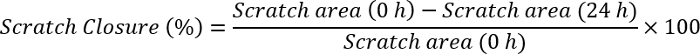
NOTE: Open the scratch image with Image J software. Draw a line that has the same magnitude as the scale bar that already exists in the image. Click analyze, set scale, and observe distance in pixels as the magnification of the drawn line. Write the size of the scale bar to the known distance part, arrange unit (pixels, cm, etc.), and click ok. Go to the analyze section again and click on measurements. This will first give the size of the scale bar as the selected unit. Click on one edge of scratch and drag until reaching the other end of the scratch. Note the value for each time point (0 h and 24 h). Plug these values into the formula above and calculate the scratch closure.
5. Cell Migration by Indirect Contact of Cancer Cells and DPSCs
- Seed 3 × 104 DPSCs onto 24-well plate inserts with 0.4 μm pore and incubate in a humidified incubator overnight at 37 °C.
- Seed PC-3 cells onto 24-well plates at a cell density of 5 × 104 and incubate in a humidified incubator overnight at 37 °C and 5% CO2.
- Scratch PC-3 cells with a sterile 200 μL tip, change medium with fresh medium, and place inserts carrying DPSCs onto PC-3 cells.
- Observe cells under an inverted microscope and take pictures at different time intervals (0 and 24 h) to analyze cell migration.
6. Co-culture Assay and Flow Cytometry Analysis
- Label PC-3 cells and DPSCs by using PKH67 (green) and PKH26 (red) fluorescent cell linker dyes, respectively26.
- Trypsinize PC-3 and DPSCs cells, respectively. Remove media from T-75 tissue culture flask, wash with 2 mL of PBS, and add 2 mL of trypsin. Incubate for 2 min in an incubator at 37 °C with humidified air atmosphere and 5% CO2. Add 2 mL of complete DMEM medium followed by 2 min incubation to inhibit trypsin.
- Centrifuge cells at 300 x g for 5 min, discard supernatant, and resuspend cell pellets in the dye solution prepared in diluent-C buffer supplied by the kit (see Table of Materials).
- Incubate cells in the dye solution for 10 min and terminate the staining reaction by adding 100 µL of FBS. Centrifuge cells at 300 x g for 5 min, discard the supernatant, and wash the cells with complete growth medium before co-culturing.
- Plate labeled cells (5 × 104/well) onto 6-well plates at 1:1 ratio (DPSCs:PC3). Maintain co-cultured cells in complete DMEM.
- Collect cells after 24 h or 48 h incubation periods by centrifugation of cells at 300 x g for 5 min and washing with PBS.
- Resuspend cells in 300 µL of fluorescence-activated cell sorting (FACS) buffer in 5 mL round bottom flow cytometry tubes. Vortex to disperse cell aggregates right before the sample analysis.
- Use a 100 µm nozzle with 45 psi sheath pressure.
NOTE: Extremely high flow rate might decrease the sensitivity of fluorescence detection. - Use unstained control cells and single colored cells to adjust appropriate forward and side scatter laser voltage for cell types and compensation as mentioned previously27.
NOTE: Use gating for live cells to exclude cell debris, dead cells, or aggregates. - Collect 10,000 events (100,000 is preferable) to determine percent positive green DPSCs and red PC-3 cells by arranging FL-1 (green) and FL-2 (red) channels.
Wyniki
Figure 1 depicts the general MSC characteristics of DPSCs under culture conditions. DPSCs exert fibroblast-like cell morphology after plating (Figure 1B). MSC surface antigens (CD29, CD73, CD90, CD105, and CD166) are highly expressed while hematopoietic markers (CD34, CD45, and CD14) are negative (Figure 1C). Changes at the morphological and molecular level related to osteo-, chondro-, and adipo-geni...
Dyskusje
Contribution of MSCs to tumor environment is regulated by several interactions including hybrid cell generation via cell fusions, entosis or cytokine and chemokine activities between stem cells and cancer cells28. Structural organization, cell-cell interactions, and secreted factors determine cancer cell behavior in terms of tumor promotion, progression, and metastasis to surrounding tissue. Proper ex vivo model systems to investigate the mechanisms behind the interactions of res...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This study was supported by Yeditepe University. All data and figures used in this article were previously published34.
Materiały
| Name | Company | Catalog Number | Comments |
| DMEM | Invitrogen | 11885084 | For cell culture |
| FBS | Invitrogen | 16000044 | For cell culture |
| PSA | Lonza | 17-745E | For cell culture |
| Trypsin | Invitrogen | 25200056 | For cell dissociation |
| PBS | Invitrogen | 10010023 | For washes |
| Dexamethasone | Sigma | D4902 | Component of differentiation media |
| β-Glycerophosphate | Sigma | G9422 | Component of osteogenic differentiation medium |
| Ascorbic acid | Sigma | A4544 | Component of osteo- and chondro-genic differentiation medium |
| Insulin-Transferrin-Selenium (ITS −G) | Invitrogen | 41400045 | Component of chondrogenic differentiation medium |
| TGF-β | Sigma | SRP3171 | Component of chondrogenic differentiation medium |
| Insulin | Sigma | I6634 | Component of adipogenic differentiation medium |
| Isobutyl-1-methylxanthine (IBMX) | Sigma | I7018 | Component of adipogenic differentiation medium |
| Indomethacin | Sigma | I7378 | Component of adipogenic differentiation medium |
| MTS Reagent | Promega | G3582 | Cell viability analyses |
| TUNEL Assay | Sigma | 11684795910 | Apoptotic analyses |
| 24-well plate inserts | Corning | 3396 | For trans-well migration assay |
| PKH67 | Sigma | PKH67GL | For co-culture cell staining |
| PKH26 | Sigma | PKH26GL | For co-culture cell staining |
| Paraformaldehyde | Sigma | P6148 | For cell fixation |
| von Kossa Kit | BioOptica | 04-170801.A | For cell staining (differentiation) |
| Alcian blue | Sigma | A2899 | For cell staining (differentiation) |
Odniesienia
- Camberlain, G., Fox, J., Ashton, B., Middleton, J. Mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 25 (11), 2739-2749 (2007).
- Demirci, S., Doğan, A., Şahin, F. . Dental Stem Cells. , 109-124 (2016).
- Fox, J. M., Chamberlain, G., Ashton, B. A., Middleton, J. Recent advances into the understanding of mesenchymal stem cell trafficking. British journal of haematology. 137 (6), 491-502 (2007).
- Chang, A. I., Schwertschkow, A. H., Nolta, J. A., Wu, J. Involvement of mesenchymal stem cells in cancer progression and metastases. Current cancer drug targets. 15 (2), 88-98 (2015).
- Dvorak, H. F. Tumors: wounds that do not heal. New England Journal of Medicine. 315 (26), 1650-1659 (1986).
- Lu, Y. -. r., et al. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer biology & therapy. 7 (2), 245-251 (2008).
- Secchiero, P., et al. Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin's lymphoma xenografts. PloS one. 5 (6), e11140 (2010).
- Hong, I. -. S., Lee, H. -. Y., Kang, K. -. S. Mesenchymal stem cells and cancer: friends or enemies?. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 768, 98-106 (2014).
- Brennen, W. N., Chen, S., Denmeade, S. R., Isaacs, J. T. Quantification of Mesenchymal Stem Cells (MSCs) at sites of human prostate cancer. Oncotarget. 4 (1), 106 (2013).
- Klopp, A. H., Gupta, A., Spaeth, E., Andreeff, M., Marini, F. Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth. Stem cells. 29 (1), 11-19 (2011).
- Albini, A., Sporn, M. B. The tumour microenvironment as a target for chemoprevention. Nature Reviews Cancer. 7 (2), 139 (2007).
- Quante, M., et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer cell. 19 (2), 257-272 (2011).
- Gronthos, S., Mankani, M., Brahim, J., Robey, P. G., Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences. 97 (25), 13625-13630 (2000).
- Mori, G., et al. Dental pulp stem cells: osteogenic differentiation and gene expression. Annals of the new York Academy of Sciences. 1237 (1), 47-52 (2011).
- Mori, G., et al. Osteogenic properties of human dental pulp stem cells. Journal of biological regulators and homeostatic agents. 24 (2), 167-175 (2010).
- Kerkis, I., et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 184 (3-4), 105-116 (2006).
- Potdar, P. D., Jethmalani, Y. D. Human dental pulp stem cells: applications in future regenerative medicine. World journal of stem cells. 7 (5), 839 (2015).
- Ahmed, N. E. -. M. B., Murakami, M., Hirose, Y., Nakashima, M. Therapeutic potential of dental pulp stem cell secretome for Alzheimer's disease treatment: an in vitro study. Stem cells international. 2016, (2016).
- Gorin, C., et al. Priming dental pulp stem cells with fibroblast growth factor-2 increases angiogenesis of implanted tissue-engineered constructs through hepatocyte growth factor and vascular endothelial growth factor secretion. Stem cells translational medicine. 5 (3), 392-404 (2016).
- Wakayama, H., et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy. 17 (8), 1119-1129 (2015).
- Doğan, A., et al. Differentiation of human stem cells is promoted by amphiphilic pluronic block copolymers. International Journal of Nanomedicine. 7, 4849 (2012).
- Taşlı, P. N., Doğan, A., Demirci, S., Şahin, F. Boron enhances odontogenic and osteogenic differentiation of human tooth germ stem cells (hTGSCs) in vitro. Biological trace element research. 153 (1-3), 419-427 (2013).
- Yalvac, M., et al. Isolation and characterization of stem cells derived from human third molar tooth germs of young adults: implications in neo-vascularization, osteo-, adipo-and neurogenesis. The pharmacogenomics journal. 10 (2), 105 (2010).
- Doğan, A., et al. Sodium pentaborate pentahydrate and pluronic containing hydrogel increases cutaneous wound healing in vitro and in vivo. Biological trace element research. 162 (1-3), 72-79 (2014).
- Doğan, A., Yalvaç, M. E., Yılmaz, A., Rizvanov, A., Şahin, F. Effect of F68 on cryopreservation of mesenchymal stem cells derived from human tooth germ. Applied biochemistry and biotechnology. 171 (7), 1819-1831 (2013).
- Rizvanov, A. A., et al. Interaction and self-organization of human mesenchymal stem cells and neuro-blastoma SH-SY5Y cells under co-culture conditions: A novel system for modeling cancer cell micro-environment. European Journal of Pharmaceutics and Biopharmaceutics. 76 (2), 253-259 (2010).
- Troiano, L., et al. Multiparametric analysis of cells with different mitochondrial membrane potential during apoptosis by polychromatic flow cytometry. Nature protocols. 2 (11), 2719 (2007).
- Melzer, C., von der Ohe, J., Lehnert, H., Ungefroren, H., Hass, R. Cancer stem cell niche models and contribution by mesenchymal stroma/stem cells. Molecular cancer. 16 (1), 28 (2017).
- Aguirre, A., Planell, J., Engel, E. Dynamics of bone marrow-derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochemical and biophysical research communications. 400 (2), 284-291 (2010).
- Bogdanowicz, D. R., Lu, H. H. . Biomimetics and Stem Cells. , 29-36 (2013).
- Plotnikov, E., et al. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. Journal of cellular and molecular. 12 (5a), 1622-1631 (2008).
- Bogdanowicz, D. R., Lu, H. H. Studying cell-cell communication in co-culture. Biotechnology journal. 8 (4), 395-396 (2013).
- Brunetti, G., et al. High expression of TRAIL by osteoblastic differentiated dental pulp stem cells affects myeloma cell viability. Oncology reports. 39 (4), 2031-2039 (2018).
- Doğan, A., Demirci, S., Apdik, H., Apdik, E. A., Şahin, F. Dental pulp stem cells (DPSCs) increase prostate cancer cell proliferation and migration under in vitro conditions. Tissue and Cell. 49 (6), 711-718 (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone