Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A Uniform Shear Assay for Human Platelet and Cell Surface Receptors via Cone-plate Viscometry
W tym Artykule
Podsumowanie
We describe an in-solution method to apply uniform shear to platelet surface receptors using cone-plate viscometry. This method may also be used more broadly to apply shear to other cell types and cell-fragments and need not target a specific ligand-receptor pair.
Streszczenie
Many biological cells/tissues sense the mechanical properties of their local environments via mechanoreceptors, proteins that can respond to forces like pressure or mechanical perturbations. Mechanoreceptors detect their stimuli and transmit signals via a great diversity of mechanisms. Some of the most common roles for mechanoreceptors are in neuronal responses, like touch and pain, or hair cells which function in balance and hearing. Mechanosensation is also important for cell types which are regularly exposed to shear stress such as endothelial cells, which line blood vessels, or blood cells which experience shear in normal circulation. Viscometers are devices that detect the viscosity of fluids. Rotational viscometers may also be used to apply a known shear force to fluids. The ability of these instruments to introduce uniform shear to fluids has been exploited to study many biological fluids including blood and plasma. Viscometry may also be used to apply shear to the cells in a solution, and to test the effects of shear on specific ligand-receptor pairs. Here, we utilize cone-plate viscometry to test the effects of endogenous levels of shear stress on platelets treated with antibodies against the platelet mechanosensory receptor complex GPIb-IX.
Wprowadzenie
Mechanoreceptors are proteins that respond to mechanical stimuli, such as pressure or mechanical perturbation/deformation. For some mechanoreceptors, sensing these mechanical perturbations is explicit to the function of the cell types in which they are expressed. Take, for example, the stretch receptors in baroreceptor neurons; these mechanosensitive ion channels regulate blood pressure by sensing vascular "stretch"1,2. In the inner ear, ion channels on hair cells detect mechanical deformations caused by sound waves3, and cutaneous low threshold mechanoreceptors (LTMRs) facilitate the transmission of tactile information4. In other cases, mechanoreceptors provide important information to the cell for the establishment of adhesion or growth. Cells can sense the rigidity of their local environment, and may rely on contractile forces via the actin cytoskeleton and integrins to dictate growth or spreading5,6.
When studying receptor-ligand interactions in cell or tissue-based models, common assays exist which can quickly and accurately report the effects of altering temperature, pH, ligand concentration, tonicity, membrane potential, and many other parameters which can vary in vivo. However, these same assays may fall short when it comes to detecting the contribution of mechanical force to receptor activation. Whether cells are sensing their microenvironment, detecting sound waves, or responding to stretch, one thing the aforementioned mechanoreceptors have in common is that they are participating in interactions where the ligand, receptor, or both, are anchored to a surface. Assays developed to test the effects of mechanical forces on receptor interactions often reflect this paradigm. Microfluidics and flow chambers are used to study the effects of shear flow on cells and receptors7,8. These types of experiments have the advantage of allowing fine-tuning of shear rates via established flow speeds. Other techniques employ fluorescent molecular probes to detect forces applied by cells on ligand-rich surfaces, yielding an accurate readout of the magnitude and orientations of forces involved in the interaction9,10.
In addition to mechanosensation occurring where one or both partners are anchored to a surface, shear stress may affect proteins and cells in solution. This is often observed in blood cells/proteins which are constantly in the circulation, and may manifest via activation of mechanoreceptors that are normally surface-anchored11, or through exposure of target sequences which would be occluded under static conditions12. However, relatively fewer techniques assay the effects of shear force on particles in solution. Some in-solution approaches introduce shear via vortexing cells in fluid suspension with varying speeds and durations, although these approaches may not allow a very precise determination of the shear stress generated. Rotational viscometers measure viscosity by applying a specific shear force to fluids. Herein we describe an applied method for determining the effect of specific laminar shear rates on cells or cell fragments in solution.
One of the most highly expressed proteins on the platelet surface is the glycoprotein (GP) Ib-IX complex. GPIb-IX is the primary receptor for the plasma protein von Willebrand Factor (VWF). Together, this receptor-ligand pair has long been recognized as the foundation of the platelet response to shear stress13. In the event of vascular damage, VWF binds to exposed collagen in the sub-endothelial matrix14, thus recruiting platelets to the site of injury via the VWF-GPIb-IX interaction. VWF engagement to its binding site in the GPIbα subunit if GPIb-IX under physiological shear stress induces unfolding of a membrane-proximal mechanosensory domain (MSD) which in turn activates GPIb-IX15. In a recent study, we have shown that antibodies against GPIbα, like those generated in many immune thrombocytopenia (ITP) patients, are also capable of inducing platelet signaling via MSD unfolding under shear stress11. However, unlike VWF, which facilitates shear-induced GPIb-IX activation by immobilizing the complex under normal circulation, the bivalent antibodies are able to crosslink platelets via GPIb-IX and unfold the MSD in circulation. In this way, a mechanoreceptor which is normally activated by surface immobilization under shear can be activated in solution. In the present report, we will demonstrate how a viscometer-based uniform shear assay was leveraged to detect the effects of specific levels of shear stress on receptor activation in solution.
Protokół
All methods using donor-derived human platelets described herein were approved by the Institutional Review Board of Emory University/Children's Healthcare of Atlanta.
1. Blood Draw and Platelet Isolation
- Draw human blood from consenting healthy adult donors via venipuncture on the day of the experiment into 3.8% trisodium citrate. One 4.5 mL tube of blood is sufficient to yield enough platelet rich plasma (PRP) for 20-25 conditions in donors whose platelet counts are close to 250 x 103 per μL.
NOTE: Avoid drawing blood via narrow gauge needles (smaller than 21 G). - Prepare PRP via centrifugation at 22 °C and 140 x g for 12 min with a long brake. This will result in two distinct layers, with red blood cells at the bottom and the light colored PRP at the top.
- Isolate the top, cloudy, yellow layer of PRP via careful pipetting through a pipette tip cut at a 45° angle and obtain the platelet count via a complete blood count (CBC).
- If necessary, wash platelets in PIPES-buffered saline (150 mM NaCl, 20 mM PIPES) in the presence of prostaglandin E1 (PGE1) and resuspend in Tyrode's buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 1 mM MgCl2, 5 mM glucose, 12 mM NaHCO3, 20 mM HEPES, pH 7.35) with 5 mM glucose, otherwise, proceed to step 1.5. The following steps describe the washing in brief.
- Adjust PRP volume to 10 mL with PIPES-buffered saline and add 0.6 μM PGE1.
- Centrifuge for 8 min at 1,900 x g, then discard supernatant and let the platelet pellet sit in 400 μL of Tyrode's and glucose solution for 5 min.
- Gently resuspend the platelet pellet and keep it undisturbed for 30 min.
- Adjust the platelet count to ~250 x 103 platelets per μL with pooled human platelet-poor plasma (PPP) and maintain the suspension at 22 °C undisturbed or under gentle rotation.
2. Ligand and uniform shear treatment
NOTE: All steps in section 2 that require pipetting should be done slowly, so as not to introduce any shear.
- Add the desired antibody or ligand to the PRP or washed platelets and mix gently by pipetting up and down or stirring with a pipette tip. Leave it undisturbed at room temperature for 5-10 min. Add an equivalent volume of PPP or Tyrode's buffer to a negative control.
- Turn on the cone-plate viscometer, set the plate temperature to 22 °C and allow time to let the plate reach this temperature.
- Pipette the treated PRP or washed platelets onto the temperature-controlled cone-plate viscometer directly at the center of the plate. Ensure that all of the sample is deposited between the cone and plate at the point of contact, and not on the outside of the cone’s rim.
- Shear at an appropriate rate and duration.
- Calculate shear as indicated by the viscometer manual, or as previously shown15,16.
- Determine shear rate from viscosity and desired shear stress via Newton's law of viscosity;
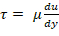 ; plasma viscosity is 1.5-1.6 centipoise (cP)17. For example, a normal shear range for human circulation is 5-30 dyn/cm2 and shear should be applied on the single digit minute time scale.
; plasma viscosity is 1.5-1.6 centipoise (cP)17. For example, a normal shear range for human circulation is 5-30 dyn/cm2 and shear should be applied on the single digit minute time scale.
- Lift the cone off of the plate slighlty (~2 mm) so that the sample remains in contact with both the plate and cone, and use a gel-loading or other long pipette tip to collect 5-10 μL from the center of the sample volume.
- Incubate the sheared samples with the desired markers for 20 min at room temperature. For markers of phosphatidylserine, β-galactose, and P-selectin exposure use Lactadherin C2 domain (LactC2) at 0.08 μM18, Erythrina cristagalli lectin (ECL) at 6.25 μg/mL, and anti-P-selectin antibody (20 µg/mL), respectively.
- Fix samples in 2% paraformaldehyde for 20 min at RT prior to dilution or cold storage, and proceed to step 3.1 or store samples at 4 °C for no longer than 12 h.
3. Detection of surface markers and crosslinking via flow cytometry
- Analyze the sample via flow cytometry, collecting at least 20,000 events for each condition.
- Quantitate signal strength of the fluorescent markers using the height value for the intensity of each fluorophore, or the geometric mean fluorescence intensity (MFI).
- If aiming to detect platelet crosslinking following shear treatment, analyze the sample on an imaging-capable flow cytometer and quantitate crosslinking by area and aspect ratio parameters.
- Plot a histogram of area and/or aspect ratio.
- Use a negative control with bovine serum albumin (BSA) or vehicle to draw a gate excluding most fully circular events (this gate is usually drawn at an aspect ratio ~0.819,20) and quantitate the percentage of events inside of this gate. Events with a lower aspect ratio are more likely to be crosslinked.
Wyniki
Figure 1 outlines how the trigger model of GPIb-IX activation, initially introduced to explain shear-dependent receptor activation when anchored to the vessel wall, may also support activation of platelets crosslinked by a multivalent ligand. Figure 2 shows readouts of human platelet activation treated by two antibodies targeting the N-terminal domain of GPIb-IX (6B4 and 11A8), and one control antibody (normal IgG) under sheared and static conditions. In
Dyskusje
The protocol described in this manuscript allows quick and versatile assessment of the effect of laminar shear on platelet and cell surface receptors. The specific representative results presented here underscore how the effects of multimeric or bivalent ligands can be affected by shear flow. In addition to this application, a uniform shear assay has broad applications in observing shear-dependent effects. In the absence of a known ligand-receptor pair, a uniform shear assay can also detect the effects of shear on factor...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
Work pertinent to this study was supported in part by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute grants HL082808, HL123984 (R.L.), and F31HL134241 (M.E.Q.). Funding also provided by NIH National Institute of General Medical Sciences grant T32GM008367 (M.E.Q.); and pilot grant funds from Children’s Healthcare of Atlanta and Emory University Pediatric Flow Cytometry Core. The authors would like to thank Dr. Hans Deckmyn for sharing the 6B4 antibody, and the Emory Children's Pediatric Research Center Flow Cytometry Core for technical support.
Materiały
| Name | Company | Catalog Number | Comments |
| APC anti-human CD62P (P-Selectin) | BioLegend | 304910 | |
| Brookfield Cap 2000+ Viscometer | Brookfield | - | |
| FITC-conjugated Erythrina cristagalli lectin (ECL) | Vector Labs | FL-1141 | |
| Pooled Normal Human Plasma | Precision Biologic | CCN-10 | |
| Vacutainer Light Blue Blood Collection Tube (Sodium Citrate) | BD | 369714 | |
| Vacutainer Blood Collection Set, 21G x ¾" Needle | BD | 367287 |
Odniesienia
- Sullivan, M. J., et al. Non-voltage-gated Ca2+ influx through mechanosensitive ion channels in aortic baroreceptor neurons. Circulation Research. 80 (6), 861-867 (1997).
- Lansman, J. B., Hallam, T. J., Rink, T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers. Nature. 325 (6107), 811-813 (1987).
- Fettiplace, R. Hair Cell Transduction, Tuning, and Synaptic Transmission in the Mammalian Cochlea. Comprehensive Physiology. 7 (4), 1197-1227 (2017).
- Zimmerman, A., Bai, L., Ginty, D. D. The gentle touch receptors of mammalian skin. Science. 346 (6212), 950-954 (2014).
- Nelson, C. M., et al. Emergent patterns of growth controlled by multicellular form and mechanics. Proceedings of the National Academy of Sciences USA. 102 (33), 11594-11599 (2005).
- Yu, C. H., Law, J. B., Suryana, M., Low, H. Y., Sheetz, M. P. Early integrin binding to Arg-Gly-Asp peptide activates actin polymerization and contractile movement that stimulates outward translocation. Proceedings of the National Academy of Sciences USA. 108 (51), 20585-20590 (2011).
- Wen, L., et al. A shear-dependent NO-cGMP-cGKI cascade in platelets acts as an auto-regulatory brake of thrombosis. Nature Communications. 9 (1), 4301 (2018).
- Marki, A., Gutierrez, E., Mikulski, Z., Groisman, A., Ley, K. Microfluidics-based side view flow chamber reveals tether-to-sling transition in rolling neutrophils. Scientific Reports. 6, 28870 (2016).
- Brockman, J. M., et al. Mapping the 3D orientation of piconewton integrin traction forces. Nature Methods. 15 (2), 115-118 (2018).
- Wang, X., et al. Constructing modular and universal single molecule tension sensor using protein G to study mechano-sensitive receptors. Scientific Reports. 6, 21584 (2016).
- Quach, M. E., et al. Fc-independent immune thrombocytopenia via mechanomolecular signaling in platelets. Blood. 131 (7), 787-796 (2018).
- Cao, W., Krishnaswamy, S., Camire, R. M., Lenting, P. J., Zheng, X. L. Factor VIII accelerates proteolytic cleavage of von Willebrand factor by ADAMTS13. Proceedings of the National Academy of Sciences USA. 105 (21), 7416-7421 (2008).
- Kroll, M. H., Hellums, J. D., McIntire, L. V., Schafer, A. I., Moake, J. L. Platelets and shear stress. Blood. 88 (5), 1525-1541 (1996).
- Pareti, F. I., Niiya, K., McPherson, J. M., Ruggeri, Z. M. Isolation and characterization of two domains of human von Willebrand factor that interact with fibrillar collagen types I and III. Journal of Biological Chemistry. 262 (28), 13835-13841 (1987).
- Deng, W., et al. Platelet clearance via shear-induced unfolding of a membrane mechanoreceptor. Nature Communications. 7, 12863 (2016).
- Ikeda, Y., et al. The role of von Willebrand factor and fibrinogen in platelet aggregation under varying shear stress. Journal of Clinical Investigation. 87 (4), 1234-1240 (1991).
- Westerhof, N., Stergiopulos, N., Noble, M. I. . Snapshots of hemodynamics: an aid for clinical research and graduate education. , (2010).
- Liang, X., et al. Specific inhibition of ectodomain shedding of glycoprotein Ibalpha by targeting its juxtamembrane shedding cleavage site. Journal of Thrombosis and Haemostasis. 11 (12), 2155-2162 (2013).
- Samsel, L., et al. Imaging flow cytometry for morphologic and phenotypic characterization of rare circulating endothelial cells. Cytometry Part B: Clinical Cytometry. 84 (6), 379-389 (2013).
- Basiji, D. A., Ortyn, W. E., Liang, L., Venkatachalam, V., Morrissey, P. Cellular image analysis and imaging by flow cytometry. Clinics in Laboratory Medicine. 27 (3), 653-670 (2007).
- Quach, M. E., Chen, W., Li, R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood. 131 (14), 1512-1521 (2018).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone