Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Laser-Induced Brain Injury in the Motor Cortex of Rats
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Erratum Notice
Podsumowanie
The protocol presented here shows a technique to create a rodent model of brain injury. The method described here uses laser irradiation and targets motor cortex.
Streszczenie
A common technique for inducing stroke in experimental rodent models involves the transient (often denoted as MCAO-t) or permanent (designated as MCAO-p) occlusion of the middle cerebral artery (MCA) using a catheter. This generally accepted technique, however, has some limitations, thereby limiting its extensive use. Stroke induction by this method is often characterized by high variability in the localization and size of the ischemic area, periodical occurrences of hemorrhage, and high death rates. Also, the successful completion of any of the transient or permanent procedures requires expertise and often lasts for about 30 minutes. In this protocol, a laser irradiation technique is presented that can serve as an alternative method for inducing and studying brain injury in rodent models.
When compared to rats in the control and MCAO groups, the brain injury by laser induction showed reduced variability in body temperature, infarct volume, brain edema, intracranial hemorrhage, and mortality. Furthermore, the use of a laser-induced injury caused damage to the brain tissues only in the motor cortex unlike in the MCAO experiments where destruction of both the motor cortex and striatal tissues is observed.
Findings from this investigation suggest that laser irradiation could serve as an alternative and effective technique for inducing brain injury in the motor cortex. The method also shortens the time for completing the procedure and does not require expert handlers.
Wprowadzenie
Globally, stroke is the second leading cause of death and the third leading cause of disability1. Stroke also leads to severe disability, often requiring extra care from medical staff and relatives. There is, therefore, a need to understand the complications associated with the disorder and improve the potential for more positive outcomes.
The use of animal models is the initial step to understanding diseases. To ensure the best research outcomes, a typical model would include a simple technique, affordability, high reproducibility, and minimal variability. The determinants in ischemic stroke models include brain edema volume, infarct size, the extent of the blood-brain barrier (BBB) breakdown, and functional impairment generally evaluated via neurological severity score2.
The most widely used stroke induction technique in rodent models occludes the middle cerebral artery (MCA) transiently or permanently3. This technique produces a stroke model similar to the ones in humans: it has a penumbra surrounding the stroked area, is highly reproducible, and regulates ischemia duration and reperfusion4. Nevertheless, the MCAO method has some complications. The technique is prone to intracranial hemorrhage and injury to the ipsilateral retina with a dysfunction of the visual cortex and common hyperthermia that often lead to additional outcomes5,6,7. Other limitations include high variations in induced stroke (arising from the probable extension of the ischemia to unintended regions, like the external carotid artery region), insufficient occlusion of the MCA, and premature reperfusion. Also, rats of different strains and sizes exhibit various infarct volumes8. In addition to all the disadvantages mentioned, MCAO model cannot induce small isolated strokes in deep brain areas, because it is limited technically in terms of its requirement of minimum vessel size for catheterization. This makes the need for an alternative model all the more critical. Another method, photothrombosis, provides a possible alternative to MCAO procedures but does not improve on the efficiency9. This technique targets stroke with light and offers some improvements on the previous models. However, photothrombosis requires an invasive craniotomy that is associated with secondary compications9.
In the light of outlined shortcomings, the protocol presented here provides a capable alternative laser technique for inducing brain injury in rodents. The mechanism of action of the laser technique is based on the laser’s photothermal effects imparted on living tissues, which leads to the absorption of light beams by body tissues and their conversion into heat. The advantages of using a laser technique are its safety and ease of manipulation. A laser’s ability to produce heat to stop bleeding makes it very important in medicine, while its ability to amplify different beams at a given meet point ensures that lasers avoid destroying healthy tissues that stands in the way of the target point10. The laser beam used in this protocol can pass through a low liquid medium, such as bone, without emitting its energy and/or causing any destruction. Once it reaches a high liquid medium, such as brain tissues, it uses up its energy to destroy the target tissues. The technique, therefore, can induce brain injury only in the appropriate area of the brain.
The technique presented here showed a tremendous amount of ability to regulate its levels of irradiation, producing the chosen variations of brain injury intended from the start. Unlike the original MCAO that impacts both the cortex and striatum, the laser technique was able to regulate the impact of brain injury, inducing injury only on the intended motor cortex. Herein, the laser-induced brain injury protocol and a summary of representative results for the procedure performed on the cerebral cortex of rats are provided.
Protokół
The following procedure was conducted according to the Guidelines of the Use of Experimental Animals of the European Community. The experiments were also approved by the Animal Care Committee at the Ben-Gurion University of the Negev.
1. Animal selection and preparation
- Select 65 male Sprague-Dawley rats weighing 300 to 350 g with no overt pathology for this procedure. The smaller size poses technical difficulties for the MCAO procedure.
- Assign 3 rats per cage and let them adapt for least 3 days.
2. MCAO procedure
- Select 25 rats for MCAO allowing for 10—20% mortality associated with the procedure11.
- Perform MCAO using a standard technique, as previously described in detail12.
3. Laser-induced brain injury experimental procedure
- Assign 20 rats to a group marked as laser group and 20 rats to another control group (sham-operated).
- Subject the laser group rats to laser irradiation at 50J X 10 points in the following manner:
- Anesthetize rat with a mixture of 2% isoflurane in oxygen allowing for the spontaneous ventilation. Check for sufficient anesthetic depth by pinching the tail with forceps to see the absence of the withdrawal reflex.
- Maintain the core body temperature of the rat at 37 ˚C throughout the experimental procedure using a rectal temperature regulated heating pad.
- Remove local hair with a shaver and disinfect with 70% alcohol and 0.5% chlorhexidine gluconate. Repeat the disinfection step two more times.
NOTE: The size of the surgical incision should be approximately 3 cm. Remove hair at least 2 cm around the incision area. - Place the rat on a stereotaxic head holder in a prone position and make a 3 cm incision to reflect the scalp laterally and to expose the area between Bregma and Lambda.
- Maintain anesthesia through the nose cone.
- Use Neodymium-YAG (Nd-YAG) laser (peak wavelength 1064 nm) to administer 50J X 10 points, with 1 s pulse duration, to the exposed area of the skull above the right hemisphere.
- Ensure that the laser generating part of the apparatus is at a 2 mm distance from the exposed area to produce a laser beam. 50J X 10 points was selected after careful evaluation of different energy/surface combinations. This combination is efficient and does not cause bone destruction of the skull after administration for less than a second10.
NOTE: 2 mm is the distance between the terminal of the laser beam (from the optical cable it is passed through) and the skull bone. In case a focusing lens is used, the distance should be calculated taking into account the angle of inclination of the lens to focus the beam in the desired area of damage. Ensure proper safety when using a laser device including appropriate training and eye protection. - Remove the rat from the device and close the scalp with 3-0 silk surgical sutures.
- Discontinue anesthesia and return the rat to its cage for recovery. Administer 0.1 mL of 0.25% bupivacaine locally to reduce the postoperative pain immediately after surgery.
NOTE: The entire procedure should last less than 5 min if performed correctly.
- Observe the rat for any signs of distress during post- anesthesia recovery. Prior to emergence from anesthesia, give 0.01mg/kg intramuscular buprenorphine for postoperative analgesia and continue with repeated doses every 12 h for at least 48 h.
- Subject control rats to the same conditions without subjecting them to the laser.
4. Neurological severity score (NSS)
- Evaluate the neurological severity score 24 h after the laser-induced brain injury using a 43-point score13. Test the animals for neurological deficits, behavior disturbances, beam-balancing task, and reflexes, assigning higher scores for more severe disabilities, as previously detailed13.
5. Post-injury manipulations
- After NSS evaluation, euthanize the rats by exposing them to 20% oxygen and 80% CO2 (via inspiration) and transcardially perfuse the rat with heparinized phosphate-buffered saline (PBS, 0.9% NaCl).
NOTE: Ensure that CO2 is delivered at a predetermined rate in accordance with the Institutional Animal Care and Use Committee guidelines. This step may also be performed under 5% isoflurane anesthesia. - Harvest brains and prepare for further examination as described in an earlier protocol11.
- Evaluate for subarachnoid hemorrhage (SAH) through visual examination of the whole brain after its isolation from the skull. If necessary, a microscope or magnifying glasses may be used for this purpose.
6. Evaluation of the brain injury
- Determining the brain infarct volume and brain edema by TTC staining
NOTE: 2,3,5-Triphenyltetrazolium chloride (TTC) staining is a convenient procedure for brain infarct detection11.- Section the harvested brains into 6 coronal slices, each 2 mm thickness.
- Incubate the set of slices from each brain for 30 min at 37 °C in 0.05% TTC.
- Following staining, scan the slices with an optical scanner with a resolution of 1600 X 1600 dpi.
- The unstained areas of the fixed brain slices are defined as infarcted12.
- Using an image processing software (e.g., freeware Image J) measure the unstained infarcted area, ipsi- and contralateral hemispheres for each of the 6 coronal slices.
- Calculate the infarcted volume as a percentage of the total brain:
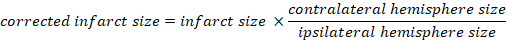
- Calculate brain edema using Kaplan method:
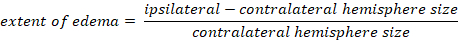
- Determining the extent of blood brain barrier (BBB) breakage
NOTE: Assess BBB breakage 24 h after the laser-induced brain injury as follows:- Administer 2% Evans Blue mixed with 4 mL/kg saline solution intravenously to rats via the cannulated tail vein and allow the solution to circulate for 1 h.
- Euthanize rats by exposing them to 20% oxygen and 80% CO2 (via inspiration) 24 h after the last NSS, as previously described13.
- Harvest the intravascularly localized dye as follows:
- Open the chests of the rats with surgical pincettes and surgical scissors.
- Perfuse the animals with cooled 0.9% saline via the left ventricle using 110 mmHg until obtaining a colorless perfusion liquid from the right atrium.
- Harvest the brains and slice them rostrocaudally into 2 mm slices.
- Separate the left brain slices from the right portions to evaluate injured and non-injured hemispheres separately.
- Weigh, homogenize using mortar and pestle, and then incubate the brain tissues in 50% trichloroacetic acid for 24 h.
- Centrifuge the homogenized brain slices at 10,000 × g for 20 min.
- Mix 1 mL of the supernatant from the centrifuged brain with 1.5 mL of 96% ethanol at 1:3 and assess blood-brain barrier breakage using a fluorescence detector at 620 nm excitation wavelength (10 nm bandwidth) and 680 nm emission wavelength (10 nm bandwidth).
NOTE: Both groups of rats undergo the same protocol for determining BBB breakdown.
Wyniki
No deaths or SAH were registered in either the control or experimental groups (Table 1). The MCAO group had a 20% rate of both mortality and SAH.
The relative body temperature changes in the rats of both groups were also similar, despite a difference in the variability of both groups (Table 1).
There was a significantly worse NSS in both the laser (16 ± 1.1) and MCAO (20 ± 1.5) models, compared to the sham-operated control...
Dyskusje
It is fair to assume that the laser technique is minimally invasive, given that no deaths or SAH occurred in the laser group. The primary cause of death and SAH is the damage to blood vessels that leads to an elevation of intracranial pressure (ICP), as shown in the original MCAO techniques10. The absence of death and SAH in the laser group is likely due to the specific effects of lasers: they do not have direct impact on blood vessels and can induce coagulation in case of leakage. Low infarct vol...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We would like to thank the Department of Anesthesiology of Soroka University Medical Center and the laboratory staff of Ben-Gurion University of the Negev for their help in the performance of this experiment.
Materiały
| Name | Company | Catalog Number | Comments |
| 2,3,5-Triphenyltetrazolium chloride | SIGMA - ALDRICH | 298-96-4 | |
| 50% trichloroacetic acid | SIGMA - ALDRICH | 76-03-9 | |
| Brain & Tissue Matrices | SIGMA - ALDRICH | 15013 | |
| Cannula Venflon 22 G | KD-FIX | 1.83604E+11 | |
| Centrifuge Sigma 2-16P | SIGMA - ALDRICH | Sigma 2-16P | |
| Compact Analytical Balances | SIGMA - ALDRICH | HR-AZ/HR-A | |
| Digital Weighing Scale | SIGMA - ALDRICH | Rs 4,000 | |
| Dissecting scissors | SIGMA - ALDRICH | Z265969 | |
| Eppendorf pipette | SIGMA - ALDRICH | Z683884 | |
| Eppendorf Tube | SIGMA - ALDRICH | EP0030119460 | |
| Ethanol 96 % | ROMICAL | Flammable Liquid | |
| Evans Blue 2% | SIGMA - ALDRICH | 314-13-6 | |
| Fluorescence detector | Tecan, Männedorf Switzerland | model Infinite 200 PRO multimode reader | |
| Heater with thermometer | Heatingpad-1 | Model: HEATINGPAD-1/2 | |
| Infusion Cuff | ABN | IC-500 | |
| Isofluran, USP 100% | Piramamal Critical Care, Inc | NDC 66794-017 | |
| Multiset | TEVA MEDICAL | 998702 | |
| Olympus BX 40 microscope | Olympus | ||
| Optical scanner | Canon | Cano Scan 4200F | |
| Petri dishes | SIGMA - ALDRICH | P5606 | |
| Scalpel blades 11 | SIGMA - ALDRICH | S2771 | |
| Sharplan 3000 Nd:YAG (neodymium-doped yttrium aluminum garnet) laser machine | Laser Industries Ltd | ||
| Stereotaxic head holder | KOPF | 900LS | |
| Sterile Syringe 2 ml | Braun | 4606027V | |
| Syringe-needle 27 G | Braun | 305620 |
Odniesienia
- World Health Organization. Global health estimates: deaths by cause, age, sex and country, 2000-2012. World Health Organization. 9, (2014).
- Meadows, K. L. Experimental models of focal and multifocal cerebral ischemia: a review. Reviews in the Neurosciences. 29, 661-674 (2018).
- Durukan, A., Strbian, D., Tatlisumak, T. Rodent models of ischemic stroke: a useful tool for stroke drug development. Current Pharmaceutical Designs. 14, 359-370 (2008).
- Fluri, F., Schuhmann, M. K., Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Design, Development and Therapy. 9, 3445-3454 (2015).
- Li, F., Omae, T., Fisher, M. Spontaneous hyperthermia and its mechanism in the intraluminal suture middle cerebral artery occlusion model of rats. Stroke. 30, 2464-2470 (1999).
- Boyko, M., et al. An experimental model of focal ischemia using an internal carotid artery approach. Journal of Neuroscience Methods. 193, 246-253 (2010).
- Zhao, Q., Memezawa, H., Smith, M. L., Siesjo, B. K. Hyperthermia complicates middle cerebral artery occlusion induced by an intraluminal filament. Brain Research. 649, 253-259 (1994).
- Braeuninger, S., Kleinschnitz, C. Rodent models of focal cerebral ischemia: procedural pitfalls and translational problems. Experimental and Translational Stroke Medicine. 1, 8 (2009).
- Choi, B. I., et al. Neurobehavioural deficits correlate with the cerebral infarction volume of stroke animals: a comparative study on ischaemia-reperfusion and photothrombosis models. Environmental Toxicology and Pharmacology. 33, 60-69 (2012).
- Boyko, M., et al. An Alternative Model of Laser-Induced Stroke in the Motor Cortex of Rats. Biological Procedure Online. 21, 9 (2019).
- Bleilevens, C., et al. Effect of anesthesia and cerebral blood flow on neuronal injury in a rat middle cerebral artery occlusion (MCAO) model. Experimental Brain Research. 224, 155-164 (2013).
- Kuts, R., et al. A Middle Cerebral Artery Occlusion Technique for Inducing Post-stroke Depression in Rats. Journal of Visualized Experiments. (147), e58875 (2019).
- Boyko, M., et al. Morphological and neuro-behavioral parallels in the rat model of stroke. Behavioural Brain Research. 223, 17-23 (2011).
Erratum
Formal Correction: Erratum: Laser-Induced Brain Injury in the Motor Cortex of Rats
Posted by JoVE Editors on 2/07/2022. Citeable Link.
An erratum was issued for: Laser-Induced Brain Injury in the Motor Cortex of Rats. The Authors section was updated.
One of the author names was updated from:
Dmitri Frank
to
Dmitry Frank
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone