Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Bovine Ovarian Cortex Tissue Culture
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
In vitro culture of bovine ovarian cortex and the effect of nutritional Stair-step diet on ovarian microenvironment is presented. Ovarian cortex pieces were cultured for seven days and steroids, cytokines, and follicle stages were evaluated. The Stair-Step diet treatment had increased steroidogenesis resulting in follicle progression in culture.
Streszczenie
Follicle development from the primordial to antral stage is a dynamic process within the ovarian cortex, which includes endocrine and paracrine factors from somatic cells and cumulus cell-oocyte communication. Little is known about the ovarian microenvironment and how the cytokines and steroids produced in the surrounding milieu affect follicle progression or arrest. In vitro culture of ovarian cortex enables follicles to develop in a normalized environment that remains supported by adjacent stroma. Our objective was to determine the effect of nutritional Stair-Step diet on the ovarian microenvironment (follicle development, steroid, and cytokine production) through in vitro culture of bovine ovarian cortex. To accomplish this, ovarian cortical pieces were removed from heifers undergoing two different nutritionally developed schemes prior to puberty: Control (traditional nutrition development) and Stair-Step (feeding and restriction during development) that were cut into approximately 0.5-1 mm3 pieces. These pieces were subsequently passed through a series of washes and positioned on a tissue culture insert that is set into a well containing Waymouth's culture medium. Ovarian cortex was cultured for 7 days with daily culture media changes. Histological sectioning was performed to determine follicle stage changes before and after the culture to determine effects of nutrition and impact of culture without additional treatment. Cortex culture medium was pooled over days to measure steroids, steroid metabolites, and cytokines. There were tendencies for increased steroid hormones in ovarian microenvironment that allowed for follicle progression in the Stair-Step versus Control ovarian cortex cultures. The ovarian cortex culture technique allows for a better understanding of the ovarian microenvironment, and how alterations in endocrine secretion may affect follicle progression and growth from both in vivo and in vitro treatments. This culture method may also prove beneficial for testing potential therapeutics that may improve follicle progression in women to promote fertility.
Wprowadzenie
The ovarian cortex represents the outer layer of the ovary where follicle development occurs1. Primordial follicles, initially arrested in development, will be activated to become primary, secondary, and then antral or tertiary follicles based on paracrine and gonadotropin inputs1,2,3,4. To better understand physiological processes within the ovary, tissue culture can be used as an in vitro model, thereby allowing for a controlled environment to conduct experiments. Many studies have utilized ovarian tissue culture for research in assisted reproductive technology, fertility preservation, and ovarian cancer5,6,7. Ovarian tissue culture has also served as a model for investigating reproductive toxins that damage the ovarian health and the etiology of reproductive disorders such as Polycystic Ovary Syndrome (PCOS)8,9,10,11. Thus, this culture system is applicable to a wide array of specialties.
In rodents, whole fetal or perinatal gonads have been used in reproductive biology experiments12,13,14,15. However, gonads from larger domestic livestock cannot be cultured as whole organs due to their large size and potential degeneration. Therefore, bovine, and non-human primate ovarian cortex is cut into smaller pieces16,17,18. Many studies have cultured small ovarian cortex pieces to study various growth factor(s) in primordial follicle initiation in domestic livestock and non-human primates1,17,18,19. The use of ovarian cortex culture has also demonstrated primordial follicle initiation in the absence of serum for bovine and primate cortical pieces cultured for 7 days20. Yang and Fortune in 2006 treated fetal ovarian cortex culture medium with a range of testosterone doses over 10 days and observed that the 10-7 M concentration of testosterone increased follicle recruitment, survival, and increased progression of early stage follicles19. In 2007, using ovarian cortex cultures from bovine fetuses (5-8 months of gestation), Yang and Fortune reported a role for Vascular Endothelial Growth Factor A (VEGFA) in the primary to secondary follicle transition21. Furthermore, our laboratory has utilized ovarian cortex cultures to demonstrate how VEGFA isoforms (angiogenic, antiangiogenic, and a combination) may regulate different signal transduction pathways through the Kinase domain receptor (KDR), which is the main signal transduction receptor that VEGFA binds16. This information allowed for a better understanding of how different VEGFA isoforms affect signaling pathways to elicit follicle progression or arrest. Taken together, culturing of ovarian cortex pieces in vitro with different steroids or growth factors can be a valuable assay to determine effects on mechanisms regulating folliculogenesis. Similarly, animals that are developed on different nutritional regimes may have altered ovarian microenvironments, which may promote or inhibit folliculogenesis affecting female reproductive maturity. Thus, our goal in the current manuscript is to report the bovine cortex culture technique and determine whether there are differences in ovarian microenvironments after in vitro culture of bovine cortex from heifers fed either Control or Stair-Step diets collected at 13 months of age as described previously16.
Therefore, our next step was to determine the ovarian microenvironment in these heifers that were developed with different nutritional diets. We evaluated ovarian cortex from heifers fed with either a Stair-Step or Control diet. Controls heifers were offered a maintenance diet of 97.9 g/kg0.75 for 84 days. The Stair-Step diet was initiated at 8 months containing a restricted fed diet of 67.4 g/kg0.75 for 84 days. After the first 84 days, while Control heifers continued to receive 97.9 g/kg0.75, the Stair-Step beef heifers were offered 118.9 g/kg0.75 for another 68 days, after which they were ovariectomized at 13 months of age16 for studying changes in follicular stages and morphology before and after culture. We also assayed for differences in steroids, steroid metabolites, chemokines, and cytokines secreted into cortex media. Steroids and other metabolites were measured to determine if there were any direct effects from treatments conducted in vivo and/or in vitro on tissue viability and productivity. Changes in the ovarian microenvironment prior to and after culture provided a snapshot of the endocrine milieu and folliculogenesis prior to culture and how culture or treatment during culture affects follicle progression or arrest.
Ovaries were collected after ovariectomies were performed at the U.S. Meat Animal Research Center (USMARC) according to their IACUC procedures from Control and Stair-Step heifers at 13 months of age16, cleaned with sterile phosphate buffer saline (PBS) washes with 0.1% antibiotic to remove blood and other contaminants, trimmed excess tissue, and transported to the University of Nebraska-Lincoln (UNL) Reproductive Physiology laboratory UNL at 37°C23. At UNL, ovarian cortex pieces were cut into small square pieces (~0.5-1 mm3; Figure 1) and cultured for 7 days (Figure 2). Histology was conducted on the cortex culture slides prior to and after culture to determine follicles stages16,24 (Figure 3 and Figure 4), and extracellular matrix proteins that may indicate fibrosis (Picro-Sirus Red, PSR; Figure 5). This allowed determination of effect of in vivo nutritional regimes on follicle stages and allowed comparison of 7 days of ovarian cortex on follicle stages and follicle progression. Throughout the culture, the medium was collected and changed daily (approximately 70% of media was collected each day; 250 µL/well) so that either daily hormones/cytokines/chemokines can be assessed or pooled over days to obtain average concentrations. Steroids such as androstenedione (A4) and estrogen (E2) can be pooled over 3 days and assessed through radioimmunoassay (RIA; Figure 6) and pooled over 4 days per animal and assayed via High Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS)24,25 (Table 1). Cytokine arrays were utilized to assess cytokine and chemokine concentrations in ovarian cortex culture medium26 (Table 2). Real-time polymerase chain reaction (RT-PCR) assay plates were conducted to determine gene expression for specific signal transduction pathways as demonstrated previously16. All of the steroid, cytokine, follicle stage and histological markers provide a snapshot of the ovarian microenvironment and clues as to the ability of that microenvironment to promote "normal" or "abnormal" folliculogenesis.
Protokół
The ovaries were obtained from U. S. Meat Animal Research Center16. As stated previously16, all procedures were approved by the U.S. Meat Animal Research Center (USMARC) Animal Care and Use Committee in accordance with the guide for Care and Use of Agricultural Animals in Agricultural Research and Teaching. The ovaries were brought to the University of Nebraska-Lincoln Reproductive Laboratory where they were processed and cultured.
1. Preparation of required media
- Waymouth MB 752/1 medium
- Fill a 1 L tissue culture bottle with 900 mL of sterile water. While the water is gently stirring on a stir plate, gradually add the powdered medium. Once the powdered medium is dissolved, add 2.24 g of sodium bicarbonate followed by 1.25 g of bovine serum albumin (BSA). Use a pH meter and adjust the pH to 7.25-7.35. Add additional sterile water to bring the final volume to 1 L.
- Move to a biological safety cabinet and add penicillin-streptomycin sulfate at a concentration of 0.1% v/v of the medium. Filter the medium with a 0.22 µm pore 33.2 cm2 500 mL bottle top filter.
- Pour off the filtered medium into several 50 mL conical tubes. Add 0.5 mL of Insulin-Transferrin-Selenium per 50 mL of aliquoted medium.
- Wrap the conical tubes and stock bottle of the medium in aluminum foil and store at 4 °C. This medium is light sensitive.
NOTE: Waymouth medium can be stored for up to 1 month.
- Leibovitz's L-15 (LB-15) medium
NOTE: LB-15 medium is used to clean tissue in preparation for culture.- Fill a 1 L tissue culture bottle with 900 mL of sterile water. While the sterile water is gently stirring on a stir plate, gradually add the prepared powdered medium. Use a pH meter and adjust the pH to 7.25-7.35. Add additional sterile water to bring the final volume to 1 L.
- Move to biological safety cabinet. Make 1 L of LB-15 with 0.1% antibiotic (see Table of Materials). Filter the medium into two 500 mL tissue culture bottles using a 0.22 µm pore 33.2 cm2 500 mL bottle top filter. Wrap bottles in aluminum foil as LB-15 medium is light-sensitive and store at 4 °C.
NOTE: LB-15 medium can be stored for up to 1 month.
- Phosphate Buffered Saline (PBS)
- Make PBS in the lab or purchase sterile PBS without calcium or magnesium (Table of Materials). To make PBS in the lab, begin with 800 mL of distilled water and add 8 g of sodium chloride (NaCl) to it. Then, add 0.2 g of potassium chloride (KCl), 1.44 g of sodium phosphate dibasic (Na2HPO4), and 0.24 g of potassium phosphate dibasic (KH2PO4). Adjust pH to ~7.4 and adjust total volume to 1 L. Sterilize the solution by autoclaving.
- Make 1 L PBS with 0.1% antibiotic (see Table of Materials) while in a biological safety cabinet.
2. Ovarian cortical culture protocol
NOTE: Ovaries were obtained from spring born USMARC heifers at 13 months of age. Ovaries were rinsed thoroughly, and all blood and other fluid were removed with PBS containing antibiotic (0.1%) and transported at 37 °C23 to University of Nebraska-Lincoln Reproduction Laboratory UNL (1.5 h away). (For comments on temperature of ovaries during transport please see Discussion)
- Prepare the ovarian tissue on a clean bench (Figure 1).
- Disinfect the clean bench with 70% ethanol. Place a fresh absorbent pad on the benchtop. Ensure that the clean bench blower is turned on half an hour prior to dissection along with UV light to sterilize anything in the clean bench, including absorbent pad and make sure appropriate PPE is used.
- Arrange the Petri dishes (60 x 15 mm) for tissue washes. Three Petri dishes are required for PBS wash, three for PBS with antibiotic, and three for LB-15 washes. An additional LB-15-containing Petri dish with accompanying lid will be utilized for final placement of pieces after washes.
- Fill each Petri dish with approximately 10 mL of appropriate fluids, either PBS or LB-15.
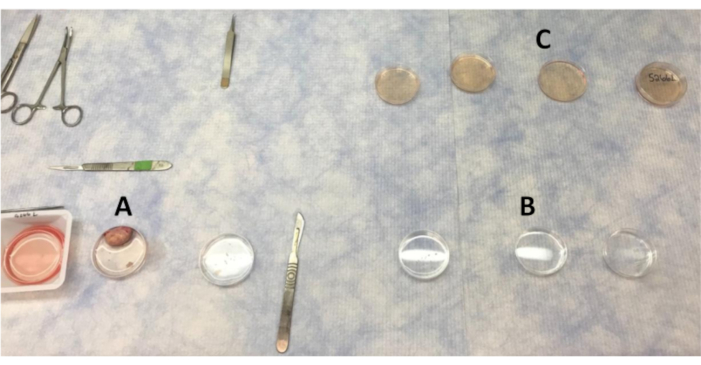
Figure 1: Layout of plates for washing the ovary and cortex pieces in the clean bench. (A) PBS used for washing the ovary as sections of the cortex are removed. (B) PBS with antibiotic washes that cortex pieces are moved through. (C) Ovarian cortex pieces are washed four times in LB-15 before moving to the biosafety cabinet for final wash in LB-15. Please click here to view a larger version of this figure.
- Remove the prepared Waymouth and LB-15 medium from the refrigerator and warm to room temperature.
- Autoclave all tools to ensure sterilization prior to use.
- Maintain ovaries at 37 °C until ovarian cortex is ready to be collected.
- Using forceps with serrated jaws, pick up the ovary and thoroughly wash in the first PBS-filled Petri dish. Transfer the ovary to the second PBS wash and thoroughly cleanse once more.
NOTE: The ovary will stay in the second PBS wash while ovarian cortical strips are removed. - Using serrated jaw forceps, secure the ovary and slice in half. At this time, the ovarian cortex will cut away from the medulla. Using a ruler, make sure that no more than 1–2 mm of depth of surface of ovary is removed away from the medulla16. Remove transverse sections of the ovarian cortex from medulla, cut 3–4 thin strips of ovarian cortex (Figure 2) with a scalpel (#11 scalpel blade; #3 handle), and place the strips in the third PBS-filled Petri dish.
NOTE: At this time, additional ovarian cortical tissue can be collected for RNA extraction or fixed and collected for histology of initial non-cultured cortex pieces. When removing strips of ovarian cortex, avoid areas with visible antral follicles or corpora lutea. In addition, avoid collecting medullary tissue. The histology of the medulla is very different as shown previously16. If the ovarian cortex is not cut to more than a 1–2 mm depth, then the medulla should not be obtained. Distinct histology allows for landmarks between the cortex and the medulla. - Cut the ovarian cortex strips in the third PBS wash into small, square pieces (~0.5–1 mm3) with a #21 scalpel blade. Use a ruler underneath the Petri dishes to ensure the pieces are of similar size and thickness to make consistent ovarian cortex pieces. Use forceps to secure the strips while cutting the pieces with a scalpel.
NOTE: The number of tissue pieces cut is dependent on the experiment. Four pieces of ovarian cortex is the minimum amount of tissue necessary for culture. Other methods for ensuring appropriate length and depth include using special slicers26 or precut plastic pieces as templates27. - Wash ovarian cortical pieces through all three PBS with antibiotic-filled Petri dishes. Use a curved tip forceps to move pieces between washes.
- Move cortex pieces through the series of LB-15 washes and place in final LB-15-filled Petri dish. Label the lid with animal ID and ovary side (left or right).
NOTE: Fully submerge the ovarian cortex pieces in each wash for thorough cleaning. - Collect four ovarian cortex pieces per ovary and fix for day zero histology. Additional pieces can also be flash frozen for RNA. The remaining tissue pieces will be used for culture. Wipe down dissecting tools with 70% ethanol after each tissue collection.
- Prepare a biological safety cabinet for final tissue wash and culture preparation. Sanitize supplies with 70% ethanol before placing in the biological safety cabinet. Use the aseptic technique when working in the biological safety cabinet.
- Move all ovarian cortex intended for culture to the biological safety cabinet and wash once more in an LB-15-filled Petri dish.
- In a 24-well tissue culture plate, pipette 350 µL of Waymouth medium per well.
- Place uncoated culture well inserts into each well using forceps. Ensure that no bubbles are formed under the base of the insert as this would result in the tissue drying out. The medium must be touching the inserts to allow for the medium to be absorbed up and surround the ovarian cortex pieces.
- Carefully position four ovarian cortex pieces onto the mesh of each insert (Figure 2). The forceps can puncture the mesh if the tissue pieces are not delicately placed. The tissue pieces should not be touching each other or the side of the insert.
- Incubate the tissue at 37 °C with 5% CO216.
NOTE: Others have used 38.8 °C28. However, no difference has been observed in the integrity of the tissue nor in the ability of follicles to progress in 37 °C tissue nor have others39,30. Thus, at this point any of these temperatures should be conducive to experiment success. Others have used 400 µL of medium. Either amount is fine as long as one is consistent, and the tissue is partially submerged allowing for adequate surface tension to allow for hydration of tissue (media surrounding ovarian cortex pieces). Fill empty wells with 500 µL of sterile water to help reduce evaporation from other wells.

Figure 2: Ovarian cortex pieces and culture plate. (A) An ovarian strip being cut from the cortex of the ovary. (B) Ruler and cortex piece shown side by side. (C) Four cortex pieces (~0.5-1 mm3) resting on the insert in the culture medium in the plate. (D) Lifting the insert to collect the culture medium from the well. Collect and replace all the culture medium daily (250 µL) to maintain proper pH.Approximately 250 µL is obtained from each well each day (about 70% of the initial culture medium). Please click here to view a larger version of this figure.
3. Media collection
- Change ovarian cortex culture medium daily for 7 days. Medium changes should be as close to 24 h apart as possible to prevent large pH and color changes in medium. Warm Waymouth medium to 37 °C prior to medium change. Approximately 250 µL is obtained from each well each day (about 70% of initial culture medium).
- During medium changes, use forceps to gently lift the insert out of the well. Collect the cultured Waymouth medium in 0.5 mL tubes (approximately 250 µL /day). Set the insert back in well and add 350 µL of fresh culture medium by dispensing the medium between the side of the insert and well.
NOTE: Change most of the media daily to obtain enough media to measure all the steroids, cytokines, and chemokines necessary to determine ovarian microenvironment. Also, daily medium changes are important to prevent large pH changes (indicated by color change) in the medium. Drops of medium were retained surrounding the ovarian cortex pieces to ensure the pieces remained wet. No problems were observed with cultured tissue due to changing 70% of the media. - Store the collected medium from tissue culture at -20 °C.
4. Imaging and downstream processing
- After 7 days of culture at 37 °C with 5% CO2, image the ovarian cortex pieces using a dissection microscope with an attached camera and a computer imaging software program.
NOTE: A dark room is usually best for achieving the best picture quality for imaging. - After imaging, fix two ovarian cortex pieces per well in Bouins for histology and flash freeze two ovarian cortex pieces in liquid nitrogen to obtain RNA for cDNA. Repeat this step for all the wells with tissue. Collect the medium from day 7 and store at -20 °C.
- Let the ovarian cortex pieces remain immersed in Bouins (picric acid 750 mL, glacial acetic acid 50 mL, and 37%–40% formalin 250 mL) for approximately 1.5 h before being washed with 70% ethanol three times. The tissue will remain in 70% ethanol and be cleared daily until the solution is no longer yellow.
NOTE: Fixatives other than Bouins as well as paraformaldehyde can be used. In this experiement Bouins is used as it is the fixative to achieve optimal morphology. If more tissue is required for other analysis, additional wells of media and pieces of ovarian cortex can be obtained from each animal.
Wyniki
This bovine cortex culture procedure can be used to determine a wide variety of hormone, cytokine, and histology data from small pieces of the ovary. Staining, such as hematoxylin and eosin (H&E), can be used to determine ovarian morphology through follicle staging16,23,31 (Figure 3). Briefly, follicles were classified as primordial, which is an oocyte surrounded by a single layer of squamous pr...
Dyskusje
The benefit of in vitro ovarian cortex culture, as described in this manuscript, is that the follicles develop in a normalized environment with adjacent stroma surrounding the follicles. The somatic cells and oocyte remain intact, and there is appropriate cell-to-cell communication as an in vivo model. Our laboratory has found that a 7-day culture system provides representative folliculogenesis and steroidogenesis data for the treatment of the ovarian cortex. Other ovarian tissue culture protocols have either relatively ...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This research was supported by National Institute of Food and Agriculture 2013-67015-20965 to ASC, University of Nebraska Food for Health Competitive Grants to ASC. United States Department of Agriculture Hatch grant NEB26-202/W3112 Accession #1011127 to ASC, Hatch–NEB ANHL Accession #1002234 to ASC. Quantitative Life Sciences Initiative Summer Postdoctoral Scholar Support – COVID-19 Award for summer funding for CMS.
The authors would like to extend their appreciation to Dr. Robert Cushman, U.S. Meat Animal Research Center, Clay Center, NE to thank him for providing the ovaries in a previous publication, which were then used in the current paper as a proof of concept in validating this technique.
Materiały
| Name | Company | Catalog Number | Comments |
| #11 Scapel Blade | Swann-Morton | 303 | Scaple Blade |
| #21 Scapel Blade | Swann-Morton | 307 | Scaple Blade |
| 500mL Bottle Top Filter | Corning | 430514 | Bottle Top Filter 0.22 µm pore for filtering medium |
| AbsoluteIDQ Sterol17 Assay | Biocrates | Sterol17 Kit | Samples are sent off to Biocrates and steroid panels are run and results are returned |
| Androstenedione Double Antibody RIA Kit | MPBio | 7109202 | RIA to determine androstenedione from culture medium |
| Belgium A4 Assay Kit | DIA Source | KIP0451 | RIA to determine androstenedione from culture medium |
| Bovine Cytokine Array Q3 | RayBiotech | QAB-CYT-3-1 | Cytokine kit to determine cytokines from culture medium |
| cellSens Software Standard 1.3 | Olympus | 7790 | Imaging Software |
| Insulin-Transferrin-Selenium-X | Gibco ThermoFisher Scientific | 5150056 | Addative to the culture medium |
| Leibovitz's L-15 Medium | Gibco ThermoFisher Scientific | 4130039 | Used for tissue washing on clean bench, and in the biosafety cabniet |
| Microscope | Olympus | SZX16 | Disection microscope used for imaging tissue culture pieces |
| Microscope Camera | Olympus | DP71 | Microscope cameraused for imaging tissue culture pieces |
| Millicell Cell Culture Inserts 0.4µm, 12,mm Diameter | Millipore Sigma | PICM01250 | Inserts that allow the tissue to rest against the medium without being submerged in it |
| Multiwell 24 well plate | Falcon | 353047 | Plate used to hold meduim, inserts, and tissues |
| Petri dish 60 x 15 mm | Falcon | 351007 | Petri dish used for washing steps prior to culture |
| Phosphate-Buffered Saline (PBS 1X) | Corning | 21-040-CV | Used for tissue washing |
| SAS Version 9.3 | SAS Institute | 9.3 TS1M2 | Statistical analysis software |
| Thomas Stadie-Riggs Tissue Slicer | Thomas Scientific | 6727C10 | Tissue slicer for preperation of thin uniform sections of fresh tissue |
| Waymouth MB 752/1 Medium | Sigma-Aldrich | W1625 | Medium used for tissue cultures |
Odniesienia
- Braw-Tal, R., Yossefi, S. Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. Journal of Reproduction and Fertility. 109, 165-171 (1997).
- Nilsson anEdson, M. A., Nagaraja, A. K., Matzuk, M. M. The mammalian ovary from genesis to revelation. Endocrine Reviews. 30 (6), 624-712 (2009).
- Fortune, J. E., Cushman, R. A., Wahl, C. M., Kito, S. The primordial to primary follicle transition. Molecular and Cellular Endocrinology. 163, 53-60 (2000).
- Ireland, J. J. Control of follicular growth and development. Journal of Reproduction and Fertility. 34, 39-54 (1987).
- Higuchi, C. M., Maeda, Y., Horiuchi, T., Yamazaki, Y. A simplified method for three-dimensional (3-D) ovarian tissue culture yielding oocytes competent to produce full-term offspring in mice. PLoS One. 10 (11), e0143114 (2015).
- Ramezani, M., Salehnia, M., Jafarabadi, M. Short term culture of vitrified human ovarian cortical tissue to assess the cryopreservation outcome: molecular and morphological analysis. Journal of Reproduction & Infertility. 18 (1), 162-171 (2017).
- McLaughlin, M., Telfer, E. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reproduction. 139 (6), 971-978 (2010).
- Stefansdottir, A., Fowler, P. A., Powles-Glover, N., Anderson, R. A., Spears, N. Use of ovary culture techniques in reproductive toxicology. Reproductive Toxicology. 49, 117-135 (2014).
- Bromfield, J. J., Sheldon, I. M. Lipopolysaccharide reduces the primordial follicle pool in the bovine ovarian cortex ex vivo and in the murine ovary in vivo. Biology of Reproduction. 88 (4), 1-9 (2013).
- Franks, S., Stark, J., Hardy, K. Follicle dynamics and anovulation in polycystic ovary syndrome. Humane Reproduction Update. 14 (4), 367-378 (2008).
- Desmeules, P., Devine, P. J. Characterizing the ovotoxicity of cyclophosphamide metabolites on cultured mouse ovaries. Toxicological Sciences. 90 (2), 500-509 (2006).
- Bott, R. C., McFee, R. M., Clopton, D. T., Toombs, C., Cupp, A. S. Vascular endothelial growth factor and kinase domain region receptor are involved in both seminiferous cord formation and vascular development during testis morphogenesis in the rat. Biology of Reproduction. 75, 56-67 (2006).
- Baltes-Breitwisch, M. M., et al. Neutralization of vascular endothelial growth factor antiangiogenic isoforms or administration of proangiogenic isoforms stimulates vascular development in the rat testis. Reproduction. 140 (2), 319-329 (2010).
- McFee, R. M., et al. Inhibition of vascular endothelial growth factor receptor signal transduction blocks follicle progression but does not necessarily disrupt vascular development in perinatal rat ovaries. Biology of Reproduction. 81, 966-977 (2009).
- Artac, R. A., et al. Neutralization of vascular endothelial growth factor antiangiogenic isoforms is more effective than treatment with proangiogenic isoforms in stimulating vascular development and follicle progression in the perinatal rat ovary. Biology of Reproduction. 81, 978-988 (2009).
- Abedal-Majed, M. A., et al. Vascular endothelial growth factor A isoforms modulate follicle development in peripbertal heifers independent of diet through diverse signal transduction pathways. Biology of Reproduction. 102 (3), 680-692 (2020).
- Wandji, S. A., Srsen, V., Voss, A. K., Eppig, J. J., Fortune, J. E. Initiation in vitro of bovine primordial follicles. Biology of Reproduction. 55, 942-948 (1996).
- Wandji, S. A., Srsen, V., Nathanielsz, P. W., Eppig, J. J., Fortune, J. E. Initiation of growth of baboon primordial follicles in vitro. Human Reproduction. 12 (9), 1993-2001 (1993).
- Yang, M. Y., Fortune, J. E. Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biology of Reproduction. 75, 924-932 (2006).
- Fortune, J. E., Kito, S., Wandji, S. A., Srsen, V. Activation of bovine and baboon primordial follicles in vitro. Theriogenology. 49, 441-449 (1998).
- Yang, M. Y., Fortune, J. E. Vascular endothelial growth factor stimulates the primary to secondary follicle transition in bovine follicles in vitro. Molecular Reproduction and Development. 74, 1095-1104 (2007).
- Barberino, R. S., Silva, J. R. V., Figueiredo, J. R., Matos, M. H. T. Transport of domestic and wild animal ovaries: a review of the effects of medium, temperature, and periods of storage on follicular viability. Biopreservation and Biobanking. 17 (1), 84-90 (2019).
- Summers, A. F., et al. Altered theca and cumulus oocyte complex gene expression, follicular arrest and reduced fertility in cows with dominant follicle follicular fluid androgen excess. PLoS One. 9 (10), e110683 (2014).
- Koal, T., Schmiederer, D., Pham-Tuan, H., Rohring, C., Rauh, M. Standardized LC-MS/MS based steroid hormone profile analysis. The Journal of Steroid Biochemistry and Molecular Biology. 129, 129-138 (2012).
- Poole, R. K., Brown, A. R., Pore, M. H., Pickworth, C. L., Poole, D. H. Effects of endophyte-infected tall fescue seed and protein supplementation on stocker steers: II. Adaptive and innate immune function. Journal of Animal Science. 97 (10), 4160-4170 (2019).
- Laronda, M., et al. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. Journal of Assisted Reproduction and Genetics. 31 (8), 1013-1028 (2014).
- Silber, S. J., et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Human Reproduction. 23 (7), 1531-1537 (2008).
- Wiedemann, C., Zahmel, J., Jewgenow, K. Short-term culture of ovarian cortex pieces to assess the cryopreservation outcome in wild fields for genome conservation. BMC Veterinary Research. 9 (37), (2013).
- Baufeld, A., Vanselow, J. Increasing cell plating density mimics an early post-LH stage in cultured bovine granulosa cells. Cell and Tissue Research. 354 (3), 869-880 (2013).
- Shimizu, T., Miyamoto, A. Progesterone induces the expression of vascular endothelial growth factor (VEGF) 120 and Flk-1, its receptor, in bovine granulosa cells. Animal Reproduction Science. 102 (3-4), 228-237 (2007).
- Tepekoy, F., Akkoyunlu, G. The effect of FSH and activin A on Akt and MAPK1/3 phosphorylation in cultured bovine ovarian cortical strips. Journal of Ovarian Research. 9 (13), 1-9 (2016).
- Beck, K., Singh, J., Arshud Dar, M., Anzar, M. Short-term culture of adult bovine ovarian tissues: chorioallantoic membrane (CAM) vs. traditional in vitro culture systems. Reproductive Biology and Endocrinology. 16 (1), 21 (2018).
- Eppig, J. J. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 122 (6), 829-838 (2001).
- Paczkowski, M., Silva, E., Schoolcraft, W. B., Krisher, R. L. Comparative importance of fatty acid beta-oxidation to nuclear maturation, gene expression, and glucose metabolism in mouse, bovine, and porcine cumulus oocyte complexes. Biology of Reproduction. 88 (5), 1-11 (2013).
- Raffel, N., et al. Is ovarian tissue transport at supra-zero temperatures compared to body temperature optimal for follicle survival?. In Vivo. 34 (2), 533-541 (2020).
- Duncan, F., et al. Ovarian tissue transport to expand access to fertility preservation: from animals to clinical practice. Reproduction (Cambridge, England). 152 (6), R201-R210 (2016).
- Liebenthron, J., et al. Overnight ovarian tissue transportation for centralized cryobanking: a feasible option. Reproductive BioMedicine Online. 38 (5), 740-749 (2019).
- Mohammed, B. T., Donadeu, F. X. Bovine granulosa cell culture. Epithelial Cell Culture: Methods and Protocols. , 79-87 (2018).
- Langbeen, A., et al. Effects of neutral red assisted viability assessment on the cryotolerance of isolated bovine preantral follicles. Journal of Assisted Reproduction Genetics. 31, 1727-1736 (2014).
- Higuchi, C. M., Maeda, Y., Horiuchi, T., Yamazaki, Y. A simplified method for three-dimensional (3-D) ovarian tissue culture yielding oocytes competent to produce full-term offspring in mice. PLoS One. 10 (11), e0143114 (2015).
- Yang, M. Y., Fortune, J. E. Changes in the transcriptome of bovine ovarian cortex during follicle activation in vitro. Physiological Genomics. 47, 600-611 (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone