Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Deep Vascular Imaging in the Eye with Flow-Enhanced Ultrasound
W tym Artykule
Podsumowanie
We present a non-invasive ultrasound technique for generating three-dimensional angiographies in the eye without the use of contrast agents.
Streszczenie
The retina within the eye is one of the most energy-demanding tissues in the body and thus requires high rates of oxygen delivery from a rich blood supply. The capillary lamina of the choroid lines the outer surface of the retina and is the dominating source of oxygen in most vertebrate retinas. However, this vascular bed is challenging to image with traditional optical techniques due to its position behind the highly light-absorbing retina. Here we describe a high-frequency ultrasound technique with subsequent flow-enhancement to image deep vascular beds (0.5-3 cm) of the eye with a high spatiotemporal resolution. This non-invasive method works well in species with nucleated red blood cells (non-mammalian and fetal animal models). It allows for the generation of non-invasive three-dimensional angiographies without the use of contrast agents, and it is independent of blood flow angles with a higher sensitivity than Doppler-based ultrasound imaging techniques.
Wprowadzenie
The high metabolism on the vertebrate retina imposes an intrinsic tradeoff between two contrasting needs; high blood flow rates and a light path devoid of blood vessels. To avoid visual disturbance of perfusing red blood cells, the retina of all vertebrates receives oxygen and nutrients via a sheet of capillaries behind the photoreceptors, the choriocapillaris1,2,3. However, this single source of nutrients and oxygen imposes a diffusion limitation to the thickness of the retina4,5, so many visually active species possess a variety of elaborate vascular networks to provide additional blood supply to this metabolically active organ6. These vascular beds include blood vessels perfusing the internal retinal layers in mammals and some fishes4,7,8,9,10, blood vessels on the inner (light-facing) side of the retina found in many fishes, reptiles, and birds4,11,12,13, and countercurrent vascular arrangements of the fish choroid, the choroid rete mirabile, that allows for the generation of super-atmospheric oxygen partial pressures14,15,16,17,18,19,20. Despite that these additional non-choroidal paths for retinal nutrient supply play an essential role in fueling the metabolic requirements of superior vision4, the three-dimensional anatomy of these vascular structures is poorly understood, limiting our understanding of the morphological evolution of the vertebrate eye.
Traditionally, retinal blood supply has been studied using optical techniques, such as fundus ophthalmoscopy. This category of techniques provides high-throughput non-destructive information on non-choroidal blood vessel anatomy in high-resolution21 and is therefore readily used in clinical diagnosis of abnormalities in retinal vessel structure22. However, the retinal pigment epithelium absorbs the transmitted light and limits the depth of view in these optical techniques, providing reduced information on choroidal structure and function without the use of contrast agent21. Similar depth limitations are experienced in optical coherence tomography (OCT). This technique can generate high-resolution fundus angiographies using light waves at the technical expense of depth penetration23, while the enhanced depth imaging OCT can visualize the choroid at the expense of retinal imaging quality24. Magnetic resonance imaging overcomes the optical limitations of ophthalmoscopy and OCT and can map vascular layers in the retina, albeit at a low resolution25. Histology and microcomputed tomography (µCT) maintain the high-resolution of the optical techniques and provide information on whole-eye vascular morphology4, but both techniques require ocular sampling and are therefore not possible in the clinic or rare or endangered species. To overcome some of the limitations of these established retinal imaging techniques, the study here presents an ultrasound protocol on anesthetized animals, where blood movement is mapped in silico on a series of equally-spaced two-dimensional ultrasound scans spanning a whole eye by applying a comparable technique as described previously for embryonic and cardiovascular imaging26,27,28 and in OCT angiography29. This approach allows for the generation of non-invasive three-dimensional deep ocular angiographies without using a contrast agent and opens up new avenues for mapping blood flow distribution within the eye across species.
Protokół
The protocol below was performed with permission from the Danish Inspectorate for Animal Experimentation within the Danish Ministry of Food, Agriculture, and Fisheries, Danish Veterinary and Food Administration (Permit number 2016-15-0201-00835).
1. Anesthesia and ultrasound medium
- Anesthetize the research animal.
NOTE: Type and dose of appropriate anesthesia are highly species-dependent. In general, immersion-based anesthetics such as MS-222 (ethyl 3-aminobenzoate methanesulfonic acid), benzocaine (ethyl 4-aminobenzoate), and propofol (2,6-diisopropyl phenol) are useful in fish and amphibians which readily absorbs the anesthetic over gills or skin (e.g., 0.05 mg·L-1 benzocaine in rainbow trout). A range of dissolved compounds that can be administered intravenously, intramuscularly, intraperitoneally is available for amniotes, as are gas-based anesthetics. Alfaxalon administered intramuscularly is useful in reptiles (e.g., 30 mg·kg-1 in lizards), and isoflurane administered as gas is useful in birds (e.g., 2% in air for pigeons). Refer to the published literature30,31,32 for a full overview of available anesthetics across species. - Test reflexes in the animal to confirm an optimal level of anesthesia. Ensure that the animal is completely motionless during the procedure as the flow-enhanced ultrasound procedure is sensitive to motion noise.
- Too deep anesthesia can alter blood flow patterns, so conduct a dose titration in the start-up phase of an experiment.
- Increase the anesthesia dosage in steps and observe blood flow in the eye aided by simple brightness mode (B-mode) ultrasound.
NOTE: An optimal level of anesthesia is obtained when the animal is motionless (except respiration) with visible ocular blood flow.
- If the type/dose of anesthetic is not permissive for respiratory movements, then ensure adequate ventilation of the animal, e.g., using an air pump to oxygenate the water for aquatic species or a ventilator for air-breathing species.
- Position the animal in a posture that allows direct access from above to the eye.
NOTE: Depending on species, this can be in either a supine or lateral position. It can be useful to construct a simple holding device using a small piece of non-reactive metal (e.g., stainless steel) and loose rubber bands (see Figure 1). - Place appropriate ultrasound medium on the eye of the animal. If scaled eyelids (ultrasound impermeable) cover the eye, then displace these gently with a cotton swab.
NOTE: For aquatic species, the best ultrasound medium is clean tank water in which the animal usually lives. For terrestrial species, a generous amount of ultrasound gel ensures free movements and imaging of the ultrasound transducer (i.e., linear array probe) across the entire surface of the eye. Vet ointment on the contralateral eye is required for terrestrial species.
2. 2D and 3D ocular ultrasound image acquisition
- Position the ultrasound transducer medial to the eye in either a dorsal/ventral or rostral/caudal orientation depending on desired image orientation.
- In B-mode, with a maximum depth of field, image the medial and deepest portion of the eye and make sure that all structures of interest are visible in the image field.
NOTE: In some species, the crystalline lens takes up a comparatively large proportion of the vitreous humor, which may absorb the ultrasound, especially at higher frequencies. - Slowly translate the transducer to each side while inspecting the real-time images. Make sure all structures of interest are visible in the image field; if not, switch to a transducer with a lower frequency and larger depth of field.
NOTE: The following center frequencies allow for the following maximum depth of field: 21 MHz: 3 cm, 40 MHz: 1.5 cm, 50 MHz: 1 cm (see Table 1). However, these maximum depth of field values can be markedly lower if the eye contains calcified or other ultrasound impermeable structures. - Adjust image depth, depth offset (distance from the top of the image to the structure of interest), image width, as well as number and position of focal zones to cover the desired region of interest in all three spatial dimensions (e.g., 1 cm image depth, 2 mm depth offset, 1 cm image width, one focal zone).
NOTE: Although specific naming of buttons that adjust these parameters may vary between ultrasound systems, most systems will have buttons with logical names for these adjustments. These image parameter settings usually affect the range of possible temporal resolutions of the ultrasound acquisition. - Set frame rate in the range of 50-120 frames·s-1.
NOTE: The temporal resolution (i.e., the time interval between successive B-scans) must be adequate to display large pixel intensity variability in imaged blood vessels, i.e., the temporal resolution must not be too high. On the other hand, to complete a full 3D recording of the eye in a reasonable time, temporal resolution cannot be too low. A temporal resolution ranging from 50-120 frames·s-1 is usually adequate for the flow-enhanced procedure in most species. On some ultrasound systems, this desired temporal resolution can be obtained by switching between the "general imaging" (high spatial/low temporal resolution) and "cardiology" (low spatial/high temporal resolution) modes. - Adjust 2D gain to a level (~5 dB), so anatomical structures are only just visible in the B-mode acquisition to increase the signal-to-noise ratio in the subsequent flow-enhanced reconstruction.
- To acquire a 2D flow-enhanced image at a single slice position, translate the transducer to this position and continue at step 3.1.
- To acquire a 3D recording of an entire region of interest, e.g., the retina, translate the transducer to one extreme of the region of interest.
- To determine the exact position of the extreme end of the region of interest, increase the 2D gain briefly.
- After correct transducer placement is complete, lower the 2D gain before recording to ensure maximal signal-to-noise ratio in the subsequent flow-enhanced reconstruction.
- For each step (slice) in the 3D recording, acquire ≥100 frames (optimally ≥1000 frames).
- Using a micromanipulator or build-in transducer motor, translate the transducer across the entire region of interest in steps of, e.g., 25 µm or 50 µm (remember to note the step size) and repeat the ≥100 frames acquisition for each step.
- Euthanize the research animal according to the animal care guidelines of the institution.
3. Flow-enhanced image reconstruction
- Export the recordings into digital imaging and communications in medicine (DICOM) file format (little-endian).
- To produce a single flow-enhanced image based on a ≥100 frames (T) cine recording, calculate the standard deviation on pixel level (STD(x,y)) using the formula:
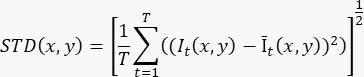
Where It(x,y) is the intensity of the pixel at the (x,y) pixel coordinate at time t, and Īt(x,y) is the arithmetic mean value of I over time. - Repeat step 3.2 for each slice in the 3D recording.
- To automate the STD-calculation and image reconstruction process for multiple slices in a 3D recording, conduct this operation in batch mode using, e.g., ImageJ and the supplementary macro script (Supplementary File 1).
- Combine all reconstructed slices into one image stack (Images to Stack command in ImageJ).
- Specify slice thickness from the step size used during acquisition (Properties command in ImageJ).
- Save the image stack as a 3D TIF file.
NOTE: Flow-weighted three-dimensional recordings of ocular blood vessels can subsequently be used to create volume renderings and build digital and physical anatomical models of vascular structures of the eye. These image processing options are outside the scope of this protocol; refer to the previously published articles for more details33,34,35.
Wyniki
The flow-enhanced ultrasound technique to image vascular beds of the eye can be applied in a range of species and has currently been used in 46 different vertebrate species (Figure 1, Table 1). The presence of nucleated red blood cells in non-adult-mammalian vertebrates provides positive contrast of flowing blood compared to static tissue in cine recordings (Supplementary File 2). However, when analyzed on a frame-by-frame basis, the clear distinction betwee...
Dyskusje
Vascular imaging using flow-enhanced ultrasound provides a new method for non-invasive imaging of the vasculature of the eye that offers several advantages over present techniques but has its intrinsic limitations. The primary advantage of flow-enhanced ultrasound is the ability to generate ocular angiographies with a depth of field that exceeds the retinal pigment epithelium, which limits the depth of field in optical techniques. In ultrasound imaging, spatial resolution and depth of field are ultimately determined by t...
Ujawnienia
The authors declare that no completing interests exists.
Podziękowania
This work has received funding from the Carlsberg Foundation (CF17-0778; CF18-0658), the Lundbeck Foundation (R324-2019-1470; R346-2020-1210), the Velux Foundations (00022458), The A.P. Møller Foundation for the Advancement of Medical Science, the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement (No. 754513), and The Aarhus University Research Foundation.
Materiały
| Name | Company | Catalog Number | Comments |
| MS-222 | Sigma | E10521-50G | |
| Benzocaine | Sigma | E-1501 | |
| Propofol | B Braun | 12260470_0320 | |
| Alfaxalon | Jurox | NA | |
| Isoflurane | Zoetis | 50019100 | |
| Ultrasound scanner | VisualSonics | Vevo 2100 |
Odniesienia
- Yu, C. Q., Schwab, I. R., Dubielzig, R. R. Feeding the vertebrate retina from the Cambrian to the Tertiary. Journal of Zoology. 278 (4), 259-269 (2009).
- Yu, D. Y., Cringle, S. J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Progress in Retinal and Eye Research. 20 (2), 175-208 (2001).
- Country, M. W. Retinal metabolism: A comparative look at energetics in the retina. Brain Research. 1672, 50-57 (2017).
- Damsgaard, C., et al. Retinal oxygen supply shaped the functional evolution of the vertebrate eye. Elife. , 8 (2019).
- Buttery, R. G., Hinrichsen, C. F. L., Weller, W. L., Haight, J. R. How thick should a retina be? A comparative study of mammalian species with and without intraretinal vasculature. Vision Research. 31 (2), 169-187 (1991).
- Ames, A., Li, Y., Heher, E., Kimble, C. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. The Journal of Neuroscience. 12 (3), 840-853 (1992).
- Chase, J. The Evolution of retinal vascularization in mammals: A comparison of vascular and avascular retinae. Ophthalmology. 89 (12), 1518-1525 (1982).
- Johnson, G. L. Ophthalmoscopic studies on the eyes of mammals. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 254 (794), 207-220 (1968).
- Johnson, G. L. I. Contributions to the comparative anatomy of the mammalian eye, chiefly based on ophthalmoscopic examination. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 194 (194-206), 1-82 (1901).
- Rodriguez-Ramos Fernandez, J., Dubielzig, R. R. Ocular comparative anatomy of the family Rodentia. Veterinary Ophthalmology. 16, 94-99 (2013).
- Copeland, D. E. Functional vascularization of the teleost eye. Current Topics in Eye Research. 3, 219-280 (1980).
- Meyer, D. B., Crescitelli, F. . The Visual System in Vertebrates. Handbook of Sensory Physiology. 7, (1977).
- Potier, S., Mitkus, M., Kelber, A. Visual adaptations of diurnal and nocturnal raptors. Seminars in Cell & Developmental Biology. 106, 116-126 (2020).
- Wittenberg, J. B., Wittenberg, B. A. Active secretion of oxygen into the eye of fish. Nature. 194, 106-107 (1962).
- Damsgaard, C. Physiology and evolution of oxygen secreting mechanism in the fisheye. Comparative Biochemistry and Physiology. 252, 110840 (2021).
- Damsgaard, C., et al. A novel acidification mechanism for greatly enhanced oxygen supply to the fish retina. Elife. 9, (2020).
- Wittenberg, J. B., Haedrich, R. L. The choroid rete mirabile of the fish eye. II. Distribution and relation to the pseudobranch and to the swimbladder rete mirabile. Biological Bulletin. 146 (1), 137-156 (1974).
- Wittenberg, J. B., Wittenberg, B. A. The choroid rete mirabile of the fish eye. I. Oxygen secretion and structure: comparison with the swimbladder rete mirabile. Biological Bulletin. 146 (1), 116-136 (1974).
- Berenbrink, M. Historical reconstructions of evolving physiological complexity: O2 secretion in the eye and swimbladder of fishes. Journal of Experimental Biology. 210, 1641-1652 (2007).
- Berenbrink, M., Koldkjaer, P., Kepp, O., Cossins, A. R. Evolution of oxygen secretion in fishes and the emergence of a complex physiological system. Science. 307 (5716), 1752-1757 (2005).
- Keane, P. A., Sadda, S. R. Retinal imaging in the twenty-first century: State of the art and future directions. Ophthalmology. 121 (12), 2489-2500 (2014).
- Yung, M., Klufas, M. A., Sarraf, D. Clinical applications of fundus autofluorescence in retinal disease. International Journal of Retina and Vitreous. 2 (1), 12 (2016).
- Ang, M., et al. Optical coherence tomography angiography: a review of current and future clinical applications. Graefe's Archive for Clinical and Experimental Ophthalmology. 256 (2), 237-245 (2018).
- Spaide, R. F., Koizumi, H., Pozonni, M. C. Enhanced depth imaging spectral-domain optical coherence tomography. American Journal of Ophthalmology. 146 (4), 496-500 (2008).
- Shen, Q., et al. Magnetic resonance imaging of tissue and vascular layers in the cat retina. Journal of Magnetic Resonance Imaging. 23 (4), 465-472 (2006).
- Tan, G. X., Jamil, M., Tee, N. G., Zhong, L., Yap, C. H. 3D reconstruction of chick embryo vascular geometries using non-invasive high-frequency ultrasound for computational fluid dynamics studies. Annals of Biomedical Engineering. 43 (11), 2780-2793 (2015).
- Ho, S., Tan, G. X. Y., Foo, T. J., Phan-Thien, N., Yap, C. H. Organ dynamics and fluid dynamics of the HH25 chick embryonic cardiac ventricle as revealed by a novel 4D high-frequency ultrasound imaging technique and computational flow simulations. Annals of Biomedical Engineering. 45 (10), 2309-2323 (2017).
- Dittrich, A., Thygesen, M. M., Lauridsen, H. 2D and 3D echocardiography in the Axolotl (Ambystoma Mexicanum). Journal of Visualized Experiments: JoVE. (141), e57089 (2018).
- Jia, Y., et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Optics Express. 20 (4), 4710-4725 (2012).
- Clarke, K. W., Trim, C. M., Trim, C. M. . Veterinary Anaesthesia E-Book. , (2013).
- Flecknell, P. . Laboratory Animal Anaesthesia. , (2015).
- West, G., Heard, D., Caulkett, N. . Zoo Animal and Wildlife Immobilization and Anesthesia. , (2014).
- Lauridsen, H., Hansen, K., Nørgård, M. &. #. 2. 1. 6. ;., Wang, T., Pedersen, M. From tissue to silicon to plastic: three-dimensional printing in comparative anatomy and physiology. Royal Society Open Science. 3 (3), 150643 (2016).
- Lauridsen, H., et al. Inside out: Modern imaging techniques to reveal animal anatomy. PLoS One. 6 (3), 17879 (2011).
- Ruthensteiner, B., Heß, M. Embedding 3D models of biological specimens in PDF publications. Microscopy Research and Technique. 71 (11), 778-786 (2008).
- Damsgaard, C., Lauridsen, H. Deep vascular imaging in the eye with flow-enhanced ultrasound. bioRxiv. , 447055 (2021).
- Mueller, R. L., Ryan Gregory, T., Gregory, S. M., Hsieh, A., Boore, J. L. Genome size, cell size, and the evolution of enucleated erythrocytes in attenuate salamanders. Zoology. 111 (3), 218-230 (2008).
- Greis, C. Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS). Clinical Hemorheology and Microcirculation. 49, 137-149 (2011).
- Urs, R., Ketterling, J. A., Tezel, G., Silverman, R. H. Contrast-enhanced plane-wave ultrasound imaging of the rat eye. Experimental Eye Research. 193, 107986 (2020).
- Walls, G. L. . The vertebrate eye and its adaptive radiation. , (1942).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone