Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Cytotoxicity Assays with Zebrafish Cell Lines
W tym Artykule
Podsumowanie
This protocol presents commonly used cytotoxicity assays (Alamar Blue [AB], CFDA-AM, Neutral Red, and MTT assays) adapted for the assessment of cytotoxicity in zebrafish embryo (ZEM2S) and liver (ZFL) cell lines in 96-well plates.
Streszczenie
Fish cell lines have become increasingly used in ecotoxicity studies, and cytotoxicity assays have been proposed as methods to predict fish acute toxicity. Thus, this protocol presents cytotoxicity assays modified to evaluate cell viability in zebrafish (Danio rerio) embryo (ZEM2S) and liver (ZFL) cell lines in 96-well plates. The cytotoxicity endpoints evaluated are mitochondrial integrity (Alamar Blue [AB] and MTT assays), membrane integrity via esterase activity (CFDA-AM assay), and lysosomal membrane integrity (Neutral Red [NR] assay). After the exposure of the test substances in a 96-well plate, the cytotoxicity assays are performed; here, AB and CFDA-AM are carried out simultaneously, followed by NR on the same plate, while the MTT assay is performed on a separate plate. The readouts for these assays are taken by fluorescence for AB and CFDA-AM, and absorbance for MTT and NR. The cytotoxicity assays performed with these fish cell lines can be used to study the acute toxicity of chemical substances on fish.
Wprowadzenie
Chemical substances need to be tested regarding their safety for human health and the environment. Molecular and cellular biomarkers have been increasingly considered in safety assessments to predict effects on living organisms by regulatory agencies and/or legislations (e.g., REACH, OECD, US EPA)1,2, since they can precede the in vivo adverse outcome (e.g., endocrine disruption, immunological response, acute toxicity, phototoxicity)3,4,5,6,7. In this context, cytotoxicity has been taken as a measurement to predict fish acute toxicity5,8; however, it can have many other applications in ecotoxicity studies, such as defining sub-cytotoxic concentrations of chemical substances to study their most diverse set of effects on fish (e.g., endocrine-disrupting effects).
In cell culture systems (in vitro systems), the cytotoxicity of chemical substances can be determined by methods differing in the types of endpoints. For instance, a cytotoxicity method can be based on an endpoint related to specific morphology observed during the cell death process, while another can determine cytotoxicity by the measurement of cell death, viability and functionality, morphology, energy metabolism, and cell attachment and proliferation. Chemical substances can affect cell viability through different mechanisms, thus cytotoxicity assessment covering different cell viability endpoints is necessary to predict chemical effects9.
MTT and Alamar Blue (AB) are assays that determine effects on cell viability based on cell metabolic activity. The MTT assay evaluates the activity of the mitochondrial enzyme succinate dehydrogenase10. The reduction of yellowish 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyltetrazolium bromide (MTT) to formazan blue occurs only in viable cells, and its optical density is directly proportional to the number of viable cells10. The AB assay is a sensitive oxidation-reduction indicator, mediated by mitochondrial enzymes that fluoresce and change color upon reducing resazurin to resorufin by living cells11; however, cytosolic and microsomal enzymes also contribute to the reduction of AB and MTT12. These enzymes may include several reductases, such as alcohol and aldehyde oxidoreductases, NAD(P)H: quinone oxidoreductase, flavin reductase, NADH dehydrogenase, and cytochromes11.
The Neutral Red (NR) assay is a cell viability assay based on the incorporation of this dye into the lysosomes of viable cells13. The uptake of NR depends on the capacity of the cells to maintain pH gradients. The proton gradient inside the lysosomes maintains a pH lower than the cytoplasm. At normal physiological pH, the NR presents a net charge of approximately zero, which enables it to penetrate cell membranes. Thus, the dye becomes charged and is retained inside the lysosomes. Consequently, the greater the amount of retained NR, the greater the number of viable cells14. Chemical substances that damage the cell surface or lysosomal membranes impair the uptake of this dye.
The CFDA-AM assay is a fluorometric cell viability assay based on the retention of 5-carboxyfluorescein diacetate acetoxymethyl ester (CFDA-AM)15. 5-CFDA-AM, an esterase substrate, is converted into carboxyfluorescein, a fluorescent substance that is polar and nonpermeable by membranes of living cells15; thus, it is retained in the inner side of an intact cell membrane, indicating viable cells.
Recently, three cytotoxicity assays (CFDA-AM, NR, and AB assays) were combined in a validated ISO (International Organization for Standardization) guideline (ISO 21115:2019)16 and OECD (Organization for Economic Co-operation and Development) test method (OECD TG 249) to evaluate fish acute toxicity using the RTgill-W1 cell line (permanent cell line from rainbow trout [Oncorhynchus mykiss] gill) in 24-well plates17. Although there is an existing cell-based method to predict fish acute toxicity, efforts have been invested in developing similar methods with other fish species and increasing the throughput of the method. Some examples include the development of ZFL cell lines transfected with reporter genes for specific toxicity pathways18,19, phototoxicity tests in the RTgill-W1 cell line20, and the use of ZFL and ZF4 cell lines (zebrafish fibroblastic derived from 1-day-old embryos) to assess toxicity by several cytotoxicity assays21.
Danio rerio (zebrafish) is one of the main fish species used in aquatic toxicity studies; thus, cell-based methods with zebrafish cell lines for fish toxicity testing may be extremely useful. The ZFL cell line is a zebrafish epithelial hepatocyte cell line that presents the main characteristics of liver parenchymal cells and can metabolize xenobiotics7,22,23,24,25. Meanwhile, the ZEM2S cell line is an embryonic zebrafish fibroblastic cell line derived from the blastula stage that can be used to investigate developmental effects on fish26,27. Thus, this protocol describes four cytotoxicity assays (MTT, AB, NR, and CFDA-AM assays), with modifications to be performed with ZFL and ZEM2S cell lines in 96-well plates.
Protokół
NOTE: See the Table of Materials for the list of materials used in this protocol and Table 1 for the composition of solutions and media used in this protocol.
1. Preparing ZFL and ZEM2S cells
- Start with a T75 flask of ZFL or ZEM2S cells with 80% confluence, cultured in the respective complete medium at 28 °C without CO2.
- Remove the culture medium from the flask and wash the cells by adding 10 mL of 1x phosphate-buffered saline (PBS) (0.01 M). Add 3 mL of 1x trypsin (0.05% v/v; 0.5 mM trypsin-EDTA) to the culture flasks. Incubate at 28 °C for 3 min.
- Gently tap the flask to release the cells, and then stop the trypsin digestion by adding 3 mL of complete culture medium to the flask.
- Transfer the cell suspension to a 15 mL conical centrifuge tube and centrifuge at 100 × g for 5 min.
- After centrifugation, carefully remove the supernatant, add 1 mL of complete medium for ZFL or ZEM2S cells, and resuspend the pellet using a micropipette.
2. Cell counting by trypan blue dye exclusion
- Add 10 µL of the cell suspension and 10 µL of trypan blue dye to a microtube to count the cells and evaluate their viability. Mix the cell suspension and dye using a pipette.
- Then, transfer 10 µL of this mixture (cell suspension + trypan blue) to a Neubauer chamber and count the cells in the four large squares (Quadrants Q) placed at the corners of the chamber, considering viable cells to be those that do not take up trypan blue. Determine the number of viable cells using equation (1):
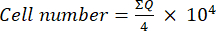 (1)
(1) - Calculate the final cell number in the cell suspension by multiplying the cell number determined using equation (1) by two (the dilution factor due to the use of trypan blue).
NOTE: Alternatively, an automated cell counting system (e.g., a cytomer with cell counting and viability function) can be used.
3. Cell plating in 96-well plates
- Calculate the cell suspension volume needed to obtain the number of cells required to perform the cytotoxicity assays. The number of viable cells for each cell line is indicated below:
- Plate 60,000 viable ZEM2S cells per well; thus, for the entire plate, use six million cells in 20 mL of complete medium (200 µL/well, 96-well plate).
- Plate 40,000 viable ZFL cells per well; thus, for the entire plate, use four million cells in 20 mL of complete medium (200 µL/well, 96-well plate).
- After that, transfer the respective volume of the cell suspension to a reagent reservoir (sterile) and fill up with the complete culture medium for ZFL or ZEM2S to 20 mL. Using a multichannel pipette, mix the solution gently up and down.
NOTE: Take care not to form foam or bubbles. - Add 200 µL of the cell suspension to each well of a transparent polystyrene 96-well plate using the multichannel micropipette. Incubate the plates at 28 °C for 24 h.
NOTE: The plate must have at least three wells without cells for the blank control, and only complete media should be added to these wells. The edge effect (caused by higher evaporation in the edge wells) commonly occurs in 96-well plate assays and can affect the viability of the cells in the edge wells of the plate28. This effect can be higher or lower depending on the 96-well plate brand and design28. Although we did not notice any cell growth/viability disturbance for ZFL and ZEM2S in the edge wells, we suggest sealing the plate with parafilm or adhesive sealing foil to prevent this effect, or culturing the cells only in the 60 inside wells and filling the edge wells with PBS.
4. Exposure of cells to test chemical
- Carefully discard the spent media from the wells using a multichannel micropipette.
- Expose the cells to test chemicals at different concentrations. Prepare the solutions of the test chemical concentrations in the culture media for ZFL or ZEM2S without fetal bovine serum (FBS) (exposure media). Then, add 100 µL per well of these solutions in technical triplicate (i.e., three wells/test chemical concentration).
- For controls, place the control groups on the same plate as the test chemical in technical triplicates (three wells/control group). Thus, for the blank control (B), add 100 µL of the exposure media in the cell-free wells, for the negative control (NC), add 100 µL of the exposure media to wells with cells, and for the positive control (PC), expose the cells to a solution of 1% Triton X-100 prepared in the exposure media. In some cases, a solvent control (SC) should be included in the plate, considering a clearly non-cytotoxic concentration as a final solvent concentration.
NOTE: It is recommended to use 0.5% DMSO as solvent; DMSO can be used up to 1% as solvent in these cell lines without exceeding the cytotoxicity threshold of 10% related to the negative control. - Incubate the plates at 28 °C for 24 h. Seal the plates with parafilm or adhesive sealing foil to prevent culture medium evaporation.
NOTE: Certain chemicals may have intrinsic background absorbance or fluorescence that may interfere with the absorbance or fluorescence of the indicator dye(s) (e.g., compounds with color may influence absorbance, serum albumin29, and compounds interfering with reduction enzymes30,31). In this case, the plate must include an additional control by adding test chemical solutions in the wells without cells. This is to verify the possible interference of the chemical auto-absorbance/autofluorescence with the dyes. If interference is detected, one should evaluate whether it can be excluded to obtain a correct prediction of cytotoxicity.
5. Cytotoxicity assays
NOTE: Prepare all solutions according to Table 1. All the steps described below (Figure 1) are carried out under sterile conditions. The use of a pipette to discard the exposure media is not recommended, because the cells can easily detach from the wells after chemical treatment.
- AB and CFDA-AM assays
- After 24 h of test chemical exposure, carefully discard the exposure media by pouring the content into a collection tray.
- Wash the plate with 200 µL of PBS. Carefully remove the PBS by pouring it into a collection tray to avoid losing cells.
- Add 100 µL per well of AB/CFDA-AM solution. Incubate the plate for 30 min in the dark at 28 °C.
- Measure the fluorescence in a fluorescence plate reader at 530 nm (excitation) and 595 nm (emission) for AB, and at 493 nm (excitation) and 541 nm (emission) for CFDA-AM.
- NR assay
NOTE: The steps for the NR assay are carried out immediately after the AB and CFDA-AM assays (Figure 1).- Centrifuge the NR working solution (40 µg/mL) at 600 × g for 10 min.
NOTE: Precipitation of NR in the tube must not be transferred to the plates. Thus, after centrifugation of the NR working solution, collect the supernatant using a pipette without aspirating the NR precipitates. Transfer the supernatant to a reagent reservoir. - Carefully remove the AB/CFDA-AM solution by pouring the content into a collection tray.
- Add 100 µL per well of the NR working solution using a multichannel micropipette. Incubate the plate at 28 °C for 3 h.
NOTE: After the 3 h incubation, observe if NR precipitation occurred in the plates using a microscope. NR precipitates may interfere with the quantification of the cell viability, thus, they should not be present. - Carefully remove the NR solution by pouring the content into a collection tray. Wash the wells by adding 150 µL of PBS per well.
- Add 150 µL per well of the NR extraction solution and incubate the plate on a plate shaker for 10 min for gently shaking. Measure the absorbance at 540 nm in a plate reader.
NOTE: A second readout at 690 nm should be carried out to exclude any background fingerprint absorbance in the plate.
- Centrifuge the NR working solution (40 µg/mL) at 600 × g for 10 min.
- MTT assay
NOTE: The MTT assay must be carried out separately from the assays described above (in a new plate) (Figure 2).- Carefully remove the exposure media by pouring the content into a collection tray.
- Add 100 µL of MTT working solution per well using a multichannel micropipette. Incubate the plate at 28 °C for 4 h.
- Discard the MTT solution by pouring the content into a collection tray.
- Add 100 µL per well of DMSO to extract the formazan crystals, incubating the plate on a plate shaker for 10 min. Measure the absorbance at 570 nm using a plate reader.
NOTE: A second readout at 690 nm should be carried out to exclude any background fingerprint absorbance in the plate. It is important to note that test chemicals may interfere with MTT, which must be evaluated to ensure the quality of the generated data32. For this, cell-free wells containing the test concentrations and MTT (0.5 mg/mL) should be exposed, followed by incubation to observe any color change in the wells that may increase the absorbance and lead to false viability results. Chemicals that interact with MTT must be avoided in this test.
6. Calculating cell viability/cytotoxicity
NOTE: The raw absorbance or fluorescence acquired is used to calculate cell viability as a percentage related to the negative control (for test chemicals prepared directly in exposure media) or solvent control (for test chemicals prepared using solvents, such as DMSO). Before determining the cell viability percentage, the raw data must be normalized by the blank control.
- Calculate the average absorbance or fluorescence for each test chemical concentration and control group (three wells/treatment).
- To determine the cell viability percentage relative to control (negative or solvent), use equation (2):
 (2)
(2)
NOTE: Absorbance (abs) or fluorescence (fluo) units represent the mean of absorbance or fluorescence measured in the three wells per concentration; blank represents wells without cells.
Wyniki
Figure 3 shows the plates of the AB, CFDA-AM, NR, and MTT assays. For the AB assay (Figure 3A), the blank wells and wells with no or a reduced number of viable cells show blue color and low fluorescence, while the wells with a high number of viable cells are pinkish and present high fluorescence values due to the transformation of resazurin (AB) into resorufin (pinkish substance) by the viable cells. For the CFDA-AM assay, there is no visible difference in the ...
Dyskusje
Cytotoxicity assays are widely used for in vitro toxicity evaluation, and this protocol article presents four commonly used cytotoxicity assays modified to be performed in zebrafish cell lines (i.e., cell density for 96-well plate, incubation time in the MTT assay, FBS depletion during the chemical exposure condition, and maximal acceptable concentration for the SC). As these assays quantify cytotoxicity by different cell viability endpoints (metabolic function, lysosomal membrane integrity, and cell membrane in...
Ujawnienia
The authors declare no conflict of interest.
Podziękowania
In memory of Dr. Márcio Lorencini, a coauthor of this work, an excellent researcher in the field of cosmetics and devoted to promoting cosmetic research in Brazil. The authors are grateful for the Multi-user Laboratory in the Physiology Department (UFPR) for equipment availability and for the financial support of the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) (Finance Code 001) and the Grupo Boticario.
Materiały
| Name | Company | Catalog Number | Comments |
| 5-CFDA, AM (5-Carboxyfluorescein Diacetate, Acetoxymethyl Ester) | Invitrogen | C1345 | |
| Cell culture plate, 96 well plate | Sarstedt | 83.3924 | Surface: Standard, flat base |
| DMEM | Gibco | 12800-017 | Powder, high glucose, pyruvate |
| FBS - Fetal Bovine Serum, qualified, USDA-approved regions | Gibco | 12657-029 | |
| Ham's F-12 Nutrient Mix, powder | Gibco | 21700026 | Powder |
| HEPES (1 M) | Gibco | 15630080 | |
| Leibovitz's L-15 Medium | Gibco | 41300021 | Powder |
| Neutral red | Sigma-Aldrich | N4638 | Powder, BioReagent, suitable for cell culture |
| Orbital shaker | Warmnest | KLD-350-BI | 22 mm rotation diameter |
| Dulbeccos PBS (10X) with calcium and magnesium | Invitrogen | 14080055 | |
| Penicillin-Streptomycin (10,000 U/mL) | Gibco | 15140122 | |
| Resazurin sodium salt | Sigma-Aldrich | R7017 | Powder, BioReagent, suitable for cell culture |
| RPMI 1640 Medium | Gibco | 31800-014 | Powder |
| Sodium bicarbonate | Sigma-Aldrich | S5761 | Powder, bioreagent for molecular biology |
| Thiazolyl Blue Tetrazolium Bromide 98% | Sigma-Aldrich | M2128 | |
| Trypan blue stain (0.4%) | Gibco | 15250-061 | |
| Trypsin-EDTA (0.5%), no phenol red | Gibco | 15400054 | |
| ZEM2S cell line | ATCC | CRL-2147 | This cell line was kindly donated by Professor Dr. Michael J. Carvan (University of Wisconsin, Milwaukee, USA) |
| ZFL cell line | BCRJ | 256 |
Odniesienia
- ECHA. Non-Animal Approaches-Current Status of Regulatory Applicability Under the REACH, CLP and Biocidal Products Regulations. ECHA. , (2017).
- Alternative Methods Accepted by US Agencies. National Toxicology Program, and US Department of Health and Human Services Available from: https://ntp.niehs.nih.gov/whatwestudy/niceatm/accept-methods/index.html (2022)
- Schirmer, K. Proposal to improve vertebrate cell cultures to establish them as substitutes for the regulatory testing of chemicals and effluents using fish. Toxicology. 224 (3), 163-183 (2006).
- Scholz, S., et al. Alternatives to in vivo tests to detect endocrine disrupting chemicals (EDCs) in fish and amphibians-screening for estrogen, androgen and thyroid hormone disruption. Critical Reviews in Toxicology. 43 (1), 45-72 (2013).
- Tanneberger, K., et al. Predicting fish acute toxicity using a fish gill cell line-based toxicity assay. Environmental Science & Technology. 47 (2), 1110-1119 (2013).
- Roesler, R., Lorencini, M., Pastore, G. Brazilian cerrado antioxidant sources: cytotoxicity and phototoxicity in vitro. Food Science and Technology. 30, 814-821 (2010).
- Ruyra, A., et al. Zebrafish liver (ZFL) cells are able to mount an anti-viral response after stimulation with Poly (I:C). Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 182, 55-63 (2015).
- Natsch, A., Laue, H., Haupt, T., von Niederhäusen, V., Sanders, G. Accurate prediction of acute fish toxicity of fragrance chemicals with the RTgill-W1 cell assay. Environmental Toxicology and Chemistry. 37 (3), 931-941 (2018).
- Freshney, R. I. Cytotoxicity. Culture of Animal Cells: A Manual of Basic Technique. , (2005).
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 65 (1-2), 55-63 (1983).
- O'Brien, J., Wilson, I., Orton, T., Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry. 267 (17), 5421-5426 (2000).
- Gonzalez, R. J., Tarloff, J. B. Evaluation of hepatic subcellular fractions for Alamar blue and MTT reductase activity. Toxicology In Vitro. 15 (3), 257-259 (2001).
- Borenfreund, E., Puerner, J. A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicology Letters. 24 (2-3), 119-124 (1985).
- Repetto, G., del Peso, A., Zurita, J. L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nature Protocols. 3 (7), 1125-1131 (2008).
- Kamiloglu, S., Sari, G., Ozdal, T., Capanoglue, E. Guidelines for cell viability assays. Food Frontiers. 1 (3), 332-349 (2020).
- Water Quality-Determination of Acute Toxicity of Water Samples and Chemicals to a Fish Gill Cell Line (RTgill-W1) (ISO 21115:2019). International Organization for Standardization Available from: https://www.iso.org/standar/69933.html (2019)
- Organisation for Economic Co-operation and Development. . Test Guideline No. 249: Fish Cell Line Acute Toxicity-The RTgill-W1 Cell Line Assay. OECD Guidelines for the Testing of Chemicals, Section 2. Effects on Biotic Systems. , (2021).
- Lungu-Mitea, S., Lundqvist, J. Potentials and pitfalls of transient in vitro reporter bioassays: interference by vector geometry and cytotoxicity in recombinant zebrafish cell lines. Archives of Toxicology. 94 (8), 2769-2784 (2020).
- Lungu-Mitea, S., Han, Y., Lundqvist, J. Development, scrutiny, and modulation of transient reporter gene assays of the xenobiotic metabolism pathway in zebrafish hepatocytes. Cell Biology and Toxicology. , 1-23 (2021).
- Schirmer, K., Chan, A. G., Greenberg, B. M., Dixon, D. G., Bols, N. C. Methodology for demonstrating and measuring the photocytotoxicity of fluoranthene to fish cells in culture. Toxicology In Vitro. 11 (1-2), 107-119 (1997).
- Lungu-Mitea, S., et al. Modeling bioavailable concentrations in zebrafish cell lines and embryos increases the correlation of toxicity potencies across test systems. Environmental Science & Technology. 55 (1), 447-457 (2021).
- Cavalcante, D. G. S. M., et al. Cytotoxic, biochemical and genotoxic effects of biodiesel produced by different routes on ZFL cell line. Toxicology In Vitro. 28 (6), 1117-1125 (2014).
- Meng, Q., Yeung, K., Chan, K. M. Toxic effects of octocrylene on zebrafish larvae and liver cell line (ZFL). Aquatic Toxicology. 236, 105843 (2021).
- Kwok, M. L., Chan, K. M. Oxidative stress and apoptotic effects of copper and cadmium in the zebrafish liver cell line ZFL. Toxicology Reports. 7, 822-835 (2020).
- Yang, J., Chan, K. M. Evaluation of the toxic effects of brominated compounds (BDE-47, 99, 209, TBBPA) and bisphenol A (BPA) using a zebrafish liver cell line, ZFL. Aquatic Toxicology. 159, 138-147 (2015).
- Bradford, C. S., Sun, L., Collodi, P., Barnes, D. W. Cell cultures from zebrafish embryos and adult tissues. Journal of Tissue Culture Methods. 16 (2), 99-107 (1994).
- He, S., et al. Genetic and transcriptome characterization of model zebrafish cell lines. Zebrafish. 3 (4), 441-453 (2006).
- Mansoury, M., Hamed, M., Karmustaji, R., Al Hannan, F., Safrany, S. T. The edge effect: A global problem. The trouble with culturing cells in 96-well plates. Biochemistry and Biophysics Reports. 26, 100987 (2021).
- Funk, D., Schrenk, H. -. H., Frei, E. Serum albumin leads to false-positive results in the XTT and the MTT assay. BioTechniques. 43 (2), 178 (2007).
- Dayeh, V. R., Bols, N. C., Tanneberger, K., Schirmer, K., Lee, L. E. J. The use of fish-derived cell lines for investigation of environmental contaminants: An update following OECD's fish toxicity testing framework no. 171. Current Protocols in Toxicology. 1, (2013).
- Stepanenko, A. A., Dmitrenko, V. V. Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene. 574 (2), 193-203 (2015).
- Ulukaya, E., Colakogullari, M., Wood, E. J. Interference by anti-cancer chemotherapeutic agents in the MTT-tumor chemosensitivity assay. Chemotherapy. 50 (1), 43-50 (2004).
- Sebaugh, J. L. Guidelines for accurate EC50/IC50 estimation. Pharmaceutical Statistics. 10 (2), 128-134 (2011).
- Weimer, M., et al. The impact of data transformations on concentration-response modeling. Toxicology Letters. 213 (2), 292-298 (2012).
- Green, J. W., Holbech, T. A., Henrik, Chapter 4: Analysis of Continuous Data (Regression). Statistical Analysis of Ecotoxicity Studies. , (2018).
- Proença, S., et al. Effective exposure of chemicals in in vitro cell systems: A review of chemical distribution models. Toxicology In Vitro. 73, 105133 (2021).
- Guidony, N. S., et al. ABC proteins activity and cytotoxicity in zebrafish hepatocytes exposed to triclosan. Environmental Pollution. 271, 116368 (2021).
- da Silva, N. D. G., et al. Interference of goethite in the effects of glyphosate and Roundup® on ZFL cell line. Toxicology In Vitro. 65, 104755 (2020).
- Yang, Y., et al. Temperature is a key factor influencing the invasion and proliferation of Toxoplasma gondii in fish cells. Experimental Parasitology. 217, 107966 (2020).
- Lopes, F. M., Sandrini, J. Z., Souza, M. M. Toxicity induced by glyphosate and glyphosate-based herbicides in the zebrafish hepatocyte cell line (ZF-L). Ecotoxicology and Environmental Safety. 162, 201-207 (2018).
- Lachner, D., Oliveira, L. F., Martinez, C. B. R. Effects of the water soluble fraction of gasoline on ZFL cell line: Cytotoxicity, genotoxicity and oxidative stress. Toxicology In Vitro. 30, 225-230 (2015).
- Morozesk, M., et al. Effects of multiwalled carbon nanotubes co-exposure with cadmium on zebrafish cell line: Metal uptake and accumulation, oxidative stress, genotoxicity and cell cycle. Ecotoxicology and Environmental Safety. 202, 110892 (2020).
- Dognani, G., et al. Nanofibrous membranes for low-concentration Cr VI adsorption: kinetic, thermodynamic and the influence on ZFL cells viability. Materials Research. , 24 (2021).
- ZEM2S (ATCC®CRL-2147™). American Type Culture Collection Available from: https://www.atcc.org/products/crl-2147 (2023)
- Chen, Y., et al. Acute toxicity of the cationic surfactant C12-benzalkonium in different bioassays: how test design affects bioavailability and effect concentrations. Environmental Toxicology and Chemistry. 33 (3), 606-615 (2014).
- Pomponio, G., et al. In vitro kinetics of amiodarone and its major metabolite in two human liver cell models after acute and repeated treatments. Toxicology In Vitro. 30, 36-51 (2015).
- Mori, M., Wakabayashi, M. Cytotoxicity evaluation of chemicals using cultured fish cells. Water Science and Technology. 42 (7-8), 277-282 (2000).
- Caminada, D., Escher, C., Fent, K. Cytotoxicity of pharmaceuticals found in aquatic systems: comparison of PLHC-1 and RTG-2 fish cell lines. Aquatic Toxicology. 79 (2), 114-123 (2006).
- Giltrap, M., et al. In vitro screening of organotin compounds and sediment extracts for cytotoxicity to fish cells. Environmental Toxicology and Chemistry. 30 (1), 154-161 (2011).
- Hollert, H., Duerr, M., Erdinger, L., Braunbeck, T. Cytotoxicity of settling particulate matter and sediments of the Neckar River (Germany) during a winter flood. Environmental Toxicology and Chemistry. 19 (3), 528-534 (2000).
- Pannetier, P., et al. Toxicity assessment of pollutants sorbed on environmental sample microplastics collected on beaches: Part I-adverse effects on fish cell line. Environmental Pollution. 248, 1088-1097 (2019).
- Ternjej, I., Srček, V. G., Mihaljević, Z., Kopjar, N. Cytotoxic and genotoxic effects of water and sediment samples from gypsum mining area in channel catfish ovary (CCO) cells. Ecotoxicology and Environmental Safety. 98, 119-127 (2013).
- Hamid, R., Rotshteyn, Y., Rabadi, L., Parikh, R., Bullock, P. Comparison of alamar blue and MTT assays for high throughput screening. Toxicology In Vitro. 18 (5), 703-710 (2004).
- Vistica, D. T., et al. Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Research. 51 (10), 2515-2520 (1991).
- Knauer, K., Lampert, C., Gonzalez-Valero, J. Comparison of in vitro and in vivo acute fish toxicity in relation to toxicant mode of action. Chemosphere. 68 (8), 1435-1441 (2007).
- Stadnicka-Michalak, J., Tanneberger, K., Schirmer, K., Ashauer, R. Measured and modeled toxicokinetics in cultured fish cells and application to in vitro-in vivo toxicity extrapolation. PLoS One. 9 (3), 92303 (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone