Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Generating Spheroids from Various Chondrocytes using Low-Adhesive Conditions under Gravity and Homemade Mini-Bioreactors
W tym Artykule
Podsumowanie
This study describes a method for producing chondrocytic spheroids by aggregating cells into spheroids under low-adhesion conditions using gravity, followed by culturing the resulting spheroids in mini-bioreactors.
Streszczenie
Cartilage repair in chronic joint diseases demands advanced cell-based therapies to regenerate damaged tissues effectively. This protocol provides a step-by-step method for differentiating induced pluripotent stem cells (iPSCs) into chondrocyte-based spheroids, supporting tissue engineering and cell therapy applications. The differentiation process is carefully structured to promote chondrogenic lineage commitment, beginning with iPSCs cultured in specific media that sequentially guide cells through critical stages of differentiation. Initially, iPSCs are expanded to reach optimal confluency before induction toward chondrogenic lineage using a series of defined media changes. By day 10, cells are transitioned to a chondrogenesis-promoting medium that enhances the formation of chondrocyte-like cells expressing key markers of mature chondrocytes. Further aggregation in 96-well agarose-coated plates leads to the formation of three-dimensional spheroids, which are then cultured in custom mini-bioreactors designed to simulate a microenvironment that encourages extracellular matrix (ECM) deposition. By enabling scalable production of chondrocyte spheroids that mimic native cartilage characteristics, this approach offers a promising, reproducible solution for developing cell-based treatments for cartilage defects, providing broad utility for clinical and research applications in musculoskeletal regenerative medicine.
Wprowadzenie
The prevalence of joint disease leads to significant economic burdens due to the increasing number of disabled patients and the costs associated with their care. Hyaline cartilage is a connective avascular tissue with limited regenerative potential1. Prolonged use of certain non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and chemotherapy or radiotherapy can further diminish cartilage's regenerative capacity, nearly eliminating its ability to heal2. This makes it challenging to obtain autologous cartilage cells for cellular therapy.
The technology of three-dimensional (3D) cell cultivation, including chondrocytes, has long been recognized as an emerging research area with significant potential. These 3D structures are being studied for applications in both fundamental biological research and regenerative medicine. Spheroids derived from autologous chondrocytes hold therapeutic promise for addressing cartilage tissue degeneration, a condition currently receiving considerable global attention3.
Spheroids derived from chondrocytes differentiated from iPSCs represent a promising alternative to primary chondrocytes, offering significant advantages for cartilage repair. iPSCs provide an almost limitless capacity for self-renewal and possess a broad differentiation potential, which allows for the production of chondrocytes in quantities sufficient for clinical applications without invasive procedures for cell collection4. Moreover, transitioning from traditional two-dimensional (2D) chondrocyte cultures to 3D culture systems, such as chondrospheres, further enhances the viability and functionality of these cells by creating a more physiologically relevant environment. Studies show that chondrocytes cultured in 3D spheroids better maintain their phenotype, displaying lower rates of dedifferentiation and higher expression levels of key hyaline cartilage markers, such as collagen type II and aggrecan5.
Despite the potential of iPSC-derived chondrospheres, standardized protocols for generating high-quality chondrospheres remain limited. Variability in protocols across studies often leads to inconsistencies in chondrocyte quality and extracellular matrix composition, affecting their effectiveness for therapeutic use6. Here, a refined protocol is presented that standardizes the generation of spheroids from iPSC-derived chondrocytes using affordable, custom-made mini-bioreactors. The mini-bioreactor culture phase is essential, as it provides a controlled, low-adhesion setting that optimizes nutrient distribution, ECM maturation, and spheroid compaction7. This phase promotes robust expression of essential chondrocyte markers, including collagen type II, aggrecan, and SOX9, along with an ECM composition that closely resembles native cartilage. Regular media changes and careful control of environmental conditions -- temperature, CO2, and rotation speed -- further ensure the viability and maturation of the chondrocyte spheroids. This protocol has been optimized to create high-quality chondrospheres with strong expression of hyaline cartilage markers in a cost-effective and scalable manner, suitable for research and clinical applications in cartilage repair.
Protokół
The study was reviewed and approved by the Ethics Committee of the LOPUKHIN FRCC PCM (protocol No. 2019/02 of April 9, 2019). All donor samples were obtained in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all participants and/or their legal guardians.
NOTE: Maintain sterile technique throughout the protocol. Warm all culture media and solutions to 37 °C before applying them to cells or spheroids. Cultivate cells in a CO2 incubator at 37 °C, with 5% CO2 upon 80% humidity. The protocol scheme is shown in Figure 1.
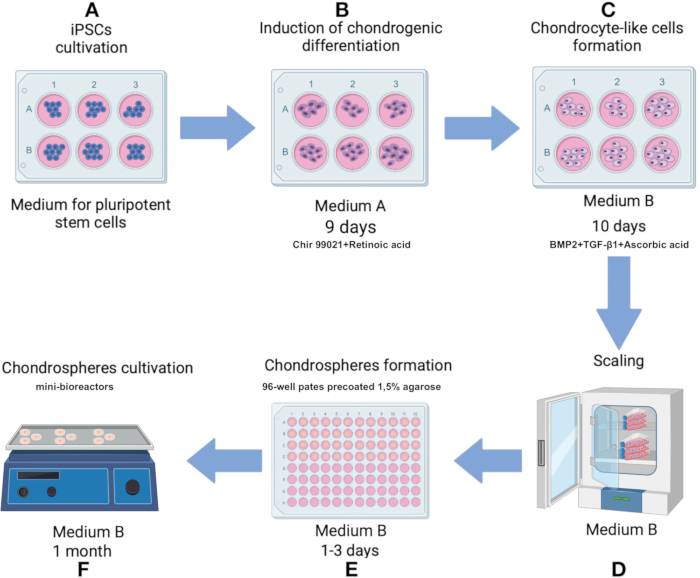
Figure 1: Protocol for producing chondrospheres from iPSC line IPSRG4S. (A) Initially, iPSCs are cultured in pluripotent stem cell media until 80% confluency is reached. (B) To induce differentiation towards the chondrocytic linage, iPSCs are then cultured in medium A for 2 days. The medium is subsequently replaced with a formulation lacking Chir 99021 and Rho kinase inhibitor, and cells are cultured for an additional 6 days with medium changes every 2 days. (C) The cells are then transferred to medium B to promote chondrogenesis for the next 10 days. (D) Once a sufficient cell number is achieved, the process advances to the spheroids production stage. (E) The cells are aggregated into spheroids in low-adhesive conditions under gravity. (F) The resulting spheroids are cultured in mini-bioreactors with medium B. Please click here to view a larger version of this figure.
1. Transformation of Petri dishes into mini-bioreactors
NOTE: Details in a previous article5.
- Slice centrifuge tubes into 7-8 mm high rings using sterile autoclave scissors (autoclave settings: 121 °C (250 °F) at 15 psi, 30 min, and post-autoclave drying for 20 min).
- Crush low-adhesion, untreated, or microbiological Petri dishes into small pieces. Dissolve approximately 1 g of plastic particles in 10 mL of chloroform overnight to create a liquid plastic solution. Conduct this procedure in a fume hood.
- Verify that the liquid plastic is sufficiently viscous for pipetting. Ensure that droplets maintain a spherical shape rather than spreading on the surface. If the solution is too thin, add more plastic particles, usually requiring 1-3 g. If it is too thick, add additional chloroform, usually requiring up to 5 mL.
- Form a plastic knob at the center of a sterile, ultra-low adhesion 60 mm Petri dish using one of the two effective methods outlined below.
- Option 1: Place the autoclaved plastic ring in the center of the Petri dish, then apply liquid plastic inside the ring; one drop will require about 100-200 µL drops of liquid plastic.
- Option 2: Apply the same number of drops of liquid plastic directly into the center of the Petri dish without using the plastic ring.
- Allow the dishes to dry uncovered for 2-3 h in a laminar flow hood. After drying, expose the dishes to ultraviolet radiation for 20-30 min.
2. Treatment of culture dishes with gelatin
- Prepare a 0.1% gelatin solution by dissolving powdered gelatin in distilled water at 95 °C. Pass the solution sequentially through 0.45 µm and 0.25 µm membrane filters, then store the filtrate at 4 °C.
- Immediately before chondrocyte reseeding, apply the 3 mL of gelatin solution to the 25 cm2 flask (up to 15 g of cartilage tissue per T25 flask is recommended). Incubate at room temperature (RT) for 30 min,remove the gelatin, rinse twice with PBS, and proceed with cell seeding.
3. Isolation and cultivation of chondrocytes
NOTE: Chondrocytes are isolated from non-weight-bearing cartilage regions from patients undergoing total knee replacement. Harvest all specimens under sterile conditions and store them in sterile tubes filled with DMEM containing 200 U/mL penicillin/streptomycin solution. Store cartilage tissue in a refrigerator at 2-8 °C.
- Begin enzymatic treatment within 2 days of cartilage harvest. Wash cartilage pieces in Hanks' solutionusing 60 mm Petri dishes, then cut cartilage into fragments of approximately 1-2 mm in diameter using autoclaved scissors.
- Transfer cartilage pieces into 15 mL tubes and digest them in 0.01% collagenase type II solution in DMEM, approximately 1 mL per 1 g of cartilage, within 8-12 h in a CO2 incubator set to 37 °C with 5% CO2, 100% humidity, under continuous agitation on an orbital shaker at 150-200 rpm.
- After digestion, wash the cartilage fragments by adding DMEM to a 15 mL tube, then centrifuge at 300 × g for 10 min. Resuspend the fragments in a chondrocyte culture medium (Supplementary Table 1) and seed them onto an adhesive T25 flask pre-coated with 0.1% gelatin solution.
- Passage chondrocytes when a confluent density of 80% is achieved.
- For passaging, wash chondrocytes with Hanks' solution, then incubate cells with 0.25% trypsin solution at 37 °C for 5 min.
- To inactivate trypsin, add an equal volume of DMEM + 10% FBS. Resuspend chondrocytes in fresh culture medium, then distribute them at a 1:3 ratio, seeding 20,000-30,000 cells per 1 cm2.
- Place the culture flask in an incubator at 37 °C with 5% CO2 and 100% humidity.
4. Cultivation of pluripotent stem cells
NOTE: In collaboration with the Stem Cell Laboratory of the Molecular Brain Research Group, Department of Neurobiology, A.I. Virtanen Institute, University of Eastern Finland, Kuopio, Finland, an iPSCs line IPSRG4S, was successfully generated in a previous study8.
- Cultivate iPSCs in 6-well plates pre-coated with a matrix containing extracellular proteins ( Figure 1A). Use a medium for pluripotent stem cells (Supplementary Table 1).
- Passage cells at 80% confluency, maintaining a 1:4 ratio (50,000-75,000 cells per 1 cm2). Approximately 2-3 h before detachment, transfer iPSCs to the medium for pluripotent stem cells containing Rho kinase inhibitor Y27632 at a final concentration 1 µM for 1 day.
- Wash iPSCs with Hanks' solution and incubate at 37 °C with 0.05% trypsin solution. To inactivate trypsin, add an equal volume of DMEM + 10% FBS. Centrifuge the cell suspension at 200 x g for 5 min in 15 mL tubes.
- Discard the supernatant and resuspend the cells in a fresh medium for pluripotent stem cells. To enhance cell viability, add Rho kinase inhibitor Y27632 to the medium at a final concentration of 1 µM for 1 day. Place the culture plate in a CO2 incubator set to 37 °C with 5% CO2 and 100% humidity.
5. Chondrogenic differentiation of iPSCs
- Initiate differentiation towards the chondrocytic lineage by culturing cells in Medium A (Supplementary Table 1) for the first 2 days. On day 3, replace Medium A with a solution that excludes Chir 99021 and Rho kinase inhibitor while keeping all other components unchanged. Change the medium every 2 days from days 3 to 9 (Figure 1B).
- On day 10, transfer chondrocyte-like cells to Medium B (Supplementary Table 1), formulated to facilitate chondrogenesis. Change the medium every 2 days for 10 days (Figure 1C).
- Prepare a 96-well cell suspension culture plate in advance by coating each well with 1.5% agarose in distilled water. Melt the agarose in a microwave until boiling (about 60-90 s at 700 W). Once agarose is liquefied, add 75 µL of agarose to each well and allow it to harden at RT (approximately 15 min).
- Add 150 µL of DMEM medium to each well and incubate the plate in a CO2 incubator for at least 12 h.
NOTE: Prepared plates can be stored for up to 1 month in a CO2 incubator at 37 °C if the medium in each well is replenished to compensate for evaporation. - On day 12, after observing phenotypical changes associated with chondrogenesis, initiate spheroids formation using the generated chondrocyte-like cells.
NOTE: At this stage, it is advisable to analyze the expression of key chondrocytic markers with immunocytochemistry (ICC) and quantitative polymerase chain reaction (qPCR) using the 2-ΔΔCT method9 to confirm successful chondrogenic differentiation of iPSCs. During passaging from 6-well plate to a 75 cm2 cell culture flask, a small portion of cells can be seeded into a 48-well plate, matching the number of markers to be analyzed. Perform ICC analysis on these samples to verify gene expression.
6. Formation of spheroids from chondrocyte-like iPSC-derived cells
- After obtaining chondrocyte-like derivatives, passage cells at 80% confluency. Detach cells from the 6-well plates using a 0.05% trypsin solution. To inactivate trypsin, add an equal volume of DMEM + 10% FBS, and then transfer cells to a 15 mL tube and centrifuge at 200 x g for 5 min.
- Remove the supernatant, resuspend the cells in 1 mL of Medium B, and transfer to a 75 cm2 cell culture flask pre-coated with 0.1% gelatin solution (Figure 1D). Once cells reach 80% confluency as a monolayer, detach cells again with trypsin and resuspend in Medium B as described.
- Transfer cells into a 96-well plate coated with 1.5% agarose at a density of 100,000 cells per well. Cultivate in Medium B, adding 150 µL of complete medium per well (Figure 1D, 3A).
- Maintain cells in the 96-well plate coated with 1.5% agarose for a minimum of 1 day and a maximum of 3 days until spheroids are formed (Figure 2B). Transfer spheroids from the wells using a 1 mL pipette tip with a trimmed end, then transfer to a 15 mL tube. Allow spheroids to settle for 2-3 min and remove the supernatant.
- Immerse the spheroids in freshly thawed, undiluted basement membrane matrix solution maintained at 4 °C. After 30 min, collect spheroids by passive sedimentation in a 15 mL tube or by gentle centrifugation at 100 x g for 1 min.
NOTE: Matrix should be thawed overnight on ice in a 2-8 °C refrigerator. After the first thaw, single-use aliquots of the matrix are made to avoid repeated freeze-thaw cycles. Polypropylene or other freezer-compatible tubes are used and stored at -70 °C or -20 °C. Be watchful of the expiration date of the batch/lot, which is 2 years from the manufacture date. - Transfer spheroids into mini-bioreactors and add 6 mL of Medium B. Place the mini-bioreactor on an orbital shaker inside a CO2 incubator set to 37 °C with 5% CO2 and 100% humidity and an agitation speed of 70-75 rpm (Figure 1F).
- Change the medium weekly or as required by medium depletion or color change of the acid-base indicator. To do this, allow spheroids to settle by gravity in a 15 mL tube, and then carefully remove the supernatant. Add fresh Medium B to the tube, and transfer spheroids back to the mini-bioreactors.
Wyniki
The outlined protocol is illustrated in Figure 1. This methodology employs two distinct culture media to drive the differentiation of iPSCs into chondrocyte spheroids over a minimum duration of 1 month (Figure 2). The differentiation process is initiated when iPSCs achieve 75%-90% confluency (Figure 1B). Early indicators of chondrogenic differentiation emerge around days 9-10 of cultivation in medium...
Dyskusje
iPSCs represent a transformative tool in regenerative medicine, offering the potential to generate patient-specific chondrocytes for cartilage repair. Current protocols leverage directed differentiation through mesodermal pathways, with key signaling molecules like TGF-β and BMP-2 promoting chondrocytic lineage commitment. These methods aim to replicate embryonic cartilage development, enabling the production of extracellular matrix components such as collagen type II and aggrecan, essential for functional cartilage...
Ujawnienia
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Podziękowania
The research was supported with allocation #22-15-00250 by the Russian Science Foundation.
Materiały
| Name | Company | Catalog Number | Comments |
| 0.05% Trypsin solution | Thermo Fisher Scientific | 25300-062 | |
| 0.25% Trypsin solution | Thermo Fisher Scientific | 25200-072 | |
| Advanced DMEM/F12 Eagle's medium | Thermo Fisher Scientific | 12634028 | |
| Aggrecan Monoclonal Antibody | Invitrogen | AHP0022 | Host: Mouse; Dilution: 1/500 |
| Ascorbic acid | Sigma | A4544 | 50 μg/mL |
| B-27 supplement | Thermo Fisher Scientific | 17504044 | 1x or 2% |
| Beta-mercaptoethanol | Serva | 28625 | 90 mM |
| BMP2 | Miltenyi biotec | 130-110-922 | 10 ng/mL |
| Chir 99021 | Miltenyi biotec | 130-103-926 | 10 μM |
| COL1A1 (E6A8E) Monoclonal antibody | CellSignalling | 39952 | Host: Rabbit; Dilution: 1/800 |
| COL2A1 (M2139) Monoclonal antibody | Santa Cruz | sc-52658 | Host: Mouse; Dilution: 1/50 |
| Collagenase type II solution | PanEco | P011-2 | 0.01% |
| DAPI (4',6-diamidino-2-phenylindole) | Sigma-Aldrich | D9542-5MG | 1 μg/mL |
| DMEM medium w/o glutamine | PanEco | ?420? | |
| Fetal bovine serum | Thermo Fisher Scientific | 10270106 | 10% |
| Hanks' solution | PanEco | ?020? | |
| Hybris 8 medium | PanEco | ?780?/?780 | |
| Insulin-Transferrin-Selenium solution | PanEco | ?065 | 1x solution has the following concentrations: Insulin: 10 µg/mL; Transferrin: 5.5 µg/mL; Selenium 5 ng/mL |
| L-alanyl-L-glutamine | Thermo Fisher Scientific | 35050038 | 2 mM |
| Matrigel Matrix | BD | 354277 | 300 μg/mL |
| Penicillin-Streptomycin solution | PanEco | ?063? | 100 U/mL |
| Retinoic acid | Miltenyi biotec | 130-117-339 | 10 nM |
| Rho kinase inhibitor Y27632 | Miltenyi biotec | 130-103-922 | 10 mM |
| Secondary Antibody Goat anti-Mouse IgG (H+L) Cross-Adsorbed, Alexa Fluor 555 | Thermo Fisher Scientific | A21422 | Host: Goat; Dilution: 1/500 |
| Secondary Antibody Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed, Alexa Fluor Plus 555 | Thermo Fisher Scientific | ?32732 | Host: Goat; Dilution: 1/500 |
| Sox9 (D8G8H) Monoclonal antibody | CellSignalling | 82630 | Host: Rb; Dilution: 1/400 |
| TeSR-1 medium | STEMCELL technologies | 85850 | |
| TGF-β1 | Miltenyi biotec | 130-095-067 | 10 ng/mL |
Odniesienia
- Cieza, A., Causey, K., Kamenov, K., Hanson, S. W., Chatterji, S., Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 396, e10267 (2021).
- Hua, C., Buttgereit, F., Combe, B. Glucocorticoids in rheumatoid arthritis: Current status and future studies. RMD Open. 6 (1), e000536 (2020).
- Wang, M., et al. Articular cartilage repair biomaterials: Strategies and applications. Materials Today Bio. 24, e100948 (2024).
- Lee, N., Bayaraa, O., Zechu, Z., Kim, H. Biomaterials-assisted spheroid engineering for regenerative therapy. BMB Rep. 54 (7), 356-367 (2021).
- Eremeev, A., Pikina, A., Ruchko, E., Sidorov, V., Ragozin, A. Fabrication of cartilage tissue substitutes from cells with induced pluripotency. Med Extrem Situat. 4, 30-41 (2022).
- Rodríguez Ruiz, A., et al. Cartilage from human-induced pluripotent stem cells: comparison with neo-cartilage from chondrocytes and bone marrow mesenchymal stromal cells. Cell Tissue Res. 386, 309-320 (2021).
- Eremeev, A., et al. Brain organoid generation from induced pluripotent stem cells in homemade mini bioreactors. J Vis Exp. 178, e62987 (2021).
- Holmqvist, S., et al. Creation of a library of induced pluripotent stem cells from Parkinsonian patients. NPJ Parkinsons Dis. 2, e16009 (2016).
- Livak, K., Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25 (40), 402-408 (2001).
- De Kinderen, P., et al. Differentiation of induced pluripotent stem cells into chondrocytes: Methods and applications for disease modeling and drug discovery. J Bone Miner Res. 37 (3), 397-410 (2022).
- Ali, E., et al. iPSCs chondrogenic differentiation for personalized regenerative medicine: a literature review. Stem Cell Res Ther. 15 (1), 185 (2024).
- Reina-Mahecha, A., Beers, M., van der Veen, H., Zuhorn, I., van Kooten, T., Sharma, P. A Review of the role of bioreactors for iPSCs-based tissue-engineered articular cartilage. Tissue Eng Regen Med. 20 (7), 1041-1052 (2023).
- Fu, L., et al. The application of bioreactors for cartilage tissue engineering: Advances, limitations, and future perspectives. Stem Cells Int. 2021, 6621806 (2021).
- Castro-Viñuelas, R., et al. Tips and tricks for successfully culturing and adapting human induced pluripotent stem cells. Mol Ther Methods Clin Dev. 23, 569-581 (2021).
- Endo, K., Fujita, N., Nakagawa, T., Nishimura, R. Effect of fibroblast growth factor-2 and serum on canine mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 25 (11-12), 901-910 (2019).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone