Method Article
Murine Model of Advanced Periodontitis Induced by Nylon Ligature in the Second Upper Molar
W tym Artykule
Podsumowanie
This study describes a modified 6-0 nylon ligation method for inducing periodontitis in mice, which is highly reproducible and represents an alternative for researchers to study periodontal disease from its development to its pathological consequences.
Streszczenie
Periodontal disease (PD) is an inflammatory disorder affecting the supporting tissues of teeth and is one of the most prevalent diseases worldwide. Its severe form, periodontitis, leads to the destruction of soft tissue, teeth, and bone. Animal models of periodontitis have been developed using primates, dogs, miniature pigs, and mice. Among these, the ligature-induced mouse model offers advantages such as rapid disease progression, reproducibility, predictability, and low cost while effectively replicating key aspects of human periodontitis. Mouse models using ligatures have provided valuable insights into the microbiological and immunological microenvironments of periodontal tissue, highlighting the critical role of biofilms in immune responses and their association with systemic diseases. This study presents a modified nylon ligation method for inducing periodontitis in mice. The modification involves using a nylon suture instead of a silk suture and placing it beneath the interproximal contact area rather than passing it through the contact point. This approach simplifies the technique while effectively inducing periodontitis. The detailed methodology for suture placement is graphically illustrated, and the progression of periodontitis is demonstrated through histological and histometric analyses.
Wprowadzenie
Periodontal disease (PD) is an inflammatory disorder of the supporting tissues of teeth and is among the most prevalent diseases in the world1; the incidence of PD has been reported to range from 20%-50% worldwide2. PD has different degrees of progression; its mild form, called gingivitis, affects only soft tissues, and its severe form, called periodontitis (PT), affects hard tissues such as bone3. Since PT is an inflammatory disease, it must be considered a complex immune response that can be modified by several risk factors that can alter the disease process4, such as diabetes mellitus5, cardiovascular diseases6, hormonal interactions such as adverse pregnancy outcomes7 or preeclampsia8, inflammatory diseases9, and even ocular alterations10 or dementia11.
Therefore, to understand the etiology associated with the development or prevalence of PT, to test new or more effective therapeutic strategies, or to identify any correlation between systemic diseases and PT or the periodontal microbiota, animal models are needed12.
Choosing an effective research method is crucial for understanding the development of PT and adequately answering research questions13. Over the years, different animal models have been developed for the study of PT; however, models such as primates, dogs, rabbits, and miniature pigs are expensive and complex to use14,15,16. Murine models of PT, particularly the ligation-induced PT model, have numerous advantages, including rapid development, reproducibility, predictability, and low cost17,18,19.
Although several methods are used to induce PT, such as oral bacterial inoculation, lipopolysaccharide injection, and ligature induction10,20, each has advantages and disadvantages17, the murine model of PT induced by nylon ligature resembled the human mechanism for its development20,21,22,23. PT occurs through the retention of the resident microbiota, causing inflammation and leading to tissue loss. Furthermore, mice can be genetically modified to study different cell populations or molecules of interest for the study of PT.
Dental ligation can be performed using different materials, such as orthodontic wires, silk sutures24,25, or nylon sutures26. The most common material for inducing PT by ligation in mice is silk; this methodology has been explained by different authors, such as Marchesan et al.18, Abe et al.27, and Chadwick et al.22, each with their own modifications, and all of these methods have been used successfully by several researchers28. However, placing a silk suture around the upper molars in mice can be complex. Marchesan et al. suggested the use of a "ligature holder"; Abe et al. and Chadwick et al. placed the suture through the contact point, although Chadwick et al. placed it around molars M1 and M2.
Nylon sutures of different thicknesses have been used for PT development in different animal models29,30,31. Lima et al.31 used 5-0 nylon sutures; in previous studies, we used 6-0 nylon sutures with similar results28.
Compared with multifilament sutures, nylon sutures are non-resorbable monofilament synthetic and exhibit a lower inflammatory tissue response32; in addition, nylon sutures also allow microbial accumulation33,34, and there is evidence of the adhesion of Fusobacterium nucleatum and Porphyromona intermedia35,36, along with facultative anaerobic bacteria in nylon sutures12,14,17,19,24,27,35,37 (Table 1).
These characteristics may allow the inflammatory response to be focused mostly on bacterial accumulation rather than material accumulation. In addition, nylon has better mechanical properties, such as tensile strength, than silk38.
Therefore, in the present study, the 6-0 nylon suture placed around M2 under the interproximal contact area induced the development of advanced-stage periodontitis in the mouse. This approach allows ligature placement with regular tweezers, and the results are consistent. After 30 days, the development of PT can be confirmed by histometric and histological analysis.
Protokół
All procedures involving experimental animals were conducted in strict compliance with the 'Ethical Framework for Biomedical Research on Laboratory Animals,' following the official Mexican standard NOM-062-ZOO-1999. This study was approved by the ethics committee of the Facultad de Estudios Superiores Iztacala (FES-Iztacala) under protocol CE/FESI/072024/1765. The mice were housed in animal chambers with free access to food and water in a pathogen-free environment at the FES-Iztacala animal facility. This protocol is a modification of the method previously described by Abe et al.27. Six- to eight-week-old female BALB/c mice (weighing 16 g) were divided into control (CTL) and periodontitis (PT) groups. Periodontitis was induced by placing a 6-0 nylon suture on Day 0 to promote sustained bacterial adhesion and trigger severe, chronic disease progression. After 30 days, all mice were euthanized (following institutionally approved protocols) to assess tissue damage and attachment loss (AL) through histometric analysis (Figure 1). Details of the reagents and equipment used are listed in the Table of Materials.
1. Anesthesia preparation
- Prepare a dilution of xylazine and ketamine (1:10) with injectable water (stock). Store at 4 °C and use within 4 weeks.
- Identify and weigh each mouse. Prepare a weight-based dose of xylazine (1 mg/kg) and ketamine (2 mg/kg) for intramuscular application using an insulin syringe.
- Inject 50% of the total dose of the anesthetic solution into the hindquarter. Repeat the procedure on the opposite side.
- Place the mice in a deep box (10 cm³) and allow them to fall asleep, which takes approximately 3-5 min.
- Prevent eye dryness and cornea damage due to anesthesia by applying a hypromellose drop to each eye every 15 min until the mice are fully awake and can blink normally.
NOTE: The mice must be kept under observation for 45 min, the approximate duration of anesthesia. If a mouse wakes up before the procedure is completed, the procedure should be suspended, and the mouse should be replaced.
2. Animal positioning
- Once the mouse is asleep, place it on a worktable facing up with its head toward the operator. Gently pull each leg without tension and secure them with micropore tape to prevent sudden involuntary movements. Cover the mouse with a blanket or gauze to maintain body heat.
- To keep the snout open, place one end of an orthodontic elastic around the upper incisors and secure the other end to an upper holder without tension. Place a second orthodontic elastic around the inferior incisors and secure it to an inferior holder with a rubber band.
- Place the cheek separators and carefully move the tongue to one side for better visibility.
- Position the microscope to allow adequate visualization of the upper molars, starting with the lowest magnification objective. Once located, adjust the magnification to ensure optimal comfort and focus for the operator.
3. Ligature placement
- Once the image is clear, identify the three upper molars: the largest and proximal molar (M1), the next molar (M2), and the smallest and distal molar (M3). Place a 6-0 nylon suture, as its diameter facilitates ligature placement and causes less mechanical damage to tissues than wider sutures.
- Locate the distal side of M2, hold the tip of the 6-0 nylon suture with tweezers, and place the tip at the base of the papilla from the palatine. Apply light pressure through the base of the interproximal palatal space toward the buccal surface (Figure 2A).
- Once the 6-0 nylon suture crosses, pull it through the interproximal space toward the buccal side (Figure 2B).
- Place the tip of the 6-0 nylon suture at the base of the interproximal area at the mesial surface of M2 from the buccal side. Gently push the tip of the suture to cross it under the interproximal space and pass it through the buccal space back to the palatine (Figure 2C).
- Pull the 6-0 nylon suture gently, hold the tip with tweezers, adjust it around M2, and secure the suture with three simple knots.
- Cut the 6-0 nylon suture with fine scissors (Figure 2D).
4. Animal recovery
- To release the mouse, remove the cheek separators, micropore tape from the legs, and orthodontic elastics. First, remove the elastic around the lower incisors, then remove the elastic around the upper incisors.
- Remove the mouse from the worktable, wrap it in gauze or cloth, and place it face up. Maintain the tongue to the side to keep the airway open and prevent blockage.
- Keep the animal warm, covered with cloth or gauze, and under observation until they are completely awake. Then, place them in their regular cages.
- Apply a hypromellose drop in each eye until the mouse can blink normally.
- Keep the animal under standard conditions.
5. Ligature check
- Check the permanence of the nylon ligature every week (Figure 3A,B).
- Take the mouse, hold the head and body firmly, and use tweezers to open the snout. Observe the 6-0 nylon suture around M2 under the light of a lamp.
- Establish the development of periodontal disease based on biofilm accumulation and mechanical irritation. Verify this through histological analysis.
6. Histology
- After 30 days, euthanize the mice in a CO2 chamber (following institutionally approved protocols). Harvest the maxillae as previously reported39. Wash the harvested tissues in a 0.9% NaCl solution and place them in new, labeled microcentrifuge tubes.
- Fix the samples in a 4% paraformaldehyde solution for 2 h with agitation.
- Wash the samples with tap water for 2 h.
- To remove minerals from bone and prepare high-quality paraffin sections, decalcify the samples in twenty volumes of a 4% EDTA (pH 7.3) solution for 20 days in microcentrifuge tubes, change the EDTA every 4 days40.
- Embed the samples in paraffin. Cut 5-µm sections of the M2 area and stain them with Hematoxylin and Eosin (H&E)41.
7. Data analysis
- Observe the stained histological sections under an optical microscope to perform a descriptive analysis of changes in the configuration of the sulcus epithelium, gingival fibers, periodontal fibers, height, and alveolar crest integrity.
- Determine attachment loss (AL) by measuring the distance between the cementoenamel junction (CEJ) and the highest point of the bone crest. Draw a line between these two points using a digital editing program. Perform histometric analysis on the buccal and palatal surfaces according to the methodology described by Semenoff et al.42 (Figure 4).
8. Statistical analysis
- Analyze the data obtained from the CTL and PT groups using the Mann-Whitney U test. Consider p < 0.05 as statistically significant. Use statistics and graphing software for analysis.
Wyniki
This methodology allows induced mice to develop periodontitis (PT) from the second week onward. The mice were monitored weekly to verify the presence of the ligature. Euthanasia was performed on day 30. The clinical characteristics of all the groups were assessed. The control group maintained normal characteristics, such as color and the structure of the marginal gingiva, over time. Compared with those in the CTL group, the PT group tissues showed inflammation, bleeding, and periodontal pocket formation, which were observed at the gingival margin in the induced mice (Figure 5).
The evolution of the disease was confirmed by performing a histological and histometric analysis of the AL of the periodontal tissue of M2 in both groups. The CTL group presented histological characteristics resembling those of healthy tissues: the epithelium of the marginal and sulcus gingiva, gingival fibers (Figure 6A, CTL blue arrow), the junctional epithelium, and the alveolar crest (Figure 6A, CTL green arrow); the connective tissue fibers were in a normal position and height; Sharpey's fibers were intact at their insertion into the alveolar bone and root cementum; and a symmetrical thickness around the root (Figure 6A, CTL orange arrow) was observed, particularly at the attachment in both the buccal and palatine views. The cell nuclei in the periodontal ligament (PDL) fibers, connective tissue, and osteocytes of the alveolar bone were clearly visible (40x). In the PT group, the periodontal tissues presented severe damage, with the presence of periodontal pockets, characterized by loss of the alveolar crest (Figure 6A, PT, green arrow), apical migration of the epithelium and detachment of Sharpey's fibers on the surface of the root (Figure 6A, PT, orange arrow). In the connective tissue, the gingiva and bone of the buccal and palatal sides lacked cell nuclei (Figure 6A, PT, blue arrow), all of which are characteristic of periodontal disease.
Histometric analysis revealed that, for the CTL group, the position of the bone crest was constant on both sides: buccal and palatine. Moreover, compared with the CTL group, the PT group presented a significant increase in the AL depth of the buccal surface (p = 0.0001, 117.5 nm ± 6 vs. 209.17 nm ± 10) due to tissue loss. Compared with that of the CTL group, the palatine surface area of the PT group was significantly greater (p = 0.03, 175.25 nm ± 8 vs. 254.03 nm ± 50). Although both sides presented tissue destruction, it was more evident on the palatal surface, with greater depth and greater attachment loss, than on the buccal surface (Figure 6B,C). These findings are consistent with our previously published results that showed similar behavior28.
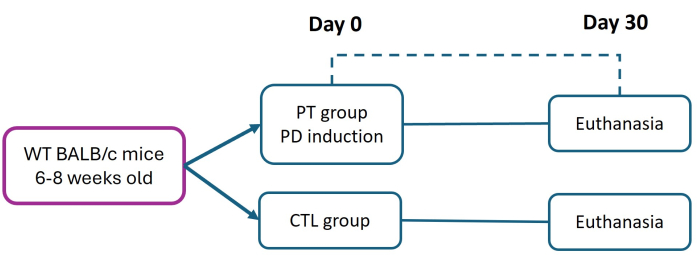
Figure 1: Experimental design. Periodontitis (PT) was induced in six- to eight-week-old BALB/c mice (16 g) using the 6-0 nylon ligature model (day 0). Thirty days postinduction, PT and healthy (CTL) mice were euthanized for periodontal tissue evaluation. Three independent experiments were performed; the total number of mice in both the CTL and PT groups was n = 9. Please click here to view a larger version of this figure.

Figure 2: Step-by-step procedure for ligature placement to induce periodontitis. Representative images of the sequence of steps used to induce periodontitis in the second upper molar of the mouse: (A) shows how the 6-0 nylon suture is inserted distal to M2, from the palatine to the buccal; (B) shows how pressure should be gently applied under the contact area; (C) shows how the nylon suture should pass through the mesial part of M2, from the palatine to the buccal; and (D) shows how the nylon suture is adjusted around M2 and secured with a triple knot to act as a ligature. Please click here to view a larger version of this figure.
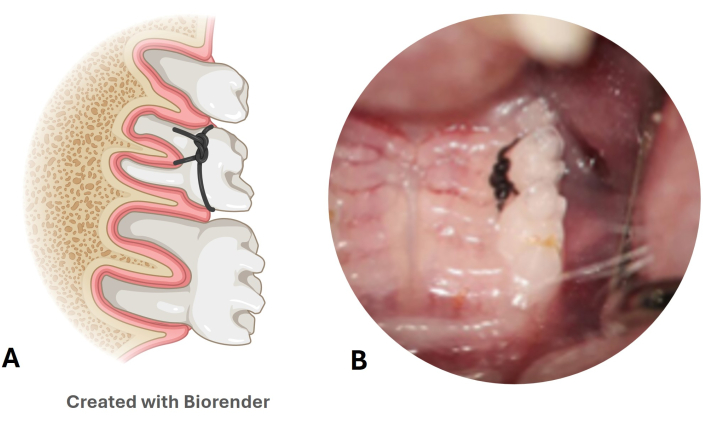
Figure 3: Nylon ligature periodontal disease model. (A) Illustration of the position of the ligature in the second upper molar. (B) Image of the nylon ligature placed in a mouse model. Please click here to view a larger version of this figure.

Figure 4: Histometric analysis. The attachment loss (AL) was determined considering the distance between the cementoenamel junction (CEJ) and the closest point of the alveolar crest, drawing a line between both points on the buccal and palatal surfaces. For reference: V(B): buccal, P palatal. Magnification: 10x; scale bar = 100 µm. Please click here to view a larger version of this figure.

Figure 5: Development of clinical signs of periodontitis in WT mice. Representative clinical image of periodontal tissues, CTL control; PT, periodontal disease day 1 after placement of the nylon 6-0 suture; day 30 after suture removal. Representative images of three independent experiments were performed; the total number of mice in both the CTL and PT groups was n = 9. Please click here to view a larger version of this figure.

Figure 6: Periodontal tissues showed attachment loss and tissue damage in the nylon-induced model of periodontitis. (A) Representative histological images with H&E staining of periodontal tissues, control (CTL); periodontitis (PT), left panel 10x, histometric analysis is shown, with a line from the cementoenamel junction (CEJ) to the edge of the alveolar crest, buccal (B), palatine (P), indicated in the blue box buccal red box palatine. Right panels buccal and palatine at close magnification at 40x. Blue arrow, connective tissue; orange arrow, periodontal ligament insertion in radicular cement; green arrow, bone crest. Scale bars = 100 µm. (B, C) Histometric analysis of the CEJ and the highest point of the alveolar crest. A representative image of three independent experiments was performed; the total number of mice in both the CTL and PT groups was n = 9. The data are expressed as the means ± SEMs, and values of *p < 0.05 and ****p < 0.0001 were considered statistically significant according to the Mann-Whitney U test. Please click here to view a larger version of this figure.
| Material | Induction | Advantages | Disadvantages |
| Silk | Passing through the interproximal space | High bacterial adherence | Requires skill to insert |
| Multifilament suture | Easily torn | ||
| Natural origin | Ligature loss | ||
| Orthodontic wire | Insertion in the interdental area | Easier to place | Need careful insertion to avoid tissue damage |
| Resistant | |||
| Nylon | Insertion under the contact point | Easier to place | Requires magnification |
| No tissue damage from material | Knot can be displaced | ||
| Resistant and flexible | |||
| Periodontal bacteria adherence |
Table 1: Different materials used to induce periodontal disease by ligature in mice.
Dyskusje
Several animal models of periodontitis have been used to evaluate different aspects, such as microbiological and immune responses, and have some similarities with human disease43. These findings provide evidence of the framework of periodontal disease, such as the role of biofilms, the immune response, and interactions with systemic conditions44,45.
The animal models of periodontitis have different complexities and similarities; for example, nonhuman primates, dogs, or miniature pigs are the most similar to human periodontal disease19, although the expense and the requisites to handle and maintain them make these models impractical. Moreover, mouse models of periodontitis are less expensive and easier to handle. Moreover, mice can be genetically modified to generate a specific molecule or cell of interest46, and the methodology is well-established47.
Although mice do not naturally develop PT, it can be induced by different methodologies; the most commonly used model is the ligature-induced model, which is highly reproducible22,23, as several materials have been used, such as orthodontic wire, silk, or nylon, among others.
In particular, nylon may be a viable option to induce PT; owing to some of its characteristics, as a synthetic monofilament, it has a minimal tissue response and allows the adhesion of periodontal bacteria such as Fusobacterium nucleatum and Porphyromona intermedia35,36. This allows the inflammatory response to lead to "normal" bacterial accumulation.
This study proposes an alternative to develop PT via the use of a 6-0 nylon suture as an induction model, and we analyzed the clinical and histological data of CTL and PT mice. We identified the characteristics of the gingival margins present in the PT group, such as bleeding, inflammation, and periodontal pocket formation. These observations were corroborated via a histometric analysis. In particular, the degree of PT loss was significantly different; although both the buccal and palatal surfaces showed tissue destruction, it was greater on the palatal surface. This is probably because the buccal wall is wider in mice than it is in humans.
Lima et al.31 reported that nylon ligatures induced PT; although they used a 5-0 suture, they placed the knot on the buccal surface and measured lineal bone loss 6 micro-CT after 15 days. While 6-0 nylon sutures were used, the knot was placed on the palatal surface, and a histometric analysis was performed after 30 days to induce severe PT. The results revealed that 6-0 nylon sutures around M2 resulted in PT. Liberman et al. reported no differences between methods used to evaluate bone loss48.
These results show that the proposed model is reproducible and has several advantages. First, it is easier to use because it is similar to using a threader; thus, it does not necessarily push the suture toward the interproximal space, increasing the risk of injury to the mouse's oral cavity owing to the limited space. Second, this model allows the development of PT through the accumulation of biofilms in a natural way and induces chronic inflammation in periodontal tissues.
This model has several limitations, including the learning curve, the necessity of visual aids, and the challenges associated with using microscopes or magnifying glasses. Additionally, the possibility of losing the knots and ligatures exists, making it essential to verify the permanence of the ligature to ensure the continued presence of the irritating stimulus.
In summary, this protocol describes the steps required to establish a robust model of advanced PT induced by a 6-0 nylon suture, which facilitates ligature placement. It also details the methodology for anesthetic administration, magnification, and attachment loss evaluation. Furthermore, this model can be used to investigate host-specific mechanisms involved in inflammatory bone loss due to PT, as well as the role of PT in the development of diseases or disorders of infectious, neoplastic, autoimmune, or hormonal origin. Therefore, using this model to study PT caused by biofilm accumulation and inflammation has the potential to generate fundamental knowledge with therapeutic implications and improve diagnosis.
Ujawnienia
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Podziękowania
This work was funded in part by the Council for Science and Technology of the State of Mexico (COMECYT) grant number [FICDTEM-2021-072] and the Support Program for Research Projects and Technological Innovation (PAPIIT)-UNAM, grant number [IN-217021]. We acknowledge the specialization program in Endoperiodontología, FES Iztacala, UNAM for the facilities provided to make this video and Rosalba Yañez Ortiz, DDS, for her support in making this video. Some figures were created with the Biorender program (Agreement number ME282NWCI1).
Materiały
| Name | Company | Catalog Number | Comments |
| 0.9% NaCl solution | PiSA | Rinse maxillae | |
| 6-0 nylon suture | Atramat, Internacional Farmacéutica | PE1946-N | Ligature placement |
| Amscope 3.7 program for digital camera | Amscope | x64, 4.11.21973.20230107 | Data colection |
| EDTA solution | Sigma–Aldrich | E5134 | Decalcification |
| Fine scissors | generic | To cut the 6-0 nylon suture | |
| Fine tweezers | generic | 6-0 nylon suture | |
| Gauze pad | generic | To keep corporal tempature | |
| GraphPad Prism | GraphPad Prism | Version 8.3.0 | Data analysis |
| Hypromellose | generic | Eye lubricant | |
| Injectable water | Pisa | Dilution of anesthetic solution | |
| Ketamine | Anesket, Pisa | Anesthetic | |
| Micro centrifuge tubes | Cellpro | 801501 | To contain tissues durin decalcification |
| Micropore | 3M | 1533 | To fix mouse |
| Microscope digital camera | Amscope | MU1603 | Histology |
| Microtubes | Axygen | MCT-150-C | Store anesthetic dilution |
| Optical microscope | UNICO | Serie G380 | Histology |
| Orhodontic elastics | TP Orthodintics, Inc | blue | To keep the snout open |
| Paraformaldehyde solution | Sigma–Aldrich | 158127 | Tissue Fixation |
| Paraplast | Leica | 39601006 | Histology |
| Surgical microscope | Carl Zeiss GmbH Berlin | 6-0 nylon suture | |
| Ultra Fine Insulin Syringes 6mm U-100, 0.3 mL | BD | 326385 | To inject the anesthetic solution |
| Universal rubber bands | generic | To keep the snout open | |
| Worktable | Generic | Wooden base to support mice | |
| Xylazine | Porcin, Pisa | Preanesthetic |
Odniesienia
- Nazir, M., et al. Global prevalence of periodontal disease and lack of its surveillance. Sci World J. 2020 (1), 2146160(2020).
- Tonetti, M. S., Jepsen, S., Jin, L., Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J Clin Periodontol. 44 (5), 456-462 (2017).
- Caton, J. G., et al. A new classification scheme for periodontal and peri-implant diseases and conditions - introduction and key changes from the 1999 classification. J Clin Periodontol. 45 (Suppl 20), S1-S8 (2018).
- Harrel, S. K., Cobb, C. M., Sottosanti, J. S., Sheldon, L. N., Rethman, M. P. Clinical decisions based on the 2018 classification of periodontal diseases. Compend Contin Educ Dent. 43 (1), 52-56 (2022).
- Stöhr, J., Barbaresko, J., Neuenschwander, M., Schlesinger, S. Bidirectional association between periodontal disease and diabetes mellitus: A systematic review and meta-analysis of cohort studies. Sci Rep. 11 (1), 13686(2021).
- Sanz, M., et al. Periodontitis and cardiovascular diseases: Consensus report. J Clin Periodontol. 47 (3), 268-288 (2020).
- Figuero, E., Han, Y. W., Furuichi, Y. Periodontal diseases and adverse pregnancy outcomes: Mechanisms. Periodontol 2000. 83 (1), 175-188 (2000).
- Jung, E., et al. The etiology of preeclampsia. Am J Obstet Gynecol. 226 (2s), S844-S866 (2022).
- Hajishengallis, G., Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 21 (7), 426-440 (2021).
- Arjunan, P., et al. Exacerbation of AMD phenotype in lasered CNV murine model by dysbiotic oral pathogens. Antioxidants. 10 (2), 309(2021).
- Ma, K. S., et al. Dementia and the risk of periodontitis: A population-based cohort study. J Dent Res. 101 (3), 270-277 (2022).
- Hajishengallis, G. Illuminating the oral microbiome and its host interactions: Animal models of disease. FEMS Microbiol Rev. 47 (3), fuad018(2023).
- Hajishengallis, G., Lamont, R. J., Graves, D. T. The enduring importance of animal models in understanding periodontal disease. Virulence. 6 (3), 229-235 (2015).
- Weinberg, M. A., Bral, M. Laboratory animal models in periodontology. J Clin Periodontol. 26 (6), 335-340 (1999).
- Shanbhag, S., et al. Peri-implant bone regeneration in pigs. Int J Implant Dent. 10 (1), 55(2024).
- Do, M. J., et al. Development of animal experimental periodontitis models. J Periodontal Implant Sci. 43 (4), 147-152 (2013).
- Khuda, F., Baharin, B., Anuar, N. N. M., Satimin, B. S. F., Nasruddin, N. S. Effective modalities of periodontitis induction in rat model. J Vet Dent. 41 (1), 49-57 (2024).
- Marchesan, J., et al. An experimental murine model to study periodontitis. Nat Protoc. 13 (10), 2247-2267 (2018).
- Martuscelli, G., Fiorellini, J. P., Crohin, C. C., Howell, T. H. The effect of interleukin-11 on the progression of ligature-induced periodontal disease in the beagle dog. J Periodontol. 71 (4), 573-578 (2000).
- Lin, P., et al. Application of ligature-induced periodontitis in mice to explore the molecular mechanism of periodontal disease. Int J Mol Sci. 22 (16), 8900(2021).
- Wong, R. L., et al. Comparing the healing potential of late-stage periodontitis and peri-implantitis. J Oral Implantol. 43 (6), 437-445 (2017).
- Chadwick, J. W., Glogauer, M. Robust ligature-induced model of murine periodontitis for the evaluation of oral neutrophils. J Vis Exp. (155), e59667(2020).
- De Almeida, K., et al. Identification of microRNAs expressed in an animal model of periodontal disease and their impact on pathological processes. Tissue Cell. 90, 102525(2024).
- Yoon, H., et al. Temporal changes of periodontal tissue pathology in a periodontitis animal model. J Periodontal Implant Sci. 53 (4), 248-258 (2023).
- Benzen, B. H., et al. A comparison of two models of experimental periodontitis in rats. Scand J Lab Anim Sci. 32 (2), 73-80 (2005).
- Franca, L. F. C., et al. Periodontitis changes renal structures by oxidative stress and lipid peroxidation. J Clin Periodontol. 44 (6), 568-576 (2017).
- Abe, T., Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 394 (1-2), 49-54 (2013).
- Ortiz-Sánchez, B. J., et al. Periodontitis exacerbation during pregnancy in mice: Role of macrophage migration inhibitory factor as a key inductor. J Periodontal Res. 59 (2), 267-279 (2023).
- Lu, H., et al. Chronic stress accelerates ligature-induced periodontitis by suppressing glucocorticoid receptor-α signaling. Exp Mol Med. 48 (3), e223(2016).
- França, A. L. Q., et al. Molecular docking study and antireabsorptive activity of a semi-synthetic coumarin derivative from Platymiscium floribundum in the ligature-induced periodontitis in rats: The involvement of heme oxygenase-1. Clin Oral Investig. 26 (2), 1701-1711 (2022).
- Lima, M. L. S., et al. The receptor at1 appears to be important for the maintenance of bone mass, and at2 receptor function in periodontal bone loss appears to be regulated by at1 receptor. Int J Mol Sci. 22 (23), 12849(2021).
- Faris, A., et al. Characteristics of suture materials used in oral surgery: Systematic review. Int Dent J. 72 (3), 278-287 (2022).
- Nadafpour, N., Montazeri, M., Moradi, M., Ahmadzadeh, S., Etemadi, A. Bacterial colonization on different suture materials used in oral implantology: A randomized clinical trial. Front Dent. 18, 25(2021).
- Naghsh, N., Yaghini, J., Arab, A., Soltani, S. Comparison of the number of bacterial colonies among four types of suture threads using simple loop method following periodontal surgery in patients with periodontitis: A single-blind randomized clinical trial. Dent Res J (Isfahan). 20, 71(2023).
- De Castro Costa Neto, O., et al. Oral bacteria adherence to suture threads: An in vitro study. Oral Maxillofac Surg. 19 (3), 275-280 (2015).
- Asher, R., Chacartchi, T., Tandlich, M., Shapira, L., Polak, D. Microbial accumulation on different suture materials following oral surgery: A randomized controlled study. Clin Oral Investig. 23 (2), 559-565 (2019).
- Banche, G., et al. Microbial adherence on various intraoral suture materials in patients undergoing dental surgery. J Oral Maxillofac Surg. 65 (8), 1503-1507 (2007).
- Kaur Randhawa, R., et al. Assessment of the mechanical properties of different suture materials for oral surgery: An in vitro tensile strength study. Cureus. 16 (8), e65952(2024).
- Liu, Y., et al. Using inducible osteoblastic lineage-specific stat3 knockout mice to study alveolar bone remodeling during orthodontic tooth movement. J Vis Exp. (197), e65613(2023).
- Wang, S. K., et al. Itgb6 loss-of-function mutations cause autosomal recessive amelogenesis imperfecta. Hum Mol Genet. 23 (8), 2157-2163 (2014).
- Sadeghipour, A., Babaheidarian, P. Making formalin-fixed, paraffin-embedded blocks. Methods Mol Biol. 1897, 253-268 (2019).
- Semenoff, T. A., et al. Histometric analysis of ligature-induced periodontitis in rats: A comparison of histological section planes. J Appl Oral Sci. 16 (4), 251-256 (2008).
- Oz, H. S., Puleo, D. A. Animal models for periodontal disease. J Biomed Biotechnol. 2011, 754857(2011).
- Zhao, P., Xu, A., Leung, W. K. Obesity, bone loss, and periodontitis: The interlink. Biomolecules. 12 (7), 865(2022).
- Graves, D. T., Corrêa, J. D., Silva, T. A. The oral microbiota is modified by systemic diseases. J Dent Res. 98 (2), 148-156 (2019).
- Yoshimi, K., Mashimo, T. Application of genome editing technologies in rats for human disease models. J Hum Genet. 63 (2), 115-123 (2018).
- Vandamme, T. F. Rodent models for human diseases. Eur J Pharmacol. 759, 84-89 (2015).
- Liberman, D. N., Pilau, R. M., Orlandini, L. F., Gaio, E. J., Rösing, C. K. Comparison of two methods for alveolar bone loss measurement in an experimental periodontal disease model in rats. Braz Oral Res. 25 (1), 80-84 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone