Method Article
Establishment of Orthotopic Patient-derived Xenograft Models for Brain Tumors using a Stereotaxic Device
В этой статье
Резюме
The development of orthotopic pediatric brain tumor models requires meticulous precision, using a stereotaxic device to implant cancer cells precisely. The methodology presented here outlines the steps involved in preparing brain tumor cells, performing intracranial injections, and implementing a post-operative monitoring system to assess brain tumor engraftment.
Аннотация
The development of clinically relevant and reliable models for central nervous system (CNS) tumors has been pivotal in advancing the field of neuro-oncology. One of the most widely used techniques is the orthotopic intracranial injection, a method that allows investigating of tumor growth, invasion, and dissemination within a controlled setting. This technique involves transplanting tumor cells from a specific patient region into the corresponding anatomical site in an animal. By doing so, these orthotopic brain tumor models offer a unique advantage, as they more accurately replicate cancer's biological behavior and its interactions with the brain environment seen in human patients. This makes them especially valuable for preclinical therapeutic testing, where a close resemblance to the clinical scenario is essential for evaluating potential treatments. This protocol shares experiences in developing patient-derived xenograft (PDX) models for pediatric brain tumors, including diffuse midline glioma (DMG), glioblastoma (GBM), medulloblastoma, and ependymoma. This method delineates the procedure for conducting intracranial stereotaxic injections in mice, ensuring the correct targeting of the injection site within the brain. Additionally, we describe the post-procedural monitoring system employed to detect signs of successful tumor engraftment. Following tumor injection, a rigorous monitoring system is implemented to observe the animals for any signs of neurological impairment, behavioral changes, and/or weight loss, which are common indicators of tumor progression. This system allows for timely intervention and provides critical data regarding the tumor's growth dynamics. By refining these models and protocols, we aim to enhance the reliability and translational potential of preclinical studies, contributing to the development of more effective treatments for pediatric CNS tumors.
Введение
Pediatric central nervous system (CNS) tumors are the most common solid tumors in children, comprising about 20%-25% of primary pediatric tumors, with distinct characteristics from adult tumors in terms of incidence and response to treatment1,2. High-grade gliomas (HGGs), such as glioblastoma (GBM) and diffuse intrinsic pontine glioma/diffuse midline glioma (DIPG/DMG), are particularly aggressive, with dismal prognoses, and despite advancements in treatment modalities, survival rates remain low3,4,5. Medulloblastomas, accounting for nearly 20% of pediatric CNS tumors, have achieved a high 5-year survival rate with standard therapy, yet certain subtypes pose challenges, especially in relapse scenarios6,7. Ependymomas, constituting about 10% of pediatric CNS tumors, exhibit varied prognoses depending on molecular characteristics and tumor location, with recurrence posing significant clinical challenges8,9. Understanding the molecular landscape and developing orthotopic brain tumor (BT) models offer promise for developing effective therapies to improve outcomes for pediatric patients.
Developing clinically relevant BT models is vital for advancing neuro-oncology research and therapeutic discovery. Orthotopic injections using a stereotaxic device are pivotal in this approach, as they allow for the precise placement of tumor cells within the brain, closely mimicking the natural environment of brain tumors10. This method enhances the reliability and reproducibility of tumor models, facilitating more accurate studies of tumor biology and key characteristics such as invasion and angiogenesis, as well as the assessment of therapeutic interventions11. The stereotaxic device's capability to control the exact location and depth of cell injections ensures that the tumor cells are consistently placed in the intended brain regions, thereby improving the validity of experimental outcomes10. Subcutaneous models provide a more accessible and cost-effective option for the initial screening of therapeutic agents. In these models, tumor cells are implanted under the skin, enabling straightforward monitoring of tumor growth and treatment responses12. Although they lack the ability to replicate the complex microenvironment of the brain, subcutaneous models remain highly valuable for high-throughput drug screening and preliminary efficacy evaluations12,13.
Multiple studies have demonstrated the successful development of orthotopic patient-derived xenograft models (PDXs) from brain tumor samples, including direct tissue implantation from autopsy or biopsy samples and primary brain tumor cultures11,14,15. However, it's crucial to acknowledge that the intracranial injection of tumor-dissociated tissue or tumor cells does not always guarantee engraftment, with reasons for failure not fully understood15. Interestingly, HGG biopsy samples tend to exhibit a higher success rate compared to other brain tumor subtypes. For instance, He et al. demonstrated a 56% success rate for HGG orthotopic models, whereas the study by Brabetz et al. reported lower engraftment rates, such as 43% and 30%, respectively, for other pediatric brain tumor samples like ependymoma and medulloblastoma11,14,16. Notably, brain tumor PDX models display a wide range of latency periods, varying from 1 month to 11 months, with later engraftments showing diminished pre-clinical utility. Despite their ability to recapitulate disease at the molecular level, some genomic disparities between biopsies and established models have been observed14,16.
PDX models serve as indispensable tools in the quest for novel therapeutic strategies against pediatric BTs. They are considered the gold standard for performing drug efficacy studies as the models faithfully recapitulate the human disease in terms of tumor growth, symptoms, and drug pharmacokinetics due to the presence of the blood-brain barrier (BBB). Research has demonstrated in the field of pediatric brainstem glioma that the maintained BBB integrity and lack of drug penetration is a feature that leads to diminished therapeutic efficacy for these tumors17,18,19. However, efficacy can be observed in selected treatments that demonstrate adequate drug penetration, such as epigenetic and metabolic inhibitors17,20.
This study presents a method for performing intracranial injections of brain tumor cells in immunocompromised animals, aimed at evaluating engraftment either for PDX development and/or assessing therapeutic drug efficacy. Comprehensive tools for monitoring tumor progression until the ethical experimental endpoint is reached are also provided.
протокол
The protocol outlined below follows the ethical guidelines for animal care and use as outlined by the University of New South Wales, and all procedures have been approved by the Institutional Animal Care and Use Committee. Animal welfare was prioritized, and all efforts were made to minimize the suffering of animals used during the intracranial injection procedure.
1. Culturing for expansion of neurosphere-forming brain tumor cultures
NOTE: Specific requirements may vary based on the brain cultures being used. The following steps outline a general culturing protocol for primary brain tumor cells as neurospheres. Brain tumor cultures may be obtained from commercial vendors or established as primary cultures from patient brain tumor biopsies or autopsies. The procedure for establishing primary cultures from patient biopsy or autopsy samples is outlined in published literature15,21.
- Place the cryopreserved stock vial in a 37 °C water bath until the contents are thawed. Wipe the external surfaces of the vial with 70% ethanol to ensure sterility.
- Prepare Neurosphere media for primary brain tumor cells as described in Mayoh et al.21. In a biological safety cabinet (BSC), use a sterile 1 mL pipette to transfer the thawed cells into a sterile 15 mL conical tube containing 5 mL of pre-warmed neurosphere media.
- Centrifuge the conical tube at 300 x g for 3 min to pellet the cells. Carefully discard as much of the supernatant with a 10 mL pipette as possible.
- Resuspend the cell pellet in 10 mL or 25 mL of neurosphere media. Plate the cell suspension into a T25 or T75 flask, depending on the expected cell number from the cryopreservation. Typically, starting cells with a density of 5x 105 cells are placed in a T25 flask.
- Place the flask in an incubator set at 37 °C with 5% CO2. Exchange the media with fresh media 1x or 2x a week. Alternatively, top up growth factors and B27 as needed, based on the cells' growth rate.
- Once neurospheres have formed in a T25 flask, they can be expanded in T75 flasks. Transfer the contents of the T25 flask with a 10 mL pipette to a 15 mL conical tube. Observe neurospheres under a light microscope, and once they grow sufficiently large (approximately 500 µm), they may also be visible to the naked eye.
- Centrifuge the tube at 300 x g for 3-5 min. Aspirate the medium above the cell pellet, leaving approximately 1 mL in the tube.
- Gently pipette the cells up and down 12x-15x with a 1 mL pipette to dissociate the cell pellet. Use a cell strainer (40 µm) to dissociate neurospheres into a single-cell mixture.
- Place the strainer on the top of a 50 mL conical tube and add dissociated spheroids with a 10 mL pipette onto the strainer. Most single cells will pass the mesh into the tube, and clump materials will stay on the mesh.
- Count cells with a cell counting chamber. Assess cell viability by mixing an equal amount of cell suspension (e.g., 5 µL) with Trypan Blue (5 µL). Dead cells will appear blue under the light microscope, whereas alive cells will remain unstained.
- Calculate cell density as follows:
Cell concentration = Average number of cells per grid × Dilution Factor/ Volume of one grid (mL). The grid volume from a standard cell counting chamber is 0.0001 mL. - To passage cells, resuspend 1 x 106 cells in 25 mL of fresh, warmed media in a T75 flask. Once cells are placed in a T75 flask, incubate them at 37 °C in a 5% CO2 humidified incubator until neurospheres grow.
- To cryopreserve brain tumor cells, resuspend 1 x 106 cells in 1 mL of cryopreservation solution and place overnight in a controlled rate freezing container and store at -80 °C. Place cryopreserved stocks in a -180 °C liquid nitrogen tank for long-term storage.
NOTE: The cryopreservation medium comprises 90% Fetal Calf Serum and 10% Dimethyl Sulfoxide (DMSO).
2. Preparation of brain tumor cells for intracranial injection
NOTE: While the following protocol outlines the preparation of cultured cells from established or short-term cultures for intracranial injection, it can also be adapted for other cell types, including freshly dissociated tissue from human brain tumors22,23.
- In a biosafety cabinet carefully transfer the cells and culture medium from the tissue culture flask with a serological pipette into a 50 mL conical tube.
- Centrifuge the conical tube at 300 x g for up to 5 min at room temperature. Aspirate with a 25 mL pipette the medium above the pellet, leaving approximately 0.5 mL in the tube.
- Gently pipette the cells up and down 10x with a 1 mL pipette to dissociate the cell pellet. Count cells using trypan blue and a hemocytometer.
- Once the desired cell count is aliquoted into a fresh tube, wash 2x with PBS by resuspending cells and pelleting them at 300 x g for 5 min at 4 °C.
NOTE: the cell count can be determined as described. For example, if 10 animals need to be injected at the cell density of 200,000 cells, then the minimum number of cells needed is 2 x 106. - During the final wash, remove as much PBS as possible. Place gently the cell pellet and the 50 µL extracellular matrix (ECM) hydrogel aliquot on ice.
NOTE: The ECM hydrogel can be pre-aliquoted into 50 µL portions on ice using 4 °C cooled pipettes and stored at -80 °C until needed. ECM hydrogel can be temporarily kept on ice to slowly thaw for subsequent experimental use. - Mix the cell pellet with the ECM hydrogel for injection, aiming to complete this step as promptly as possible before initiating intracranial injections. As a standard procedure, use 2 µL of the suspension containing 200,000 cells suspended in ECM hydrogel.
NOTE: If intracranial injections take longer than 2-3 h, a new stock of cells should be prepared, as prolonged incubation on ice may reduce the cells' viability. It is possible to intracranially inject up to 1,000,000 cells and in volumes up to 10 µL; however, this will require the cells to be resuspended in PBS rather than ECM hydrogel and added at a very slow injection rate.
3. Set up of stereotaxic and anesthetic equipment
NOTE: The procedure listed below might vary slightly depending on the manufacturer of the stereotaxic equipment and the type of anesthesia preferred.
- Start the BSC by sanitizing its surface first with polysorbate 20 detergent and letting it sit for 10 min. Subsequently, wipe the surface with 80% ethanol. Prior to placement within the BSC hood, wipe each piece of equipment with 80% ethanol (avoid wetting any electrical inputs).
- Position the base plate within the BSC hood according to Figure 1, with the nose cone positioned on the right-hand side (for right-handed users). Ensure the XYZ manipulators are already affixed to the base plate and secured.
- Connect the digital display console to the inlet at the rear of the base plate and plug its power cable into the provided power supply within the BSC hood (see Figure 1). This console registers the coordinates of the XYZ manipulator's movements.
- Secure the carbon filter canister (for anesthetic output) and the anesthetic input to the two valves on the detachable nosepiece. Slide the nosecone piece onto the mounting section and tighten the screw onto the stereotaxic apparatus.
- Attach the ear bars to their respective holders on each side of the mounting section, securing them into place by tightening the screws affixed to the holders. Ear bars can be covered with gauze pads or electrical tape.
- Introduce the surgical bed into the device, adjusting its height to align with the ear bars, which secure the animal in position. Wrap the surgical bed with a gauge pad to keep the animal warm.
- Connect the heating pad to the power supply and position it beneath the animal to maintain warmth during the anesthetic procedure.
- Inside the BSC hood, arrange sterile cotton tips, 20 µL pipette tips, eye ointment, individually packaged isopropanol wipes, surgical glue, and a 20 µL pipette. Dampen a gauze pad with 80% ethanol and lay sterile forceps and a scalpel on top of it.
- Clean the glass microsyringe by flushing it 5x in cold 80% ethanol and subsequently in cold 0.9% Saline solution.
- For drilling purposes, connect the drill motor to the power supply and mount the drill onto the XYZ manipulator, ensuring a secure fit by tightly screwing it.
- Replace the drill component with the glass microsyringe holder for cell injection purposes. Remove the drill by loosening the screw on the XYZ manipulator and replacing it with the syringe holder. Ensure the screw is tightened before aligning the glass microsyringe with the previously drilled hole.
- After using the stereotaxic device, dismantle all components and sanitise them with 80% ethanol before storing them in a clean storage box or dust-free cabinet. Dispose of all sharps safely and appropriately into a designated sharps container.
4. Intracranial injection
NOTE: Intracranial injections can be performed in both male and female mice, though female mice are generally preferred, as they are less prone to wound complications caused by aggression among males. The procedure is typically carried out in mice aged 7-10 weeks, allowing sufficient time for tumor development. Typically, animals are randomized in groups of 4-6 per cage. Immunocompromised strains such as BALB/c Nude, NOD-SCID, and NSG are commonly used for patient-derived xenograft (PDX) development, as their immune-deficient status supports tumor engraftment and growth24. While this protocol specifies the use of isoflurane for anesthesia induction, alternative anesthetics such as ketamine and xylazine may also be used25. If isoflurane is not utilized, steps involving the anesthetic chamber and related equipment can be omitted from the procedure.
- Weigh and monitor the overall well-being of the animals before the intracranial procedure. Administer an analgesic to the animals one cage at a time (e.g., buprenorphine 0.05 mg/kg, intraperitoneally) for up to 1 h before commencing the intracranial injection.
- Set up anesthetic equipment according to the guidelines provided with the instrument. Before starting the procedure, ensure the vaporizer is at least halfway full. Ensure the oxygen supply is open.
- If animals have fur, it can be removed the day before the intracranial procedure or simultaneously. Remove the fur from the surgical site at the dorsum or mouse skull with electric clippers or a razor.
- Transfer the animal to the anesthetic induction chamber. Ensure the flow meter at the anesthetic machine is set to 1 L/min flow rate, and the oxygen regulator is between 40-50 psi. Ensure medium anesthesia is maintained by checking that the muscles are relaxed, and reflexes are absent.
- Monitor the animal's response to stimuli and observe chest wall movement to ensure steady breathing. If intracranial injections exceed 10 min, monitor temperature with a rectal probe and temperature monitoring system and track pulse using a pulse oximeter
- Mount the animal onto the stereotaxic device, ensuring the front teeth are fixed in the incision bar and the nose cone has secured the animal in place.
- Disinfect the mouse's head with an iodine-soaked cotton tip and then an isopropanol wipe. Apply corneal eye ointment to both eyes to prevent drying during the surgery.
- Begin the incision at the base of the cerebellum and extend it across the cranium to the midpoint, making a small cut approximately 1 cm in length along the superior aspect of the skull.
- Tighten the skin between the ears to expose the skull and secure the head by tightening the ear bars. Clean the skull surface and dry it with a cotton bud.
- Secure the drill onto the stereotaxic frame and locate the bregma (or lambdoid structure) using the drill.
NOTE: Specific stereotaxic coordinates are selected based on the anatomical region required for the engraftment study. These precise coordinates can be sourced from published literature or determined using resources like the Mouse Brain in Stereotaxic Coordinates26 atlas, ensuring accurate targeting for successful implantation. - Once the coordinates are established, use a drill to carefully create a small burr hole in the bone at the designated site of Diffuse midline glioma (DMG): X = +0.5, Y = -5.5, Z = -3.1 from bregma; Supratentorial brain tumor (GBM or ependymoma): X = +1.5, Y = +1, Z = -3 from bregma; Infratentorial brain tumor (Medulloblastoma, ependymoma): X = +2, Y = - 2, Z = -2 from lambdoid structure.
- Resuspend the cells multiple times with a pipette while avoiding air bubbles. Draw 2 µL of the cell mixture into a prewashed cold glass micro syringe. Attach the syringe to the stereotaxic frame and adjust the needle to the skull tip.
- Inject the cells within 30 s into the drilled region, drying any refluxed cell suspension during the injection.
NOTE: Since the cells are suspended in ECM hydrogel, the injection needs to occur fast enough so that the cell suspension does not solidify in the syringe. Varying cell densities can be used to assess different engraftment rates, with higher cell densities generally leading to faster tumor engraftment. - Leave the syringe in place for 1 min to avoid backflow and allow ECM hydrogel to settle. Remove the syringe and wipe the wound with an isopropanol wipe. Use skin glue or wound clips to seal the incision if necessary.
- Place the animal in a recumbent position in a clean recovery cage with a heat lamp or on a heating pad to keep warm until recovery. Monitor the animal continuously until it starts moving on its own and frequently thereafter until it shows normal behavior.
- If an animal is found to have a previously sealed wound reopened during recovery, anesthetize the animal again, clean the wound with an isopropanol wipe to remove any remaining glue, and then re-secure with staples.
5. Post-surgical monitoring for engraftment
- Following the intracranial injection, begin monitoring mice 5 days per week (or more if required). Provide aftercare, such as seeds or mushy food, during the first couple of weeks after the intracranial injection. Monitor animals carefully for any potential wound opening.
NOTE: If female animals have their wounds clipped, they remain in the litter box, as they rarely exhibit aggression. For male animals, we provide two igloos in the cage along with extra enrichment to help reduce aggression. In the rare event that aggression occurs and leads to wounds reopening, the wounds are re-secured with staples, and the aggressor is removed from the group and housed separately. - Record general well-being parameters such as weight loss, activity level, posture, signs of dehydration, and fur condition on the monitoring sheet.
- Record on the monitoring sheet intermittent, mild, or severe neurological symptoms such as head tilting, ataxia, and/or circling behavior.
- Identify neurological symptoms as follows:
- Observe and categorize the following as intermittent symptoms: Head Tilting: Observed 1-2 times during a 5 min inspection, overall well-being satisfactory. Ataxia: Observed 1-2 times during a 5 min inspection; overall well-being satisfactory. Circling: Observed 1-2 times during a 5 min inspection; overall well-being satisfactory. Behavior: Mice show normal behavior, capable of reaching food/water and climbing.
- Observe and categorize the following as mild symptoms: Head Tilting: Regularly displayed during a 5 min inspection, overall well-being satisfactory. Ataxia: Regularly displayed during a 5 min inspection, overall well-being satisfactory. Circling: Regularly displayed during a 5 min inspection, overall well-being satisfactory. Behavior: Mice might have a ruffled coat or dry skin but can still exhibit normal behavior, feed, and climb.
- Observe and categorize the following as severe symptoms : Head Tilting: Continuously displayed during inspection, nearly 90° tilt, ruffled coat, less active. Ataxia: Continuously displayed during inspection, drunk movements, ruffled coat, less active. Circling: Continuously displayed during the inspection, ruffled coat, less active. Behavior: Mice might have a very ruffled coat or dry skin. They may exhibit weight loss of over 15%. Provide animals with seeds or mushy food at this stage.
- Establish the humane endpoint for the mice when they exhibit any of the following clinical signs indicating tumor growth that affects their well-being: weight loss of 20% or more from their initial weight, severe head tilting, severe ataxia, severe circling, lethargy, or labored breathing. At this stage, humanely euthanize the mice using CO2 asphyxiation.
6. Harvesting of brains
- Ensure the animal is humanely euthanized either by CO2 asphyxiation or cervical dislocation.
- Make a 2 cm midline incision along the scalp using scissors or a scalpel. Carefully separate and peel back the skin to expose the skull.
- Using fine scissors or a scalpel, make a careful 2 cm incision along the midline of the skull. Gently pry open the skull using forceps, working from the back of the head towards the nose.
- Continue cutting along the sides of the skull, ensuring no damage to the brain tissue underneath. Once the skull is fully opened, carefully detach the brain from the cranial nerves and blood vessels using forceps and scissors.
- Lift the brain gently from the cranial cavity, starting from the back and moving forward. Store the brain under appropriate conditions based on the requirements of subsequent experimental procedures, such as snap freezing or fixation. Immunohistochemical analysis of brains may vary between different labs. A typical workflow for proliferative cell marker KI67 is described in published literature20.
Результаты
Orthotopic injections using a stereotaxic device have become essential for developing brain tumor models. This technique allows for the precise and reproducible placement of tumor cells, which is crucial for generating consistent and reliable data in neuro-oncology research. The successful implementation of orthotopic injections relies heavily on the accurate setup of stereotaxic equipment, which typically includes a stereotaxic frame, a digital micromanipulator for precise movements, an injection syringe, and an anesthesia system to immobilize the animal (Figure 1A,B). The use of a glass microsyringe enables the intracranial injection of small volumes, typically between 2-5 µL. Proper maintenance and frequent washing are essential to prevent the buildup of biological material, which can block the injection (Figure 1C).
The procedure for intracranial injection of brain tumor cells using stereotaxic equipment in mice involves several key steps. Firstly, the mouse is anesthetized to remain still and comfortable throughout the procedure. Typical cues indicating that the animal is anesthetized include the absence of movement and reflexes upon toe pinching, as well as the presence of a regular breathing pattern. Then, the mouse is placed in a stereotaxic frame, securing its head in a fixed position (Figure 2A). Using landmarks on the skull, such as bregma and lambda, precise coordinates for injection are determined. A small incision is made in the scalp, and a burr hole is drilled through the skull at the chosen coordinates (Figure 2B). A microinjection needle attached to the stereotaxic arm is inserted through the burr hole to deliver the tumor cells into the desired brain region (cortex, cerebellum, or brainstem; Figure 2C). Careful monitoring and adjustment of the needle position ensure accurate targeting. After injection, the needle is slowly withdrawn, and the incision is closed (Figure 2D). The sealed wound is expected to heal within two weeks following intracranial injection.
Following intracranial injection, animals require careful monitoring for overall well-being and the development of neurological symptoms, which often indicate tumor progression (Supplementary Table 1). Typically, animals may experience a slight weight loss during the 1st-week post-injection (up to 10% of their highest weight), which typically stabilizes once the injection sites are fully sealed by the end of the 2nd week. Generally, animals exhibit a steady increase in weight and maintain good overall health until tumor progression occurs. The manifestation of neurological symptoms varies depending on factors such as the type of brain tumor, injection site, and animal strain. For instance, cortical gliomas or ependymomas may present with progressive weight loss and potential forebrain enlargement, while brainstem gliomas and medulloblastomas might exhibit symptoms such as ataxia and head tilting, particularly in immunocompromised animals (Figure 3A). Additionally, circling behavior may be observed in animals with brainstem tumors, especially in NSG or NOD/SCID strains. The progression of neurological symptoms often coincides with gradual weight loss. Proper post-care measures, such as providing water bottles with long nozzles or offering soft food (mushy food pellets) or seeds, are essential for supporting the animals' well-being. Upon reaching the experimental endpoint and the subsequent humane euthanasia of the animals, potential alterations in the brain may become apparent, including enlargement at the injection site and/or the presence of hemorrhagic areas (Figure 3B).
Kaplan-Meier graphs are commonly employed to depict the survival outcomes of brain tumor animal models. They offer a visual depiction of the probability of survival over time following intracranial injection or anticancer treatments. These graphs serve as crucial tools for analyzing and presenting survival data in pre-clinical research settings15,27. We performed intracranial injections of medulloblastoma cell culture D425 at varying cell densities in the cerebellum. All animals intracranially injected developed tumors. Our results indicated that higher cell densities, specifically 50,000 and 100,000 cells per mouse, engrafted more rapidly than 10,000 cells per mouse (Figure 4). Furthermore, we intracranially injected patient-derived high-grade glioma (HGG) cells into the cortex and brainstem to compare overall survival and the pattern of tumor growth, as well as immunohistochemical features, in these different locations. Irrespective of the location, all animals were injected with intracranially developed tumors. The culturing of HGG cells was carried out following the protocol detailed in steps 1.1 to 1.10. Preparation of the cells for intracranial injection was performed as described in steps 2.1 to 2.6, while the intracranial injection procedure adhered to the method outlined in steps 4.1 to 4.1627. When injected into the cortex, HGG cells displayed a median survival of approximately 25 days post-intracranial injection (Figure 5A). Animals were monitored as outlined in steps 5.1 to 5.4. These animals did not exhibit any neurological symptoms but did show progressive weight loss. Animals were humanely euthanized upon reaching the humane endpoint outlined in step 5.5. Brains were harvested following the protocol detailed in steps 6.1 to 6.5 and sent for further analysis27. Immunohistochemical analysis revealed a large, highly nucleated tumor mass with evidence of increased vascularization (Figure 5B) and many proliferative Ki67 cells (Figure 5C). When injected into the brainstem, HGG cells displayed a median survival of approximately 26 days post-intracranial injection (Figure 5D). Immunohistochemical analysis indicated a large tumor mass in the 4th ventricle/upper pons region, as well as leptomeningeal infiltration into the lateral ventricle (Figure 5E). Further immunohistochemical analysis indicated many proliferative cells in the brainstem and other infiltrated areas (Figure 5F). This experiment highlights that injecting the same tumor cells into different anatomical locations does not affect the tumor growth rate, although some differences in the growth pattern are observed.

Figure 1: Representative images of the stereotaxic setup for intracranial injection of brain tumor cells. (A) The anesthetic apparatus is typically positioned next to the BSC hood, necessitating dual administration of anesthesia to both the induction chamber and the stereotaxic apparatus. (B) Glass microsyringe wash station and stereotaxic holder; the syringe requires flushing with saline, ethanol, and subsequently water after each intracranial injection. (C) Stereotaxic setup inside the BSC hood, illustrating the anesthetic chamber, drilling machine, stereotaxic device, stereotaxic coordinate console, cotton tips, scalpel, tweezers, pipette, and tips. Please click here to view a larger version of this figure.
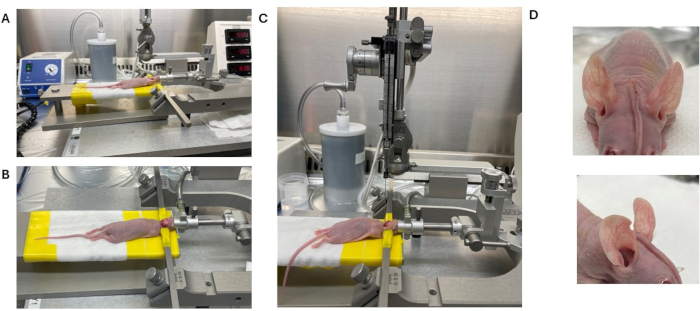
Figure 2: Representative images of intracranial injection being performed on an immunocompromised mouse. (A) Anesthetized animal positioned in an anesthetic cone. (B) Skin incision followed by securing skin to each side with ear bars. (C) Glass microsyringe positioned atop the drilled hole for injection. (D) Skin forms a Mohawk style once glued. Please click here to view a larger version of this figure.
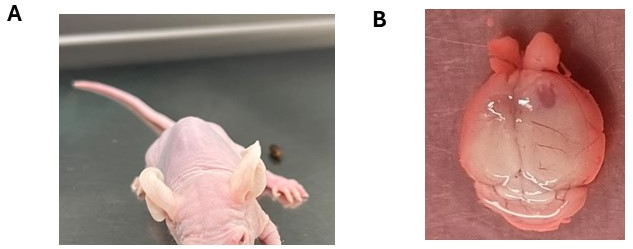
Figure 3: Potential neurological symptoms observed following engraftment of brain tumor cells. (A) Animal exhibiting a head tilt following intracranial injection of DMG cells in the brainstem. (B) Enlargement of the right cortical hemisphere with the injection side revealing tumor formation. The animal was injected with ependymoma cells. Please click here to view a larger version of this figure.
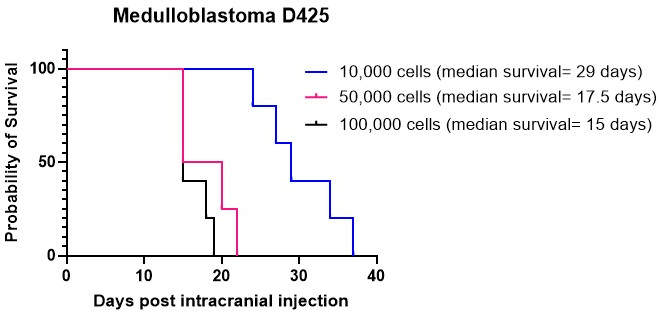
Figure 4: Engraftment of the medulloblastoma culture D425 at different cell densities. Medulloblastoma cells were intracranially injected using stereotaxic equipment at densities of 10,000 cells in 2 µL of ECM hydrogel, 50,000 cells, and 100,000 cells. Animals were monitored for progressive weight loss and were humanely euthanized once they reached 20% below their highest recorded weight. The median survival for animals injected with 10,000 cells was 29 days, while the median survival for those injected with higher cell densities of 50,000 and 100,000 cells was 17.5 days and 15 days, respectively. All animals injected in this study successfully developed tumors. Please click here to view a larger version of this figure.

Figure 5: Representative survival curves and histological analysis of NSG animals intracranially injected with HGG cells in the cortex and brainstem. Approximately 100,000 HGG cells (passage 3) were injected into 2 µL of ECM hydrogel using stereotaxic equipment. Animals were monitored for progressive weight loss and humanely euthanized once they reached 20% below their highest recorded weight. No neurological symptoms were observed for this brain tumor subtype. (A) The median survival of animals with HGG cells injected into the cortex is approximately 25 days post-injection (N=2). (B) H&E staining of the cortex shows a highly nucleated region with increased vascularization, indicated by the presence of red blood cells. (C) The tumor-engrafted area in the cortex displays a high level of proliferative cells, as shown by KI67 staining. (D) The median survival of animals with HGG cells injected into the brainstem is approximately 26 days post-injection (N=2). All animals injected in this study successfully developed tumors. (E) H&E staining of the brainstem shows a highly nucleated region with tumor infiltration observed in the leptomeninges in the lateral ventricle. (F) The tumor-engrafted area in the brainstem displays a high level of proliferative cells, as shown by KI67 staining. The black scale bar in the main images indicates 2 mm, whereas the magnified inserted images indicate 20 µm. Please click here to view a larger version of this figure.
Supplementary Table 1: Monitoring form used for the inspection of animals' post-intracranial injections and potential treatment with anticancer agents. Please click here to download this table.
Обсуждение
While the intracranial injection technique detailed in this study provides a robust method for establishing orthotopic patient-derived xenograft (PDX) models for pediatric brain tumors, several areas need improvement. One approach to improving the technique involves optimizing the tumor cell preparation process. This includes refining the materials used for the implantation of the tumor cells. While ECM hydrogel is considered the gold standard, utilizing hydrogels composed of components that more closely resemble those of the brain or tumor ECM could enhance BT cell survival and engraftment. This approach particularly benefits tumor types with longer engraftment periods, such as medulloblastomas and ependymomas. For instance, collagen has been shown to play a critical role in the growth of Group 3 medulloblastoma, whereas laminin supports the SHH type of medulloblastoma28. Additionally, the stiffness of the ECM may also influence tumor growth. High-grade glioblastomas, for example, exhibit higher stiffness in their core compared to normal brain tissue, which may support tumor growth and survival29. By tailoring the ECM composition and stiffness to match the specific requirements of different tumor types, the engraftment and growth of PDX models may be significantly improved.
A few recent studies have provided valuable insights into patient tumor sample handling and the development of PDX models. Tsoli et al. highlighted that direct implantation of DMG tissue typically results in higher engraftment rates than primary cultures15. Smith et al. established 37 PDX models through intracranial injection of brain tumor biopsies or autopsy samples, reporting an overall success rate of 43%, with HGGs demonstrating the highest engraftment rate (100%), followed by medulloblastomas (45%) and ependymomas (25%). Tumor latency varied widely, from 1 to 11 months, with more aggressive tumors engrafting more quickly11. In contrast, Brabetz et al. reported a lower overall success rate of 30%, with HGGs exhibiting a 31% engraftment rate and lower rates for medulloblastomas (37%) and ependymomas (17%)16. Despite these differences, the last two studies conducted by Smith et al. and Brabetz et al. confirmed that the PDX models generally recapitulated the patient tumors at immunohistochemical and molecular levels, although minor genomic discrepancies were observed between the original tumor samples and the established models. These findings underscore the challenges in developing representative PDX models and highlight the variability in engraftment success across tumor types11,16.
One significant limitation of the orthotopic approach is the inability to accurately measure tumor size unless an imaging system is used, such as Xenogen Imaging, if the tumor cells are tagged with luciferase or Magnetic Resonance Imaging (MRI). Tagging tumor cells with luciferase could provide a solution by allowing for bioluminescent imaging to confirm engraftment and monitor tumor progression non-invasively. This method would enhance the ability to track the response to treatment dynamically, thereby offering a more precise evaluation of therapeutic efficacy17,30. An alternative method involves utilizing MRI technology; however, the detectability of tumors is contingent upon their size. Larger tumors are more readily identified, which is often at the experimental endpoint, and detection may also vary depending on the subtype of the tumor, with GBM being more easily detected than diffuse gliomas27,31. Particularly for diffuse gliomas, the presence of an intact blood-brain barrier (BBB) leads to a lack of contrast enhancement. In GBM, however, leaky vasculature and the presence of edema can amplify the contrast between the tumor tissue and the surrounding healthy brain tissue, potentially aiding in the detection process. Generally, contrasting agents are needed to improve detection30,31,32. Alternative imaging techniques such as diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR) are sometimes used to improve the detection of non-enhancing tumor regions. However, these methods also have their limitations and may not always provide a precise delineation of tumor boundaries33. Amide Proton Transfer-weighted (APTw) imaging is a non-invasive MRI technique that detects diffuse gliomas by tracking protein-water proton exchange, aiding in tumor grading. While promising, it faces challenges like susceptibility to artifacts and has not yet been adapted for small animal imaging, limiting its broader application34.
Another crucial aspect to consider when utilizing orthotopic BT PDX models for drug efficacy studies is the BBB integrity in the PDX models used. The BBB's permeability significantly affects drug delivery and efficacy in brain tumor treatment. For example, the maintained BBB integrity in brainstem gliomas often results in poor drug penetration, limiting the effectiveness of systemic therapies17. On the other hand, in Group 3 medulloblastomas, BBB integrity has been observed to be heterogeneous on MRI imaging, and it was similarly found to be leaky in the patient-derived xenograft (PDX) models35. Therefore, characterizing the BBB status-whether it is intact or leaky-in each PDX model is essential for designing appropriate therapeutic strategies and accurately interpreting the results of drug efficacy studies.
Overall, the intracranial injection procedure outlined offers a robust approach for establishing BT PDX models and conducting efficacy studies for therapeutic agents. Continued refinements, coupled with a comprehensive understanding of these models, will augment their utility and facilitate the development of therapeutic interventions for pediatric BT patients.
Раскрытие информации
The authors have nothing to disclose.
Благодарности
The authors thank the patients, clinicians, and researchers of the Sydney Children's Hospital Network, the Sydney Children's Tumour Bank Network, and the Zero Childhood Cancer Program for generously providing the essential samples for establishing this methodology. Children's Cancer Institute Australia is affiliated with the University of New South Wales Sydney and the Sydney Children's Hospital Network. This protocol stems from research efforts supported by grants from the National Health and Medical Research Council (Synergy Grant #2019056, Leadership Grant APP2017898 to DZ) and Cancer Institute New South Wales Program Grant (TPG2037 to DZ and MT), as well as support from the Levi’s Project, The Kids Cancer Project, The Cure Starts Now, DIPG Collaborative, Cure Brain Cancer Foundation, The Robert Connor Dawes Foundation and The Benny Wills Foundation.
Материалы
| Name | Company | Catalog Number | Comments |
| Anaesthetic agent (e.g. Isoflurane) | Merck | 792632 | |
| 100% Ethanol | Merck | 459844 | |
| 70% ethanol | NA | NA | Make 70% ethanol from 100% ethanol diluting it with sterile water |
| 80% ethanol | NA | NA | Make 80% ethanol from 100% ethanol diluting it with sterile water |
| Adhesive microscope slides | Merck | Z681156 | |
| Analgesic (Buprenorphine 0.3mg/mL) | Jurox | NA | |
| Carbon filter canister | Vet Equip | 931401 | |
| Conical tubes 15 mL (Falcon) | Fisher Scientific | 14-959-53A | |
| Conical tubes 50 mL (Falcon) | Fisher Scientific | 14-432-22 | |
| DMSO | Merck | 472301 | |
| Ear bars | Kopf | 1921 | |
| ECM hydrogel (Matrigel, Corning) | Merck | E1270 | |
| Eye Ointment (Poly Visc) | ChemistDirect | 719986 | |
| Fetal Calf Serum | Merck | F0926 | |
| glass microsyringe | Hamilton | 7002 | |
| Isopropanol wipes | Medisa | SUL_LWMS-1 | |
| KI67 antibody | Abcam | ab209897 | |
| Microcentrifuge tubes | Eppendorf | 211-2130 | |
| Microdrill bit 0.028” | Kopf | 8170 | |
| Microinjection unit | Kopf | 5004 | |
| PBS | Merck | P4474 | |
| Pipette 10mL | Corning | CLS4488 | |
| Pipette 1mL | Eppendorf | 3123000063 | |
| Pipette 200uL | Eppendorf | 3123000055 | |
| Pipette 20uL | Eppendorf | 3123000098 | |
| Pipette 25mL | Corning | CLS4489 | |
| Pipette tip 1mL | Corning | CLS4868 | |
| Pipette tip 200uL | Corning | CLS4860 | |
| Polysorbate 20 detergent (F10SC) | Health and Hygiene | G3070 | |
| Pulse oximeter (MouseSTAT) | Kent Scientific | SPO2-MSE | |
| Riodine (Povidone-Iodine) | Merck | Y0000466 | |
| Scalpel (disposable) | Medisa | SUL_SCALPEL | |
| Skin Glue Vetbond | Medisa | 3M_1469SB | |
| Small Animal stereotactic instrument with digital display console | Kopf | 940 | |
| Stereotactic Drill | Kopf | 1474 | |
| Temperature monitoring system | Harvard Apparatus | 55-7020 | |
| Trypan Blue | Merck | 93595 |
Ссылки
- Mueller, S., Chang, S. Pediatric brain tumors: current treatment strategies and future therapeutic approaches. Neurotherapeutics. 6 (3), 570-586 (2009).
- Packer, R. J. Brain tumors in children. Arch Neurol. 56 (4), 421-425 (1999).
- Paugh, B. S., et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 28 (18), 3061-3068 (2010).
- MacDonald, T. J., Aguilera, D., Kramm, C. M. Treatment of high-grade glioma in children and adolescents. Neuro Oncol. 13 (10), 1049-1058 (2011).
- Rutka, J. T. Malignant brain tumours in children : Present and future perspectives. J Korean Neurosurg Soc. 61 (3), 402-406 (2018).
- Cho, Y. J., et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 29 (11), 1424-1430 (2011).
- Thomas, A., Noel, G. Medulloblastoma: optimizing care with a multidisciplinary approach. J Multidiscip Healthc. 12, 335-347 (2019).
- Pajtler, K. W., et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 27 (5), 728-743 (2015).
- Thorp, N., Gandola, L. Management of ependymoma in children, adolescents and young adults. Clin Oncol. 31 (3), 162-170 (2019).
- Upton, D. H., Ziegler, D. S., Tsoli, M. Development of orthotopic patient-derived xenograft models of pediatric intracranial tumors. Methods Mol Biol. 2806, 75-90 (2024).
- Smith, K. S., et al. Patient-derived orthotopic xenografts of pediatric brain tumors: a St. Jude resource. Acta Neuropathol. 140 (2), 209-225 (2020).
- Akter, F., et al. Pre-clinical tumor models of primary brain tumors: Challenges and opportunities. Biochim Biophys Acta Rev Cancer. 1875 (1), 188458 (2021).
- Stribbling, S. M., Ryan, A. J. The cell-line-derived subcutaneous tumor model in preclinical cancer research. Nat Protoc. 17 (9), 2108-2128 (2022).
- He, C., et al. Patient-derived models recapitulate heterogeneity of molecular signatures and drug response in pediatric high-grade glioma. Nat Commun. 12 (1), 4089 (2021).
- Tsoli, M., et al. International experience in the development of patient-derived xenograft models of diffuse intrinsic pontine glioma. J Neurooncol. 141 (2), 253-263 (2019).
- Brabetz, S., et al. A biobank of patient-derived pediatric brain tumor models. Nat Med. 24 (11), 1752-1761 (2018).
- Khan, A., et al. Dual targeting of polyamine synthesis and uptake in diffuse intrinsic pontine gliomas. Nat Commun. 12 (1), 971 (2021).
- Chen, Y., et al. Preclinical evaluation of protein synthesis inhibitor omacetaxine in pediatric brainstem gliomas. Neurooncol Adv. 6 (1), vdae029 (2024).
- Ung, C., et al. Doxorubicin-loaded gold nanoarchitectures as a therapeutic strategy against diffuse intrinsic pontine glioma. Cancers (Basel). 13 (6), 1278 (2021).
- Ehteda, A., et al. Dual targeting of the epigenome via FACT complex and histone deacetylase is a potent treatment strategy for DIPG. Cell Rep. 35 (2), 108994 (2021).
- Mayoh, C., et al. High-throughput drug screening of primary tumor cells identifies therapeutic strategies for treating children with high-risk cancer. Cancer Res. 83 (16), 2716-2732 (2023).
- Lin, G. L., Monje, M. A Protocol for rapid post-mortem cell culture of diffuse intrinsic pontine glioma (DIPG). J Vis Exp. (121), e55360 (2017).
- Whitehouse, J. P., et al. In vivo loss of tumorigenicity in a patient-derived orthotopic xenograft mouse model of ependymoma. Front Oncol. 13, 1123492 (2023).
- Hermans, E., Hulleman, E. Patient-derived orthotopic xenograft models of pediatric brain tumors: In a mature phase or still in its infancy. Front Oncol. 9, 1418 (2019).
- Kim, M. P., et al. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 4 (11), 1670-1680 (2009).
- Paxinos, G., Franklin, K. . Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates. , (2019).
- Tsoli, M., et al. Integration of genomics, high throughput drug screening, and personalized xenograft models as a novel precision medicine paradigm for high risk pediatric cancer. Cancer Biol Ther. 19 (12), 1078-1087 (2018).
- Yang, H., et al. Roles and interactions of tumor microenvironment components in medulloblastoma with implications for novel therapeutics. Genes Chromosomes Cancer. 63 (4), e23233 (2024).
- Kondapaneni, R. V., et al. Glioblastoma mechanobiology at multiple length scales. Biomater Adv. 160, 213860 (2024).
- Mahmoudian, E., Jahani-Asl, A. Establishing brain tumor stem cell culture from patient brain tumors and imaging analysis of patient-derived xenografts. Methods Mol Biol. 2736, 177-192 (2024).
- Oudin, A., Moreno-Sanchez, P. M., Baus, V., Niclou, S. P., Golebiewska, A. Magnetic resonance imaging-guided intracranial resection of glioblastoma tumors in patient-derived orthotopic xenografts leads to clinically relevant tumor recurrence. BMC Cancer. 24 (1), 3 (2024).
- Bauer, S., Wiest, R., Nolte, L. P., Reyes, M. A survey of MRI-based medical image analysis for brain tumor studies. Phys Med Biol. 58 (13), R97-R129 (2013).
- Lasocki, A., Gaillard, F. Non-contrast-enhancing tumor: A new frontier in glioblastoma research. AJNR Am J Neuroradiol. 40 (5), 758-765 (2019).
- Nichelli, L., Zaiss, M., Casagranda, S. APT weighted imaging in diffuse gliomas. BJR Open. 5 (1), 20230025 (2023).
- Genovesi, L. A., et al. Patient-derived orthotopic xenograft models of medulloblastoma lack a functional blood-brain barrier. Neuro Oncol. 23 (5), 732-742 (2021).
Перепечатки и разрешения
Запросить разрешение на использование текста или рисунков этого JoVE статьи
Запросить разрешениеThis article has been published
Video Coming Soon
Авторские права © 2025 MyJoVE Corporation. Все права защищены