A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Testing Protozoacidal Activity of Ligand-lytic Peptides Against Termite Gut Protozoa in vitro (Protozoa Culture) and in vivo (Microinjection into Termite Hindgut)
* These authors contributed equally
In This Article
Summary
We present procedures for demonstrating that ligands bind to the surface membrane of the cellulose-digesting protozoa in the gut of Formosan subterranean termites using fluorescent microscopy and that ligands coupled with lytic peptides kill these protozoa in vitro (anaerobic protozoa culture) and in vivo (injection into the termite hindgut).
Abstract
We are developing a novel approach to subterranean termite control that would lead to reduced reliance on the use of chemical pesticides. Subterranean termites are dependent on protozoa in the hindguts of workers to efficiently digest wood. Lytic peptides have been shown to kill a variety of protozoan parasites (Mutwiri et al. 2000) and also protozoa in the gut of the Formosan subterranean termite, Coptotermes formosanus (Husseneder and Collier 2009). Lytic peptides are part of the nonspecific immune system of eukaryotes, and destroy the membranes of microorganisms (Leuschner and Hansel 2004). Most lytic peptides are not likely to harm higher eukaryotes, because they do not affect the electrically neutral cholesterol-containing cell membranes of higher eukaryotes (Javadpour et al. 1996). Lytic peptide action can be targeted to specific cell types by the addition of a ligand. For example, Hansel et al. (2007) reported that lytic peptides conjugated with cancer cell membrane receptor ligands could be used to destroy breast cancer cells, while lytic peptides alone or conjugated with non-specific peptides were not effective. Lytic peptides also have been conjugated to human hormones that bind to receptors on tumor cells for targeted destruction of prostate and testicular cancer cells (Leuschner and Hansel 2004).
In this article we present techniques used to demonstrate the protozoacidal activity of a lytic peptide (Hecate) coupled to a heptapeptide ligand that binds to the surface membrane of protozoa from the gut of the Formosan subterranean termite. These techniques include extirpation of the gut from termite workers, anaerobic culture of gut protozoa (Pseudotrichonympha grassii, Holomastigotoides hartmanni,
Spirotrichonympha leidyi), microscopic confirmation that the ligand marked with a fluorescent dye binds to the termite gut protozoa and other free-living protozoa but not to bacteria or gut tissue. We also demonstrate that the same ligand coupled to a lytic peptide efficiently kills termite gut protozoa in vitro (protozoa culture) and in vivo (microinjection into hindgut of workers), but is less bacteriacidal than the lytic peptide alone. The loss of protozoa leads to the death of the termites in less than two weeks.
In the future, we will genetically engineer microorganisms that can survive in the termite hindgut and spread through a termite colony as "Trojan Horses" to express ligand-lytic peptides that would kill the protozoa in the termite gut and subsequently kill the termites in the colony. Ligand-lytic peptides also could be useful for drug development against protozoan parasites.
Protocol
Experiment 1: Extraction of termite gut protozoa under anaerobic conditions
- Use a fan box (Coy Laboratories) in a glove box to constantly circulate air through a desiccant and type D catalyst Stak-Paks to control humidity and oxygen levels and eliminate uneven temperatures. Fill the glove box with a continuous stream of nitrogen for 20 to 30 min. Monitor oxygen levels with an oxygen sensor (C-squared, Inc.) for 1 h. Use nitrogen to reach and maintain anaerobic conditions when needed.
- Prepare Trager U media (Trager 1934) and adjust pH to 7.0. Sparge the filter sterilized media in the glove box with a mixture of 2.5% hydrogen, 5% carbon dioxide and 92.5% nitrogen for 1 h to remove oxygen residues.
- Silanize all materials including microscope slides, microcentrifuge tubes, pipettes, glassware etc. used in the experiments using Sigmacote to prevent adsorption of protozoa or peptides to surfaces (Sigmacote ,Sigma, # SL-2, http://www.sigmaaldrich.com/etc/medialib/docs/ Sigma/Product_Information_Sheet/1/sl2pis.Par.0001.File.tmp/sl2pis.pdf).
- Since termite gut protozoa are strictly anaerobic organisms they must not be exposed to oxygen. Therefore the following steps are performed under anaerobic conditions in a glove box (see 1.1). With a forceps submerge the whole body of a termite worker in 70% ethanol and gently swirl for approximately 10 s to remove surface contaminants.
- Remove the worker from the ethanol and allow to dry on a clean Kimwipe for about 20 s. Use a sterile fine-tipped forceps to hold the worker abdomen and grab the tip of the abdomen with another pair of forceps to gently pull the gut upward or downward in a 45 degree angle. If the gut is pulled at a straight angle and with too much force it is likely to break apart. Place 10 guts in a drop of 100 μl Trager U media on a microscope slide.
- Pierce the guts with a pair of sterile fine dissecting probes to release the protozoa and gently transfer the gut contents with a 200 μl pipette into a 1 ml microcentrifuge tube containing 900 μl Trager U media. After allowing for sedimentation of gut wall fragments (5 s), transfer 900 μl of the supernatant to a fresh tube.
- Transfer 10 μl of protozoa culture to a sigmacoted microscope slide and check the condition of protozoa under a microscope at 200 X magnification.
- Prepare control cultures of the aerobic protozoa Tetrahymena pyriformis, Amoeba sp., Euglena sp., and Paramecium sp. (Carolina Biological Supply Company, Burlington, NC) as well as an overnight culture of Escherichia coli in the culture media recommended by the supplier.
Experiment 2: Add ligand coupled with a fluorescent dye to the protozoa and the bacteria cultures to test for binding to the surface membranes and cell walls
We previously used phage display libraries (New England Biolabs Inc, Ipswich, MA) to identify 19 heptapeptide sequences that bind to protozoa (protocols available at http://www.neb.com/nebecomm/ManualFiles/manualE8110.pdf). A ligand with a peptide sequence (ALNLTLH) that showed similarities to putative glycoproteins known from the Trypanosoma brucei membrane was synthesized and coupled to a C-terminal fluorescent probe (EDANS, 5-((2-Aminoethyl) amino) naphthalene-1-sulfonic acid, λmax = 341 nm, λem = 471 nm) via solid state peptide synthesis (SSPS) using the EDANS NovaTag resin (EMD Biosciences). Here we demonstrate that the ligand binds to the protozoa that were isolated from the termite gut and other free-living protozoa, but not to bacteria.
- Culture the protozoa as described in Exp. 1. Fix protozoa with 10 % formaldehyde at 4 °C for 12 h.
- Centrifuge the protozoa solution (30 x g, 10 min), discard the supernatant and wash the pellet containing the fixed protozoa twice in 1ml Trager U media. Re-suspend the pellet in 1 ml Trager U media. Also, fix other protozoa, and E. coli bacteria for controls.
- Incubate the fixed microorganisms for 1-2 h with solution of synthesized ligand coupled to the fluorescent dye EDANS (prepared in water) at a final concentration of 50 μM. The ligand dissolves better in water than Trager U media.
- Observe the microorganisms under a fluorescent microscope at 400 X magnification at an absorbance maximum at 341 nm and an emission in the blue region at 471 nm.
Experiment 3: Testing protozoacidal activity of the ligand coupled to a lytic peptide in vitro (protozoa culture)
A conjugate of the ligand and the lytic peptide Hecate (Mutwiri et al. 2000) was previously synthesized at the LSU Protein Facility.
- Silanize materials and prepare termite gut protozoa culture in the anaerobic glove box as described in Exp. 1.
- Prepare cultures of aerobe control microorganisms (e.g., E. coli and the free-living protozoa species T. pyriformis).
- In the anaerobic environment of the glove box, pipette 6 aliquots of 198 μl of protozoa culture into 0.5 ml Eppendorf tubes. Add 2 μl of a 100 μM solution of ligand-lytic peptide to half of the aliquots (end concentration 1 μM) of the termite gut protozoa culture. Add 2 μl of water to the other half of the aliquots (controls).
- On the benchtop, prepare similar aliquots of E. coli and T. pyriformis with 1 μM lytic peptide, ligand-lytic peptide or water.
- After 1 h, transfer 10 μl of each protozoa culture to the slides. Compare the survival of treated protozoa to that of controls under a microscope at 200 X magnification.
- After 1 h, plate 100 μl of approx. 10-4 dilution of the E. coli cultures on BHI and incubate at 37°C overnight. Compare the numbers of colony forming units on the plates.
Experiment 4: Injection of the ligand coupled to a fluorescent dye in into the termite hindgut
- Pull needles (Model GD-1, 1 X 900 mm) using a Narishige PC-10 glass micropipette puller with a dual stage heat level (65 and 48) to obtain a tip size of 20-30 μm. Confirm the tip size by measuring it under a microscope using a micrometer.
- Fill one needle with ca. 30 μl of 50 μM of fluorescently marked ligand suspended in water using an attached syringe. Fill another needle with water for the control. Attach a micropipette (0.5 μl capacity and 32 mm length, Drummond Scientific Company) to a holder in a micromanipulator. Attach a needle to the injection system holder in a second micromanipulator. Set initial injection parameters to approximately 1 s pulse length and 10-12 psi injection. Advance the needle slowly into the micropipette and inject the solution using a pedal-driven high-speed electronic injection system with a pulse length control attached to a controlled nitrogen gas flow, which ensures that a constant volume is reproducibly injected. After injection, remove the micropipette and record the length of the injected solution using a Vernier caliper. Calculate the injected volume from the known parameters of the micropipette. Adjust the pressure of nitrogen gas and the pulse length to expel 0.3 μl solution in a single injection.
- Squeeze the end of a termite worker abdomen with soft forceps to remove any excreta present in the rectum. Immobilize termite worker by chilling them on ice for 5 min.
- Make receivers for holding termite workers by cutting off 100 μl pipette tips using a scalpel blade. Cut the tip to a length of 10 - 12 mm and use according to the size of termites.
- Attach the receiver to the micromanipulator. Place a worker on a Petri dish on its dorsal side and aspirate the worker head first into the receiver using a nitrogen suction pump so that the terminus of the worker protrudes from the receiver.
- Holding the termite in the receiver, carefully advance the filled needle using the micromanipulator to insert it into worker anus. Inject 0.3 μl of the solution (fluorescently marked ligand or water for controls).
- Place the workers injected with ligand or water into separate petri dishes with damp filter paper and keep them at 26±2 °C with 78% R.H.
- Extirpate guts from the injected termites and collect the protozoa after 24 h as shown above in Exp. 1. Fix and observe the protozoa as shown in Exp. 2.
Experiment 5: Testing protozoacidal activity of the ligand coupled to a lytic peptide in vivo (injection into termite hindgut)
- Silanize materials as described in Exp. 1.
- Prepare a 500 μM solution of the ligand-lytic peptide in water.
- Follow steps 3.1) through 3.4) to prepare glass needles, receivers and termite workers.
- Following the methods described in 3.5) inject 0.3 μl of ligand-lytic peptide solution (treatment) or water (control) into the hindgut of 20 termite workers.
- Hold termites for 24 h, extirpate guts from several workers and observe gut contents under the microscope as described in 3.6). and 3.7).
- As soon a death of protozoa in the termite gut is confirmed, keep remaining treated termites and controls in Petri dishes with moist filter paper and observe mortality daily.
Representative Results:
Experiment 1: Usually, foregut, midgut and hindgut are obtained in one piece when the procedure is followed correctly (Figure 1a). The protozoa reside in high density in the anaerobic portions of the hindgut and can be released by piercing the hindgut with forceps (Figure 1b 1&2). The largest protozoa species in the gut of the Formosan subterranean termite is the spindle-shaped P. grassii, which is 200-300 μm long and 150 μm wide and can be seen with the naked eye. The second largest species is the pear-shaped H. hartmanni (50-140 μm long and 30-80 μm wide). The smallest species is the cone-shaped S. leidyi (15-50 μm long and 8-30 μm wide; Lai et al. 1983). The protozoa species are shown in Figure 2.
Under optimal culture conditions the three species of protozoa isolated from the gut of Formosan subterranean termites will stay alive and healthy for at least 72 h in anaerobic Trager U media (Figure 3a). However, if culture conditions are not optimal protozoa will die fast. If there are oxygen residues in the media, the movement of protozoa will cease immediately. If osmotic pressure is too high or membrane integrity is compromised the surface membrane of the protozoa will bulge out and the cells rupture (Figure 3b). If osmotic pressure is too low or membranes are compromised, protozoa will shrivel and shrink (Figure 3c).
Experiment 2: We confirmed that the ligand coupled to a fluorescent probe bound to all three species of protozoa from the hindgut of Formosan subterranean termites in detectable densities. Ligand binding occurs on the whole cell surface (Figure 4). Binding sites are concentrated in the anterior region of the protozoa on the axostyle (a sheet of microtubules) and nucleus in P. grassii.
We observed some patchy autofluorescence of wood particles ingested by the protozoa. However, autofluorescence is usually easy to discern from specific binding of the ligand, since there is no autofluorescence of the surface, the axostyle and the nucleus (Figure 4).
We also detected fluorescence in all tested free-living aerobic protozoa species (Figure 5), which suggests that the ligand binds to structures generic to protozoa. However, no ligand binding was observed for E. coli.
Experiment 3: One μM of ligand-lytic peptide killed all three species of protozoa from the gut of Formosan subterranean termite workers and the free-living T. pyriformis in vitro in less than 10 min, while controls stayed alive. Figure 6 shows the progressive loss of membrane integrity of the termite gut protozoa treated with ligand-lytic peptide. Membranes bulge and rupture, protozoa shrivel and die. No difference was observed in the number of E. coli colonies between treatments of ligand-lytic peptide and water. Lytic peptide with no ligand, however, reduced the number of E. coli colonies considerably (Figure 7). This suggests that attachment of the ligand to some degree protects non target microorganisms from lysis.
Experiment 4: When 0.3 μl 50 μM of the fluorescently marked ligand was injected into the hindgut of termite workers, binding to P. grassii, S. leidyi and H. hartmanni was confirmed via fluorescence microscopy similar to Exp. 2 (Figure 4). Termite gut tissue did not show fluorescence.
Experiment 5: Injection of 0.3 μl 500 μM ligand-lytic peptide killed all three species of protozoa in the gut of Formosan subterranean termites within 24h. Termites died within 10 days after loss of their symbiotic protozoa. Previously, Husseneder and Collier (2009) injected the same concentration of lytic peptide into termite guts. Without the attached ligand, it took more time until the protozoa in the gut (72 h) and the termites were dead (six weeks). This suggests that the ligand increases protozoacidal efficiency of lytic peptides, most likely by binding the lytic peptides to the protozoa.
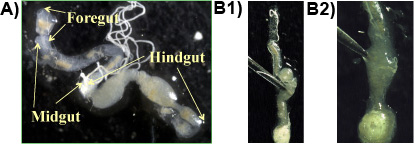
Figure 1. a: Formosan subterranean termite gut on a slide showing the main sections of the gut (fore-, mid-, hindgut);
b 1& 2: Hindgut is pierced with forceps to release the gut content containing the protozoa.

Figure 2. The three species of flagellate protozoa found in the hindgut of the Formosan subterranean termite: a) Pseudotrichonympha grassii, b) Holomastigotoides hartmanni, and c) Spirotrichonympha leidyi.

Figure 3. Protozoa in culture, a) Healthy protozoa, b) Protozoa with bulging membranes, c) Shriveled protozoa.

Figure 4. Confirmation of the binding of the ligand coupled to a fluorescent probe to termite gut protozoa (from top to bottom: P. grassii, H. hartmanni, S. leydi), treated with fluorescently marked ligand and untreated controls (showing autofluorescence).

Figure 5. Ligand binding to free-living aerobic protozoa, a) Tetrahymena, b) Amoeba, c) Euglena, and d) Paramecium.
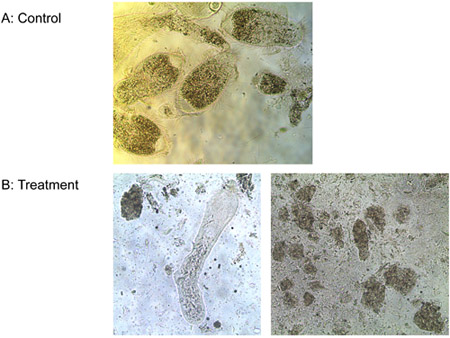
Figure 6. Treatment of protozoa with a) water (control) and b) 1 μM ligand-lytic peptide.
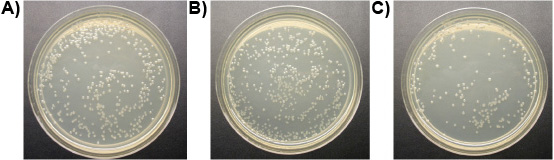
Figure 7. E. coli colonies on plates (10-4 dilution): a)Treated with water (control), b)Treated with 1 μM ligand-lytic peptide, c)Treated with 1 μM lytic peptide.
Access restricted. Please log in or start a trial to view this content.
Discussion
Ligand-lytic peptides have been successfully used to effectively target and destroy cancer cells (Hansel and Leuschner 2004, Hansel et al. 2007). Based on this concept, we developed a heptapeptide ligand that binds to the surface of protozoa in the gut of Formosan subterranean termites and coupled it to a lytic peptide with the goal to destroy these obligate cellulose-digesting symbionts in the gut of termites to achieve termite control (Husseneder and Collier 2009).
We successfully ...
Access restricted. Please log in or start a trial to view this content.
Disclosures
No conflicts of interest declared.
Acknowledgements
We thank Dr. Allison Richard, former director of the LSU peptide facility for the fluorescent ligand synthesis, the Interdisciplinaray Center for Biotechnology Research, UF for the ligand-lytic peptide synthesis, and the Socolovsky Microscope facility for providing access to fluorescence microscopes. Funding was provided by the SERDP Exploratory Development Program (SEED) of the Department of Defense, Department of Energy and Environmental Protection Agency, the Biotechnology AgCenter Interdisciplinary Team Program and the state of Louisiana.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Sigmacote | Sigma-Aldrich | SL-2 | |
| EDANS | Novabiochem, EMD Millipore | ||
| Anaerobic glove box | Coy Laboratories, Inc. | Custom made | |
| Intellus environmental controller | Percival Scientific, Inc. | I36NL | |
| PC-10 Glass micropipette puller | Narishige International | PC-10 | |
| Glass needles (Model GD-1, 1 X 900 mm) | Narishige International | GD-1 | |
| Leitz micromanipulators | Vermont Optechs, Inc. | ACS01 | |
| Microinjector | Tritech Research, Inc. | MINJ-1 | |
| Microcaps | Drummond Scientific | 1-000-0005 | |
| LEICA fluorescence imaging system | Leica Microsystems | DMRxA2 | |
| LEICA dissecting scope | Leica Microsystems | MZ16 | |
| LEICA microscope | Leica Microsystems | DMLB | |
| Olympus dissecting scope | Olympus Corporation | SZ61 |
References
- Hansel, W., Leuschner, C., Enright, F. Conjugates of lytic peptides and LHRH or βCG target and cause necrosis of prostate cancers and metastases. Mol. Cell. Endocrinol. 269, 26-33 (2007).
- Husseneder, C., Collier, R. E. Paratransgenesis for termite control. Insect Symbiosis. Bourtzis, K., Miller, T. A. 3, CRC Press LLC. Boca Raton, Florida. Volume 3 361-376 (2009).
- Husseneder, C., Grace, J. K., Oishi, D. E. Use of genetically engineered bacteria (Escherichia coli) to monitor ingestion, loss and transfer of bacteria in termites. Curr. Microbiol. 50, 119-123 (2005).
- Husseneder, C., Grace, J. K. Genetically engineered termite gut bacteria deliver and spread foreign genes in termite colonies. Appl. Microbiol. Biotechnol. 68, 360-367 (2005).
- Javadpour, M. M., Juban, M. M., Lo, W. C., Bishop, S. M., Alberty, J. B., Cowell, S. M., Becker, C. L., Mc Laughlin, M. L. De novo antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 39, 3107-3113 (1996).
- Lai, P. Y., Tamashiro, M., Fuji, J. K. Abundance and distribution of the three species of symbiotic protozoa in the hindgut of Coptotermes formosanus (Isoptera). Proc. Haw. Entomol. Soc. 24, 271-276 (1983).
- Leuschner, C., Hansel, W. Membrane disrupting lytic peptides for cancer treatments. Curr. Pharm. Des. 10, 2299-2310 (2004).
- Mutwiri, G. K., Henk, W. G., Enright, F. M., Corbeil, L. B. Effect of the Antimicrobial Peptide, d-Hecate, on Trichomonads. J. Parasitol. 86, 1355-1359 (2000).
- Trager, W. The cultivation of a cellulose-digesting flagellate, Trichomonas termopsidis, and of certain other termite protozoa. Biol. Bull. 66, 182-190 (1934).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved