A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Imaging G-protein Coupled Receptor (GPCR)-mediated Signaling Events that Control Chemotaxis of Dictyostelium Discoideum
In This Article
Summary
Here, we describe detailed live cell imaging methods for investigating chemotaxis. We present fluorescence microscopic methods to monitor spatiotemporal dynamics of signaling events in migrating cells. Measurement of signaling events permits us to further understand how a GPCR-signaling network achieves gradient sensing of chemoattractants and controls directional migration of eukaryotic cells.
Abstract
Many eukaryotic cells can detect gradients of chemical signals in their environments and migrate accordingly 1. This guided cell migration is referred as chemotaxis, which is essential for various cells to carry out their functions such as trafficking of immune cells and patterning of neuronal cells 2, 3. A large family of G-protein coupled receptors (GPCRs) detects variable small peptides, known as chemokines, to direct cell migration in vivo 4. The final goal of chemotaxis research is to understand how a GPCR machinery senses chemokine gradients and controls signaling events leading to chemotaxis. To this end, we use imaging techniques to monitor, in real time, spatiotemporal concentrations of chemoattractants, cell movement in a gradient of chemoattractant, GPCR mediated activation of heterotrimeric G-protein, and intracellular signaling events involved in chemotaxis of eukaryotic cells 5-8. The simple eukaryotic organism, Dictyostelium discoideum, displays chemotaxic behaviors that are similar to those of leukocytes, and D. discoideum is a key model system for studying eukaryotic chemotaxis. As free-living amoebae, D. discoideum cells divide in rich medium. Upon starvation, cells enter a developmental program in which they aggregate through cAMP-mediated chemotaxis to form multicullular structures. Many components involved in chemotaxis to cAMP have been identified in D. discoideum. The binding of cAMP to a GPCR (cAR1) induces dissociation of heterotrimeric G-proteins into Gγ and Gβγ subunits 7, 9, 10. Gβγ subunits activate Ras, which in turn activates PI3K, converting PIP2 into PIP3 on the cell membrane 11-13. PIP3 serve as binding sites for proteins with pleckstrin Homology (PH) domains, thus recruiting these proteins to the membrane 14, 15. Activation of cAR1 receptors also controls the membrane associations of PTEN, which dephosphorylates PIP3 to PIP2 16, 17. The molecular mechanisms are evolutionarily conserved in chemokine GPCR-mediated chemotaxis of human cells such as neutrophils 18. We present following methods for studying chemotaxis of D. discoideum cells. 1. Preparation of chemotactic component cells. 2. Imaging chemotaxis of cells in a cAMP gradient. 3. Monitoring a GPCR induced activation of heterotrimeric G-protein in single live cells. 4. Imaging chemoattractant-triggered dynamic PIP3 responses in single live cells in real time. Our developed imaging methods can be applied to study chemotaxis of human leukocytes.
Protocol
1. Preparation of chemotactic competent cells of Dictyostelium discoideum
- To generate D. discoideum cells that are chemotactic to the chemoattractant cAMP, harvest cells growing in D3-T rich media from a shaking culture at 22°C.
- Wash the cells twice in non-nutrient developmental buffer (DB buffer containing 5 mM Na2HPO4, 5 mM KH2PO4, 2 mM MgCl2, and 0.1 mM CaCl2).
- Re-suspend cells in DB buffer at a density of 2x107 cells/ml.
- Shake 10 ml cells in a 250 ml flask at 100 rpm at 22°C for one hour.
- Deliver 100 μl of 7.5 μM cAMP stock to the 10 ml cells every six minutes over 6 hours to achieve a final concentration of 75 nM cAMP, a process designated as cAMP pulsing treatment. After 5-6 hours of cAMP pulsing treatment, D. discoideum cells become chemotactic competent toward cAMP gradient.
- Collect cells by centrifugation at 200 g for 5 min and then resuspend cells with DB buffer containing 2.5 mM caffeine, and shake at 200 rpm at 22°C for 20 min to basolate cell to a chemotactic situation.
2. Imaging chemotaxing cells in a visible and manipulatable chemoattractant gradient
- Backfill a micropipette with a freshly prepared 30 μl solution of 1 μM cAMP and Alexa 594 at 0.1μg/μl in DB buffer.
- Attach the Femtotip to a micropipette holder and connect the tubing to a pressure supply apparatus, Eppendorf FemtoJet system.
- Attach the micropipette assembly to a micromanipulator (Eppendorf TransferMan NK2) motorized micromanipulator to provide a steady pressure in order to establish a stable gradient.
- Mount a one-well LabTek chamber filled with 6 ml of DB buffer over a 40X oil lens on a confocal microscope and use bright-field optics, center the Femtotip into the field of view.
- Turn on the pressure supply and set the compensation pressure (Pc) for 70 hPa to establish a gradient of the cAMP/Alexa 594 mixture.
- Visualize cAMP gradient by monitoring the mixture of desired concentration of cAMP and Alexa 594 fluorescence using excitation with a 543 nm laser line.
- Use auto-positioning function of the micromanipulator to put micropipette to the desired positions and set them as Position 1, Position 2, and Position 3 to manipulate the gradient to which cell are exposed to.
3. Immobile nonpolarized cell system facilitates imaging signaling events involved in cAMP gradient sensing
- After caffeine treatment, remove an aliquot of cells and centrifuge at 500g for 3 min.
- Remove buffer and dilute cells to 5x105 cells/ml with fresh DB buffer containing 2.5 mM caffeine.
- Apply 1 ml of cell suspension to a single-well chamber or 0.4 ml to each well of a four-well chamber.
- Allow cells to adhere for 10 min, carefully pipette off the buffer to remove unattached cells and replace with the same volume.
- Locate the desired cells under microscope and start imaging.
- For an experiment designed to monitor dynamics of signaling components in the cells exposing to a steady gradient, treat the cells with 5.0 μM (final concentration) Latrunculin B for 10 min prior to the experiments.
4. Simultaneous monitoring heterotrimeric G protein activation and PIP3 production
- cAMP pulsing develop cells co-expressing GαCFP and YFPGβ (G cells) and cells expressing PIP3 indicator PH-GFP (PH cells) 7.
- Mix these two types of cells with 1:1 ratio and plate them in one-well or 4-well chambers.
- Create and save the emission fingerprint reference curve CFP, YFP and GFP using Lambda Stack Acquisition mode within the spectral range from 464 to 624 nm with a 10 nm width.
- Simultaneously image G protein activation in G cells and PIP3 production in PH cells using with same Lambda Stack Acquisition mode within the spectral range from 464 to 544 nm with a 10 nm increments.
- Apply Linear Unmixing function of Zeiss 510META software using saved CFPand YFP and emission fingerprints to mathematically calculate the contribution of each fluorophore in the Lambda Stack to separate the CFP and YFP intensity into individual channels in G.
- With the same strategy, apply Linear Unmixing function using saved GFP and background emission fingerprints to mathematically calculate GFP intensity in PH cells.
5. Representative results:
- An excellent model system of D. discoideum for GPCR mediated chemotaxis. A social amoeba, D. discoideum exhibits a striking chemotaxis during the life cycle. Due to its genetic and biochemical advantages, D. d provides a powerful system to study chemotaxis.
- Chemotaxis of cells under a visible and manipulatable chemoattract fields. Here, we first show a simple methodology to obtain a linear relationship between cAMP concentration and the intensity of a fluorescent dye Alexa 594 by a dilution series of 2 μM cAMP mixed with 10 μg/mL Alexa 594 (Fig. 2A). Next, we provide an easy way to visualize the gradient, moreover, to establish a quantitative measurement of cAMP concentration of a gradient by the intensity of Alexa 594 (Fig. Fig. 2B).
- Cell motility is uncoupled with cell polarization and directional sensing. An actin polymerization inhibitor eliminates pre-existing morphological polarity and also prevents cell movement while maintaining the cells' capability of directional sensing (Fig. 3A). Employment of visible and manipulatable cAMP stimulation guarantees the input, for example uniformly applied stimulations or a gradient. This method allows a quantitative analysis of cAMP-induced redistribution of key signaling components in the gradient sensing machinery. Measured spatiotemporal dynamics of these signaling components allow us to understand how the signaling network achieves adaptation to uniform stimulations while generating polarized biochemical responses to gradients (Fig. 3B).
- Systemic measurements of kinetics of chemosensing signaling network upon exposure to a steady gradient. It is critical to measure the dynamics/kinetics of directional-sensing signaling components to understand how each component contributes to the establishment of intracellular polarization when cells experience first gradient. Application of live cell imaging with a high tempo-spatial resolution, we first show a biphasic PIP3 production of cell which is exposed to a steady cAMP gradient (Fig. 4A-C). Applying live cell imaging, we have systematically measured dynamics of directional-sensing specific signaling network from cAMP stimulation to PIP3 production (Fig. 4D, E).
- Simultaneous monitoring heterotrimeric G protein activation and PIP3 production upon uniformly applied cAMP stimulation. Förster resonance energy transfer (abbreviated FRET) provides an efficient approach to monitor heterotrimeric G protein activation (dissociation) upon cAMP stimulation. Here, we described an convenient easy-adopt system for a simultaneous measurement of heterotrimeric G protein activation and PIP3 production by monitoring the FRET change and membrane translocation of PIP3 probe, PH-GFP in G and PH cells, respectively (Fig. 5). A uniformly applied cAMP stimulation triggers a persistent G protein activation while which triggers a transient PIP3 production.
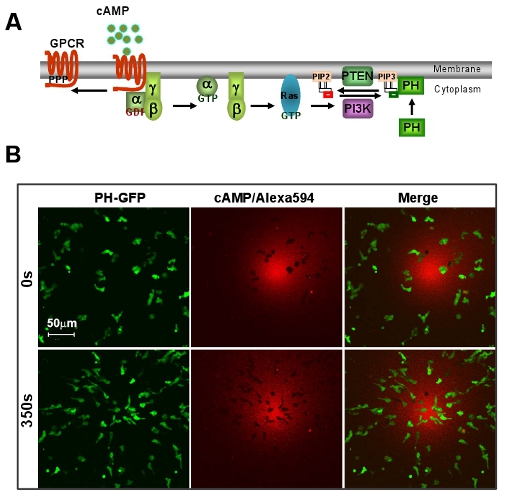
Figure 1: An excellent model system of D. discoideum for GPCR mediated chemotaxis. A. Scheme shows a brief signaling pathway of directional sensing. B. cAMP gradient induces rapid chemotaxis of D. discoideum cells. Cells express PIP3 probe, PH-GFP (Green). Gradient (Red) is visualized by Alexa 594. Scale bar=50μm.
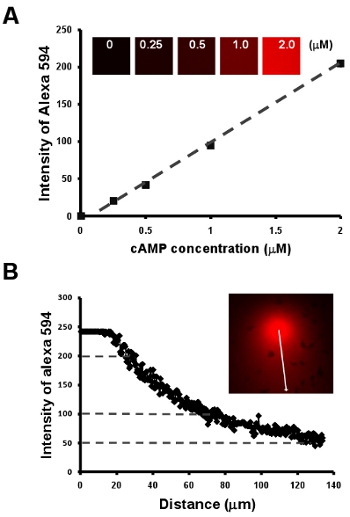
Figure 2: Chemotaxis of cells under a visible and manipulatable chemoattract fields. A. Graph shows a linear relationship between cAMP concentration and the intensity of a fluorescent dye Alexa 594 by a dilution series of 2 μM cAMP mixed with 10 μg/mL Alexa 594. B. Quantitative measurement of cAMP concentration of a gradient by the linear relationship of cAMP concentration and intensity of fluorescent dye Alexa 594 in A.

Figure 3: Cell motility is uncoupled with cell polarization and directional sensing. A. Image shows that immobile cells by the treatment of actin polymerization inhibitor Latrunculin B maintain the capability of directional sensing. Cells express PIP3 probe, PH-GFP (Green). Gradient (Red) is visualized by Alexa 594. B. Manipulatable cAMP stimulation and immobile cell system allows to address key questions of directional sensing. Scale bar=10μm.

Figure 4: Systemic measurements of kinetics of chemosensing signaling network upon exposure to a steady gradient. A. Montage shows a biphasic PIP3 production (Green) of cell which is exposed to a steady cAMP gradient (Red). B. Image shows the regions of interests (ROIs) for measurement of kinetics of PIP3 production presented in C. C. Kinetics of PIP3 production in the cells exposed to a steady gradient. D. Scheme shows the signaling network of directional sensing from cAMP stimulation to PIP3 production. Their kinetics upon exposure to a steady gradient is presented in the same color solid lines in E.

Figure 5: Simultaneous monitoring multi-events of GPCR signaling networks. A. Scheme shows simultaneous measurement of heterotrimeric G protein activation and PIP3 production by monitoring the FRET change and membrane translocation of PIP3 probe, PH-GFP in G and PH cells, respectively. B. Montage of rainbow images of G and PH cells shows that a uniformly applied cAMP stimulation triggers a persistent G protein activation at the cell peripheral, while which triggers a transient PIP3 production. The time points are before (0s) and after stimulation for 4.9s, 10.2s and 20.4s. C. Kinetics of G protein activation and PIP3 production upon a uniformly applied cAMP stimulation.
Discussion
The processes of reaching chemotactic competent stage of cells
For wild type D. discoideum cells, it takes about 5 ˜ 6 hours pulsing development at room temperature to induce them into a well-chemotactic competent stage during which cells display a well polarized cellular morphology and rapid cell migration (Fig. 1). Several factors, such as cAMP concentration for pulsing, temperature, and different genetic backgrounds, may affect the process of reaching chemotactic competent stag...
Disclosures
No conflicts of interest declared.
Acknowledgements
This work is supported by the intramural fund from NIAID, NIH.
Materials
| Name | Company | Catalog Number | Comments |
| D3-T Growth Media | KD Medical | ||
| Caffeine | Sigma-Aldrich | ||
| Latrunculin B | Molecular Probes, Life Technologies | ||
| Alexa 594 | Molecular Probes, Life Technologies | ||
| cAMP | Sigma-Aldrich | ||
| ChronTrol XT programmable timer | ChronTrol Corp | ||
| Miniplus 3 peristaltic pump | Gilbson | ||
| Platform rotary shaker | |||
| FemtoJet microcapillary pressure supply | Eppendorf | ||
| Single- and four-well Lab-Tek II coverglass chambers | Nalge Nunc international | ||
| LSM 510 META or equivalent fluorescent microscope | Carl Zeiss, Inc. | a 40X 1.3 NA or 60X 1.4 NA oil DIC Plan-Neofluar objective lens | |
| Olympus X81 or equivalent | Olympus Corporation | Requires a 100X 1.47 NA TIRF objective lens |
References
- Lijima, M., Huang, Y. E., Devreotes, P. Temporal and spatial regulation of chemotaxis. Dev Cell. 3, 469-469 (2002).
- Murphy, P. M. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 12, 593-593 (1994).
- Devreotes, P. N. G protein-linked signaling pathways control the developmental program of Dictyostelium. Neuron. 12, 235-235 (1994).
- Jin, T., Xu, X., Hereld, D. Chemotaxis, chemokine receptors and human disease. Cytokine. 44, 1-1 (2008).
- Meier-Schellersheim, M. Key role of local regulation in chemosensing revealed by a new molecular interaction-based modeling method. PLoS Comput Biol. 2, e82-e82 (2006).
- Xu, X. Coupling mechanism of a GPCR and a heterotrimeric G protein during chemoattractant gradient sensing in Dictyostelium. Sci Signal. 3, 71-71 (2010).
- Xu, X., Meier-Schellersheim, M., Jiao, X., Nelson, L. E., Jin, T. Quantitative imaging of single live cells reveals spatiotemporal dynamics of multistep signaling events of chemoattractant gradient sensing in Dictyostelium. Mol Biol Cell. 16, ra71-ra71 (2005).
- Xu, X., Meier-Schellersheim, M., Yan, J., Jin, T. Locally controlled inhibitory mechanisms are involved in eukaryotic GPCR-mediated chemosensing. J. Cell Biol. 178, 141-141 (2007).
- Jin, T., Zhang, N., Long, Y., Parent, C. A., Devreotes, P. N. Localization of the G protein betagamma complex in living cells during chemotaxis. Science. 287, 1034-1034 (2000).
- Janetopoulos, C., Jin, T., Devreotes, P. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science. 291, 2408-2408 (2001).
- Funamoto, S., Milan, K., Meili, R., Firtel, R. A. Role of phosphatidylinositol 3' kinase and a downstream pleckstrin homology domain-containing protein in controlling chemotaxis in dictyostelium. J. Cell Biol. 153, 795-795 (2001).
- Li, Z. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 287, 1046-1046 (2000).
- Sasaki, A. T., Chun, C., Takeda, K., Firtel, R. A. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J. Cell Biol. 167, 505-505 (2004).
- Meili, R. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18, 2092-2092 .
- Parent, C. A., Blacklock, B. J., Froehlich, W. M., Murphy, D. B., Devreotes, P. N. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 95, 81-81 (1998).
- Funamoto, S., Meili, R., Lee, S., Parry, L., Firtel, R. A. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 109, 611-611 (2002).
- Iijima, M., Devreotes, P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 109, 599-599 (2002).
- Haastert, P. J. V. a. n., Devreotes, P. N. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol. 5, 626-626 (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved