A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Investigating Intestinal Inflammation in DSS-induced Model of IBD
In This Article
Summary
Experimental models of inflammatory bowel disease have allowed us to examine the complex innate and adaptive immune responses associated with pathogenesis. Using histological scoring, quantification of pro-inflammatory cytokines and myeloperoxidase activity, one can begin to assess these responses seen in inflammatory bowel disease.
Abstract
Inflammatory bowel disease (IBD) encompasses a range of intestinal pathologies, the most common of which are ulcerative colitis (UC) and Crohn's Disease (CD). Both UC and CD, when present in the colon, generate a similar symptom profile which can include diarrhea, rectal bleeding, abdominal pain, and weight loss.1 Although the pathogenesis of IBD remains unknown, it is described as a multifactorial disease that involves both genetic and environmental components.2
There are numerous and variable animal models of colonic inflammation that resemble several features of IBD. Animal models of colitis range from those arising spontaneously in susceptible strains of certain species to those requiring administration of specific concentrations of colitis-inducing chemicals, such as dextran sulphate sodium (DSS). Chemical-induced models of gut inflammation are the most commonly used and best described models of IBD. Administration of DSS in drinking water produces acute or chronic colitis depending on the administration protocol.3 Animals given DSS exhibit weight loss and signs of loose stool or diarrhea, sometimes with evidence of rectal bleeding.4,5 Here, we describe the methods by which colitis development and the resulting inflammatory response can be characterized following administration of DSS. These methods include histological analysis of hematoxylin/eosin stained colon sections, measurement of pro-inflammatory cytokines, and determination of myeloperoxidase (MPO) activity, which can be used as a surrogate marker of inflammation.6
The extent of the inflammatory response in disease state can be assessed by the presence of clinical symptoms or by alteration in histology in mucosal tissue. Colonic histological damage is assessed by using a scoring system that considers loss of crypt architecture, inflammatory cell infiltration, muscle thickening, goblet cell depletion, and crypt abscess.7 Quantitatively, levels of pro-inflammatory cytokines with acute inflammatory properties, such as interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF)-α,can be determined using conventional ELISA methods. In addition, MPO activity can be measured using a colorimetric assay and used as an index of inflammation.8
In experimental colitis, disease severity is often correlated with an increase in MPO activity and higher levels of pro-inflammatory cytokines. Colitis severity and inflammation-associated damage can be assessed by examining stool consistency and bleeding, in addition to assessing the histopathological state of the intestine using hematoxylin/eosin stained colonic tissue sections. Colonic tissue fragments can be used to determine MPO activity and cytokine production. Taken together, these measures can be used to evaluate the intestinal inflammatory response in animal models of experimental colitis.
Protocol
1. Murine model of DSS-induced acute colitis
- Add dextran sulphate sodium (DSS) to autoclaved drinking water to the desired final concentration (1-5%) (wt/vol) (i.e. To make a 5% DSS solution, add 25 g of DSS powder to 500 mL of autoclaved water). Stock solution can be left at room temperature for up to one week or at 4°C until use.
- In a biosafety hood, pour stock DSS solution into 50 mL Falcon tubes (one needed per cage). Keep the stock solution to refill tubes when needed.
- Replace drinking water in each mouse cage with the DSS solution (in the 50 mL Falcon tubes) (The duration will depend on the DSS regimen that is used. For example, using 6-8 week old male C57BL/6 mice, we administer a 5% DSS solution for a total of five days). Mice should not have access to any other water source. Control mice are given autoclaved drinking water without DSS.
- Weigh mice daily and record the amount of DSS consumed per day. Top each bottle to 50 mL after recording DSS levels. This is to measure the approximate volume of DSS consumed per cage per mouse throughout the duration of the experiment. In our studies, we use a 5% DSS solution for 5 days with male C57BL/6 mice. Significant weight loss, altered stool consistency and signs of fecal blood are seen as early as day 3 using this particular DSS regimen. During the DSS administration, mice exhibit pronounced weight loss (around 5-10% of their initial weight on day 5) with weight loss greater than 20% of initial weight with dehydration and diarrhea to be the significant physiological indicator that an animal is at or near endpoint. If the animal is given access to normal water after the 5 days of 5% DSS, it will recover within 7 days. All experiments should be approved by the institution's animal ethics committee and be in accordance with the approved Animal Utilization Protocol (AUP).
- During the duration of the experiment, a disease activity index (DAI) score can be assessed to evaluate the clinical progression of colitis. The DAI is the combined score of weight loss compared to initial weight, stool consistency, and bleeding. Scores are defined as follows: weight loss: 0 (no loss), 1 (1-5%), 2 (5-10%), 3 (10-20%), and 4 (>20%); stool consistency: 0 (normal), 2 (loose stool), and 4 (diarrhea); and bleeding: 0 (no blood), 1 (Hemoccult positive), 2 (Hemoccult positive and visual pellet bleeding), and 4 (gross bleeding, blood around anus). DAI can be scored daily during the duration of the DSS treatment.
- At the time point of choice, weigh and sacrifice mice. Mice can be euthanized by cervical dislocation following inhalation of isoflurane or by another method approved by the institution's animal facility.
2. Collect colonic tissue samples
- Expose the ventral side of the animal and wet the abdomen area with a 70% ethanol solution. At this point, refer to Table 1 and make note of any signs of rectal bleeding (blood present at the anal orifice) or rectal prolapse in each animal.
- Use standard dissecting scissors to incise the abdomen by making a ventral midline incision.
- Locate the colon and transect the colon as close to the colorectal margin as possible to free the distal colon.
- Carefully and slowly pull out the whole colon, detaching it from the surrounding mesentery.
- Transect the colon at the colonocecal margin to free the proximal end of the colon. Feces can be removed by rinsing colon with sterile PBS by using a gavage needle attached to a 3 or 5 mL syringe or by carefully squeezing it out using a pair of bent tweezers/forceps. Using the whole colon, assess for damage (See Section 3.1)
- Tissue sampling for histological analysis and other assays can be done by cutting 0.5 cm to 1.0 cm long colonic fragments making note of which area the sample is from (i.e. proximal, middle, or distal).
- Tissue samples to be used for assays can be individually placed in 1.5 mL eppendorf tubes and frozen in liquid nitrogen and stored until use at -70°C.
3. Assessment of colitis severity
- Macroscopic or disease severity score is assessed terminally by an unbiased observer using a previously published scoring system (Table 1).9 Stool consistency can be assessed by using a pair of forceps and pressing down on the feces to determine consistency. To determine a score for blood in the feces, note the colour of the feces (i.e. black stool versus light brown stool) and further validate using a Hemoccult test. Using the scoring system, determine a score for each of the conditions. The final macroscopic score for each animal is the sum of each individual score.
- To evaluate histological damage of colitis severity, cut a small fragment (0.5 cm) of the colon, place in a tissue cassette and submerge in buffered 10% formalin solution. Prepare 5 μm paraffin embedded cross sections and stain sections with hematoxylin/eoxin (H&E) using the appropriate procedures. Colon fragments can be taken from the proximal, mid-colon, or distal section of the colon.
- H&E stained colonic tissue sections are scored by a blinded observer using a previously published system for the following measures: crypt architecture (normal, 0 - severe crypt distortion with loss of entire crypts, 3), degree of inflammatory cell infiltration (normal, 0 - dense inflammatory infiltrate, 3), muscle thickening (base of crypt sits on the muscularis mucosae, 0 - marked muscle thickening present, 3), goblet cell depletion (absent, 0- present, 1) and crypt abscess (absent, 0- present, 1).7 The histological damage score is the sum of each individual score. It should be noted that unlike human UC, crypt abscesses are not characteristic of this model and are rarely seen; microscopic ulcerations are also rare. If multiple colon sections were stained, histological scores between similar sections should be used to determine the final score for each area (i.e. histological score in proximal colon versus histological score in distal colon).
4. Prepare stock solutions of reagents for assays
- Prepare a 50 mM solution of Potassium Phosphate buffer by adding solution B (K2HPO4, 8.7 g of dibasic potassium phosphate in 1 L of dH2O) to solution A (KH2PO4, 6.8 g of monobasic potassium phosphate in 1L of dH2O) until a pH of 6.0 is achieved. Remaining solutions can be stored in fridge (2-8°C) until future use.
- Prepare hexadecyltrimethylammonium bromide (HTAB) buffer by adding 5 g HTAB into 1 L of Potassium Phosphate buffer (50 mM, pH=6.0). Gently heat to dissolve and store at 2-8°C until use. When required, heat to re-dissolve.
- Prepare lysis buffer for tissue homogenization for protein analysis by adding 10 mL of 1M tris-hydrochloric acid (pH=8.0), 6 mL of 5M sodium chloride and 2 mL of Triton X-100 to 182 mL of sterilized distilled water. Triton X-100 is very viscous at room temperature and thus, should be gently warmed prior to use. The prepared lysis buffer can be stored at -20°C until use.
5. Sample preparation for assays
- Sample preparation for MPO analysis.
- Remove samples from -70°C and place on ice. Record the weight of each sample after removing any visible feces or fat by using a bent forcep/tweezer and place into a 2mL Eppendorf Safe-Lock microcentrifuge tube (or any tube that can be used with a homogenizer). Samples should be kept on ice at all times. It is important to note that similar colon fragments should be used from each biological replicate (i.e. distal sections only or proximal sections only).
- Add homogenizer bead to each sample tube.
- Add the appropriate amount of HTAB buffer according to tissue weight. If tissue weight is less than 25 mg, add the buffer at a ratio of 12.5mg/mL; if tissue weight is between 25-50, add at a ratio of 25mg/mL. If tissue weight is greater than 50, add buffer at a ratio of 50mg/mL.
- Homogenize with a tissue homogenizer for 4 min at 30 Hz. Repeat if tissue is not fully homogenized.
- Remove homogenizer bead and centrifuge solution for 6 min (13400 x g, 4°C).
- Collect supernatant and discard the resulting pellet. Supernatent can be stored at -70°C until use.
- Sample preparation for cytokine analysis.
- Repeat steps 5.1.1. to 5.1.2.
- Add 50 μl of protease inhibitor cocktail (PIC) to 10 mL of prepared lysis buffer.
- Add 1 mL of the PIC and lysis buffer solution to each sample irrespective of weight.
- Homogenize for 5 min at 30 Hz. Repeat if tissue is not fully homogenized.
- Remove homogenizer bead and centrifuge solution for 5 min at 3300 x g.
- Collect supernatant and discard the resulting pellet. Supernatant can be stored at -70°C until use.
6. Quantification of inflammatory markers
- MPO activity assay
- Prepare o-dianisidine dihydrochloride (o-dianisidine) solution by combining 16.7 mg of o-dianisidine dihydrochloride, 90 mL of dH2O, and 10 mL of potassium phosphate buffer. This solution should be prepared fresh for every assay.
- Add 7 μL of tissue homogenate (prepared in section 5.1) in triplicate into a 96-well plate.
- Add 50 μL of diluted H2O2 (4 μL of 30% H2O2 diluted in 96 μL of dH2O) to the o-dianisidine mixture.
- Use a multi-channel pipette to add 200 μL of the o-dianisidine mixture containing H2O2 to each of the wells.
- Measure absorbance at 450 nm using a spectrophotometer. Take three readings at 30 second intervals.
- Calculate MPO activity. MPO activity is measured in units (U) of MPO/mg tissue, where one unit of MPO is defined as the amount needed to degrade 1 μmoL of H2O2 per minute at room temperature. Considering that one unit (U) of MPO= 1 μmoL of H2O2 split and that 1 μmoL of H2O2 gives a change of absorbance of 1.13 x 10-2 nm/min, units of MPO in each sample is determined as change in absorbance [ΔA(t2-t1)]/Δmin x (1.13 x 10-2). To get units per mg of tissue, use the tissue: buffer ratio. For example, if a tissue: buffer ratio of 50 mg/mL was used, in 7 μL of homogenate, there is 0.35 mg of tissue. Therefore, to get units per mg tissue, divide the units of MPO by 0.35. A sample calculation using absorbance values (nm) is included below (assuming that the sample has been added in triplicate):
| Sample | Time 0 sec | Time 30 sec | Time 60 sec | ||||||
| A | 1 | 2 | 3 | 1' | 2' | 3' | 1" | 2'' | 3" |
| 0.048 | 0.048 | 0.051 | 0.061 | 0.061 | 0.065 | 0.074 | 0.073 | 0.078 | |
- Average at Time 0 sec= (0.048 + 0.048 + 0.051)/3= 0.049 nm
- Average at Time 30 sec= 0.0623 nm
- Average at Time 60 sec= 0.075 nm
- Change in absorbance (ΔA) from 0 to 30 sec/ mg tissue (assuming 50 mg/mL of tissue: buffer ratio)= [(0.0623-0.049)/(1.13 x 10-2)]/0.35= 3.363
- Change in absorbance (ΔA) from 30 to 60 sec/ mg tissue= 3.211
- *MPO activity (U/mg tissue)= average of ΔA(0-30) and ΔA(30-60)= 3.287
- Quantification of pro-inflammatory cytokines by ELISA
- Cytokine (IL-1β, IL-6 and TNF-α) levels are determined using commercially available enzyme-linked immunosorbent assay (ELISA) kit (Quantikine Murine; R&D Systems).
- Absorbance values from each ELISA is normalized using a Bradford protein assay respective to each sample and is expressed in units of pg/mg of protein.
7. Representative Results
Administration of the appropriate DSS regimen will induce acute colitis in mice. During the duration of the DSS treatment, DAI can be used to assess and evaluate the clinical progression of disease. Animals treated with DSS will show significant weight loss compared to their initial weights, loose stools and fecal bleeding (Figure 1). Upon sacrifice and examination of the colon, the severity of colitis is macroscopically scored based on shortening of colon length, colonic bleeding, fecal bleeding, loosening of stool consistency, and signs of rectal bleeding compared to controls treated with water only (Figure 2 & Table 1). Cross sections of colonic tissue samples stained with H&E will have higher histological scores for DSS-treated colons versus water-treated controls (Figure 3). To further characterize the extent of inflammation in DSS-treated mice, MPO activity can be assessed from homogenized colonic tissue samples. DSS-treated colons will have higher MPO activity compared to controls (Figure 4). In addition, this is associated with increased levels of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) (Figure 5).
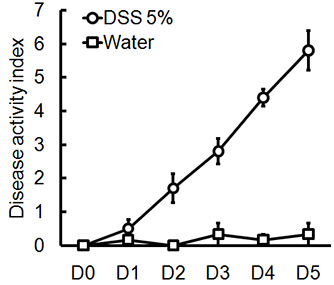
Figure 1. Male, C57BL/6 mice were given 5% DSS in drinking water for 5 days. DAI scores were assessed daily for each animal and were averaged per day for each group (mean ± SEM, n=4 mice/group).
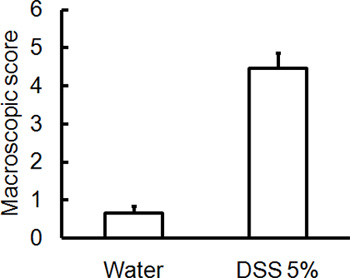
Figure 2. C57BL/6 mice were given 5% DSS in drinking water for 5 days. Control mice received water without DSS. Macroscopic damage score/disease severity scores were blindly assessed on day 5 post DSS-induced colitis. Colons isolated from mice that received DSS have higher macroscopic damage scores (rectal bleeding, rectal prolapse, diarrhea, colonic bleeding) indicating greater disease severity (mean ± SEM, n=4 mice/group).
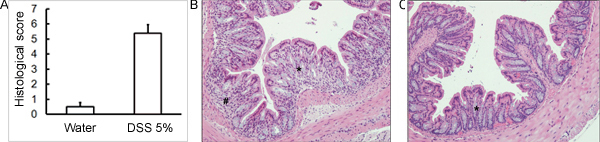
Figure 3. C57BL/6 mice were given 5% DSS solution in drinking water in order to induce colitis. Control mice received water without DSS. (A) Histological scores were blindly scored using H&E stained colonic tissue sections collected on day 5 post-DSS administration. (B) DSS-treated samples show more histological damage (more cellular infiltration, more goblet cell depletion, greater distortion/damage to crypt architecture) compared to (C) controls (mean ± SEM, n=4 mice/group). In (B) and (C), asterisk (*) indicates area of goblet cell depletion and distortion of crypt architecture; number sign (#) indicates cellular infiltration.
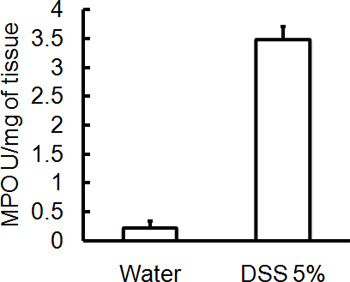
Figure 4. All mice were sacrificed on day 5 post administration of DSS and colonic tissue samples were collected to assess MPO activity. Severity of DSS induced colitis is associated with higher levels of MPO activity compared to controls (mean ± SEM, n=4 mice/group).
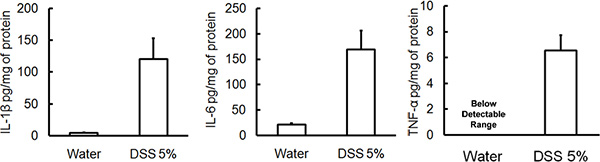
Figure 5. In addition to higher MPO levels, severity of DSS-induced colitis is also associated with an increased level of pro-inflammation cytokines such as IL-1β, IL-6, TNF-α (mean ± SEM, n=4 mice/group).
| Score | Rectal bleeding | Rectal Prolapse | Stool Consistency | Blood |
| 0 | None | None | Normal | Normal |
| 1 | Red | Signs of prolapse | Soft | Red |
| 2 | Dark Red | Clear prolapse | Very Soft | Dark red |
| 3 | Gross Bleeding | Extensive prolapse | Diarrhea | Black |
Table 1. Macroscopic/Disease Severity Score
Discussion
DSS colitis is a widely used chemically induced model of intestinal inflammation. In this model, mice are given drinking water supplemented with DSS, which is thought to be toxic to gut epithelial cells and disrupt the integrity of the mucosal barrier.10 Administration of DSS induces an acute colitis that is characterized by loose stool, fecal bleeding, and infiltration with granulocytes.10 During DSS administration, colitis is usually associated with significant weight loss and presence of blood in...
Disclosures
No conflicts of interest declared.
Acknowledgements
This work is supported by grants from Canadian Institutes of Health Research (CIHR) and by Crohn's and Colitis Foundation of Canada (CCFC).
Materials
| Name | Company | Catalog Number | Comments |
| Eppendorf Safe-Lock Microcentrifuge Tube (2mL) | Eppendorf | 0030 120.094 | |
| Biotek EL808 Absorbance plate reader | BioTek | EL808 | |
| Dextran sulphate sodium salt reagent grade (molecular weight 36,000-50,000 Da) | MP Biomedicals | 160110 | |
| Gen5 (software) | BioTek | Version 1.10.8 | |
| Hexadecyltrimethylammonium bromide (HTAB) | Sigma-Aldrich | H5882-100G | |
| Hydrogen peroxide, 30 wt.% solution in water | Sigma-Aldrich | 216763 | Store at 2-8°C |
| Microtest plate 96-well flat bottom | Sarstedt Ltd | 82.1581 | For single use only |
| o-Dianisidine | Sigma-Aldrich | D-3252 | Light sensitive. Store at 2-8°C |
| Potassium phosphate, dibasic | Caledon | 6620-1 | |
| Potassium phosphate, monobasic | EMD Millipore | PX1565-1 | |
| Protease inhibitor cocktail | Sigma-Aldrich | P8340 | Store at -20°C |
| Triton X-100 | Sigma-Aldrich | T8787 | |
| Tungsten Carbide beads for Tissue Lyser II | Qiagen | 69997 |
References
- Sands, B. E. From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology. 126, 1518-1532 (2004).
- Danese, S., Fiocchi, C. Etiopathogenesis of inflammatory bowel diseases. World J. Gastroenterol. 12, 4807-4812 (2006).
- Wirtz, S., Neufert, C., Weigmann, B., Neurath, M. F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2, 541-546 (2007).
- Axelsson, L. G., Landstrom, E., Goldschmidt, T. J., Gronberg, A., Bylund-Fellenius, A. C. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: effects in CD4(+)-cell depleted, athymic and NK-cell depleted SCID mice. Inflamm. Res. 45, 181-191 (1996).
- Egger, B., Bajaj-Elliott, M., MacDonald, T. T., Inglin, R., Eysselein, V. E., Büchler, M. W. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 62, 240-248 (2000).
- Krawisz, J. E., Sharon, P., Stenson, W. F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 87, 1344-1350 (1984).
- Cooper, H. S., Murthy, S. N., Shah, R. S., Sedergran, D. J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 69, 238-249 (1993).
- Smith, J. W., Castro, G. A. Relation of peroxidase activity in gut mucosa to inflammation. Am. J. Physiol. 234, R72-R79 (1978).
- Ghia, J. E., Blennerhassett, P., Kumar-Ondiveeran, H., Verdu, E. F., Collins, S. M. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 131, 1122-1130 (2006).
- Okayasu, I., Hatakeyama, S., Yamada, M., Ohkusa, T., Inagaki, Y., Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 98, 694-702 (1990).
- Solomon, L., Mansor, S., Mallon, P., Donnelly, E., Hoper, M., Loughrey, M., Kirk, S., Gardiner, K. The dextran sulphate sodium (DSS) model of colitis: an overview. Comparative Clinical Pathology. 19, 235-239 (2010).
- Mähler, M., Bristol, I. J., Leiter, E. H., Workman, A. E., Birkenmeier, E. H., Elson, C. O., Sundberg, J. P. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am. J. Physiol. 274, G544-G551 (1998).
- Hans, W., Scholmerich, J., Gross, V., Falk, W. The role of the resident intestinal flora in acute and chronic dextran sulfate sodium-induced colitis in mice. Eur. J. Gastroenterol. Hepatol. 12, 267-273 (2000).
- Rath, H. C., Schultz, M., Freitag, R., Dieleman, L. A., Li, F., Linde, H., Schölmerich, J., Sartor, R. B. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect. Immun. 69, 2277-2285 (2001).
- Ghia, J. E., Li, N., Wang, H., Collins, M., Deng, Y., El-Sharkawy, R. T., Côté, F., Mallet, J., Khan, W. I. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 137, 1649-1660 (2009).
- Elson, C. O., Beagley, K. W., Sharmanov, A. T., Fujihashi, K., Kiyono, H., Tennyson, G. S., Cong, Y., Black, C. A., Ridwan, B. W., McGhee, J. R. Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J. Immunol. 157, 2174-2185 (1996).
- Dieleman, L. A., Ridwan, B. U., Tennyson, G. S., Beagley, K. W., Bucy, R. P., Elson, C. O. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 107, 1643-1652 (1994).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved