A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Deriving the Time Course of Glutamate Clearance with a Deconvolution Analysis of Astrocytic Transporter Currents
In This Article
Summary
We describe an analytical method to estimate the lifetime of glutamate at astrocytic membranes from electrophysiological recordings of glutamate transporter currents in astrocytes.
Abstract
The highest density of glutamate transporters in the brain is found in astrocytes. Glutamate transporters couple the movement of glutamate across the membrane with the co-transport of 3 Na+ and 1 H+ and the counter-transport of 1 K+. The stoichiometric current generated by the transport process can be monitored with whole-cell patch-clamp recordings from astrocytes. The time course of the recorded current is shaped by the time course of the glutamate concentration profile to which astrocytes are exposed, the kinetics of glutamate transporters, and the passive electrotonic properties of astrocytic membranes. Here we describe the experimental and analytical methods that can be used to record glutamate transporter currents in astrocytes and isolate the time course of glutamate clearance from all other factors that shape the waveform of astrocytic transporter currents. The methods described here can be used to estimate the lifetime of flash-uncaged and synaptically-released glutamate at astrocytic membranes in any region of the central nervous system during health and disease.
Introduction
Astrocytes are one of the most abundant cell types in the brain with star-shaped morphology and fine membrane protrusions that extend throughout the neuropil and reach neighboring synaptic contacts 1,2. The astrocytes' cell membrane is densely packed with glutamate transporter molecules 3. Under physiological conditions, glutamate transporters rapidly bind glutamate at the extracellular side of the membrane and transfer it to the cell cytoplasm. By doing so, the transporters maintain low the basal concentration of glutamate in the extracellular space 4. Glutamate transporters in fine astrocytic processes adjacent to excitatory synapses are ideally positioned to bind glutamate released during synaptic events as it diffuses away from the synaptic cleft. By doing so, the transporters also limit glutamate spillover towards peri- and extra-synaptic regions and onto neighboring synapses, reducing the spatial spread of excitatory signals in the brain 5-7.
Glutamate transport is an electrogenic process stoichiometrically coupled to the movement of 3 Na+ and 1 H+ along their electrochemical gradient and to the counter-transport of 1 K+ 8. Glutamate transport is associated with (but not stoichiometrically coupled to) an anionic conductance permeable to SCN- (thiocyanate) > NO3- (nitrate) ≈ ClO4- (perchlorate) > I- > Br- > Cl- > F-, not to CH3SO3- (methane sulfonate) and C6H11O7- (gluconate) 9-11. Both currents (stoichiometric and non-stoichiometric) can be recorded by obtaining whole-cell patch-clamp recordings from astrocytes, visually identified under Dodt illumination or infra-red differential interference contrast (IR-DIC) in acute brain slices 12. The stoichiometric component of the current associated with glutamate transport across the membrane can be isolated by using CH3SO3-, or C6H11O7- based intracellular solutions and can be evoked by flash-uncaging glutamate on astrocytes 13,14, or by activating glutamate release from neighboring synapses, either electrically 12 or with a targeted optogenetic control.
The time course of the stoichiometric component of the transporter current is shaped by the lifetime of the glutamate concentration profile at astrocytic membranes (i.e. glutamate clearance), the kinetics of glutamate transporters, the passive membrane properties of astrocytes, and during synaptic stimulations, by the synchronicity of glutamate release across the activated synapses 13. Here we describe in full detail: (1) an experimental approach to isolate the stoichiometric component of glutamate transporter currents from whole-cell patch-clamp recordings from astrocytes using acute mouse hippocampal slices as an example experimental preparation; (2) an analytical approach to derive the time course of glutamate clearance from these recordings 13,14. These methods can be used to record and analyze glutamate transporter currents from astrocytes in any region of the central nervous system.
Protocol
1. Slice Preparation
- Prepare 500 ml slicing/storage solution containing (in mM): 119 NaCl, 2.5 KCl, 0.5 CaCl2, 1.3 MgSO4·7H2O, 4 MgCl2, 26.2 NaHCO3, 1 NaH2PO4, and 22 glucose, 320 mOsm, pH 7.4
- Use a 250 ml beaker to prepare a submersion chamber for the slices, fill it with 200 ml slicing/storage solution, warm it in a water bath at 34 °C and bubble it with 95% O2, 5% CO2.
- Keep the remaining slicing/storage solution in a glass bottle at 4 °C.
- Use a cyanoacrylate adhesive to attach a small agar block (6%, prepared in ACSF) on the vibratome sample holder and store it at 4 °C.
- 30 min before starting slicing, place the glass bottle containing the slicing/storage solution in a bucket filled with ice and bubble it with 95% O2, 5% CO2.
Note: speed and precision are paramount for the dissection steps described below.
- Anesthetize the mouse (P14-21, C57BL/6) with halothane/isoflurane (isoflurane has been reported to enhance astrocytic glutamate uptake15), decapitate it, and dip the head in a 50 ml beaker containing oxygenated, cold slicing/storage solution.
- Perform the dissection of the brain on an ice pack wrapped with paper towels and stored at -20 °C.
- Use a scalpel to make a mid-sagittal skin incision on the dorsal side of the head, from the frontal to the caudal end, and expose the skull.
- Place the lower shear blade of a small pair of surgical scissors in the occipital hole and make two cuts, towards the left and on the right hand side (45° angle).
- Cut the skull along the mid-sagittal line, from the caudal to the frontal end.
- Remove the brain with a spatula and dip it in oxygenated, cold slicing/storage solution.
- With a scalpel, make five cuts to: (1) remove the olfactory bulbs and the frontal cortex; (2) remove the cerebellum; (3) remove the left and (4) right temporal lobes; (5) separate the two brain hemispheres with a mid-sagittal cut.
- Dab the two parts of the brain with paper towels to remove any excess solution.
- Glue the lateral surface of each brain section to the cold vibratome base plate: the dorsal side of the brain should be facing the vibratome blade; the ventral side of the brain should be in contact with the agar block, away from the vibratome blade.
Note: to obtain the highest-quality slices, it is important that the two parts of the brain are firmly glued to the vibratome base plate. To do this, use a cyanoacrylate adhesive that is not too liquid and that does not dry out too quickly. - Secure the vibratome base plate to the dissecting chamber, set the slice thickness to 250 μm and adjust the width of the blade run. Proceed with slicing.
Note: if everything is oriented correctly, the vibratome should be cutting parasagittal slices from the dorsal towards the ventral and from the medial towards the lateral side of the brain. - Once the blade has passed through the cortex and hippocampus, use a scalpel to cut the cortex/hippocampus from the midbrain and place each slice in the submersion chamber at 34 °C.
- Discard the first couple of slices. Typically, 12 slices (250 μm thick) can be obtained from a P14-21 mouse brain.
- Keep the slices at 34 °C for 30 min and let them cool down to room temperature for 30 min before using them for the electrophysiology recordings.
2. Astrocyte Identification and Recordings
- Prepare an internal solution containing (in mM): 120 KCH3SO3, 10 EGTA, 20 HEPES, 2 MgATP, 0.2 NaGTP, 5 QX-314Br, and 5 NaCl, 290 mOsm, pH 7.2.
- Prepare an extracellular recording solution containing (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4·7H2O, 1 MgCl2, 26.2 NaHCO3, 1 NaHPO4, and 22 glucose, 300 mOsm, pH 7.4, saturated with 95% O2, 5% CO2.
- Add the following drugs to the extracellular recording solution, to block activation of GluA, GluN, mGluRII, mGluRIII, GABAA, GABAB, and A1 adenosine receptors (in μM): 10 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[ƒ]quinoxaline-7-sulfonamide disodium salt (NBQX), 10 (RS)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP), (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid disodium salt (LY341495), 100 (R,S)-α-methylserine-O-phosphate (MSOP), 100 picrotoxin, 5 3-[[(3,4-Dichlorophenyl)methyl]amino]propyl] diethoxymethyl)phosphinic acid (CGP52432), 1 8-cyclopentyl-1,3-dipropylxanthine (DPCPX).
- Set the temperature of the extracellular solution in the recording chamber; typical temperatures are between 34 - 36 °C.
- Prepare patch-clamp electrodes from borosilicate glass capillaries (R ≈ 2.5 MΩ) using a dual-stage, glass micro-pipette puller.
- Take one of the slices and place it in the recording chamber. Hold it down with a metal harp made with platinum wire and nylon strings.
- Visually inspect the slices under Dodt illumination or IR-DIC. Astrocytes can be identified by their small cell body (Ø = 10 μm) and prominent nucleus (Figure 1).
- For synaptic stimulations, place a bipolar stainless steel electrode, ~100 μm away from the astrocyte that you plan to patch.
- Patch the astrocyte and break in the whole-cell configuration by applying a very gentle suction.
Note: Astrocytes typically have low input resistance (~10 MΩ), hyperpolarized resting membrane potential (~-90 mV), and no firing activity. The astrocytes are kept at its resting membrane potential throughout the experiments in voltage-clamp mode (i.e. the holding current should read 0 pA). Before each stimulation, a 10 ms hyperpolarizing voltage step (-3 mV) is used to monitor the series and input resistance of the astrocyte. - Discard the recordings if the series resistance changes >20% or if the membrane potential of the astrocyte becomes depolarized. The membrane potential of the astrocyte can be directly measured by switching from voltage-clamp to current-clamp mode. Alternatively, while in voltage-clamp mode, the membrane potential can be monitored by reading the value of the holding potential that results in 0 pA holding current.
- If synaptic stimulations are employed, alternate single and paired stimuli (e.g. 100 msec apart), every 10 - 20 sec.
- For UV photolysis experiments, connect an uncaging Xe lamp to the epifluorescence port of the microscope and add the caged compound to the extracellular solution. Transporter currents of ~100 pA amplitude can be obtained when flash-uncaging 100 μM MNI-L-glutamate in the whole field of view (Ø = 662.5 μm when using a 40X objective 14). Alternate stimulations with the light path open and blocked, every 10 - 20 sec.
- Evoke glutamate transients and record transporter currents in control conditions and in the presence of a sub-saturating concentration of the broad-spectrum glutamate transporter antagonist D,L-threo-β-Benzyloxyaspartic acid (TBOA; 10 μM) to reduce the transporter current amplitude to at least 30% of its control value (see sections 4, 5) or in the presence of a high concentration of TBOA (50 - 100 μM) to block transporter currents completely and isolate the sustained K+-current (see section 3).
3. Pharmacological Isolation of the Sustained K+-current
- Record astrocytic currents in control conditions and in the presence of a high, saturating concentration of TBOA (50 - 100 μM).
- Average at least 20 sweeps in TBOA (50 - 100 μM).
- Fit the averaged traces obtained in 3.2 with the function:
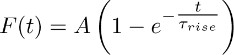
Note: the TBOA-insensitive component (i.e. the one that is recorded in the presence of TBOA (50 - 100 μM)) represents the sustained K+-current. Its time course is approximated by the mono-exponential function described above. - Repeat this fit across different astrocytes and derive an average value of Τrise (i.e. the average rise time of the sustained K+-current (see section 5)).
4. Isolation of the Facilitated Portion of Synaptically-Activated Transporter Currents (fSTCs)
- Average at least 20 sweeps obtained with paired stimulations, in control conditions and in TBOA (10 μM) (Figure 2a left).
- Average an identical number of sweeps obtained with single stimulations, in control conditions and in TBOA (10 μM) (Figure 2a middle).
- Compare the amplitude of the average current response to the -3 mV voltage step in the four averaged traces (control single pulse, control paired-pulses, TBOA single pulse, TBOA paired-pulses).
- If the amplitude of the current response is the same in all four traces, proceed to step 4.7.
- If the amplitude of the average current response is different in any of the four traces, check that all individual traces were appropriate to be included in the average.
- If the amplitude of the average current is different in any of the four traces, but all individual traces are appropriate to be included in the average, verify that in each trace the size of the fSTC scales linearly with the size of the current response to the test voltage step. If this is the case, scale all traces with respect to each other so that the current responses to the test voltage step are all equal in amplitude.
Note: In the recording conditions described here (i.e. intracellular CH3SO3- or C6H11O7-), synaptic stimulations of excitatory axons generate astrocytic currents with complex waveforms consisting of a fast-rising transient inward stoichiometric current and a slow-rising, sustained inward K+-current reflecting K+ re-equilibration in the extracellular space following action potential propagation along neighboring axons. It is critical to remove this sustained K+-current, as any residual current would lead to an overestimate of glutamate lifetime. - Subtract the average trace obtained with single stimulations from the average trace obtained with paired stimulations (Figure 2a right). This step allows isolating the stoichiometric and the sustained K+-current evoked by the second stimulus.
- Shift the average trace obtained with single stimulations by a time interval that matches the inter-pulse interval used to deliver paired-pulses (i.e. 100 msec) (Figure 2b) .
- Subtract the time-shifted average response obtained with single stimulations from the average response to the second stimulus obtained in 4.7 (Figure 2c). This step isolates the facilitated portion of the transporter current evoked by the second stimulus (the fSTC).
- In most cases, the previous step removes entirely the sustained K+-current. If this is the case, the isolation of the fSTC is complete and you can proceed with the deconvolution analysis and derive the time course of glutamate clearance from astrocytes. In some cases, however, a small sustained K+-current is still present (Figure 2d) and further analysis is required (see section 5).
Note: remember that the analysis described in 4.7-4.10 must be performed on the average traces obtained in control conditions and in TBOA (10 μM).
5. Subtraction of the Residual zdustained K+-current from fSTCs
- Measure the amplitude of the sustained K+-current remaining after performing the analysis described in section 4.
- Scale the amplitude of the mono-exponential function described in 3.3 to the amplitude of the residual sustained K+-current measured in 5.1 (Figure 2d left and middle). To do this, in equation 3.3, set the term A equals to the amplitude of the sustained K+-current measured in 5.1 and the term Τrise equals to the average value of Τrise estimated in 3.4.
- Subtract the resulting mono-exponential function from the fSTC and sustained K+-current obtained in 4.10 (Figure 2d right). This step completes the isolation of the fSTC.
6. Isolation of Flash-activated Transporter Currents (FTCs)
- Average at least 20 sweeps obtained with the light path open, in control conditions and in TBOA (10 μM).
- Average an identical number of sweeps obtained with the light path closed, in control conditions and in TBOA (10 μM).
- Compare the amplitude of the current response to the -3 mV voltage step in the four averaged traces (control single pulse, control paired-pulses, TBOA single pulse, TBOA paired-pulses).
- If the amplitude of the current response is the same in all four traces, proceed to step 6.7.
- If the amplitude of the average current response is different in any of the four traces, check that all individual traces were appropriate to be included in the average.
- If the amplitude of the average current is different in any of the four traces, but all individual traces are appropriate to be included in the average, verify that in each trace with the light path open the size of the FTC scales linearly with the size of the current response to the test voltage step. If this is the case, scale all traces with respect to each other so that the current responses to the test voltage step are all equal in amplitude.
Note: in the recording conditions described here (i.e. intracellular CH3SO3- or C6H11O7-), flash stimuli generate astrocytic currents consisting only of the fast-rising transient stoichiometric inward current. - Subtract the average trace obtained with the light path blocked from the average trace obtained with the light path open. This step allows removing the stimulus artifact and isolating the FTCs.
Note: the analysis described in 6.7 must be performed on the average traces obtained in control conditions and in TBOA (10 μM).
7. Deconvolution analysis
- Fit the transporter current (fSTC or FTC) recorded in control conditions (Figure 3a left) and in TBOA (10 μM) (Figure 3a right) and isolated as described in sections 4 - 6, with the multi-exponential function:

- Create an instantaneously rising function that decays mono-exponentially according to the function:
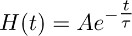
and that best describes the decaying phase of the transporter current recorded in TBOA (10 μM) (Figure 3b).
Note: the function described in 7.2 (i.e. H(t)) represents the time course of glutamate clearance from astrocytes in the presence of TBOA (10 μM). - Deconvolve the mono-exponential function obtained in 7.2 (Figure 3b and 3c middle) from the fit of the transporter current recorded in TBOA (10 μM) obtained in 7.1 (Figure 3a right, c left).
Note: deconvolution is a mathematical operation included in many analysis software packages like (e.g. Matlab, Python). In IgorPro, the programming code that we typically use to perform this kind of analysis, the deconvolution operation can be efficiently computed as described below, by using discrete fast Fourier transforms. First, use the FFT operation to compute the discrete fast Fourier transform of the mono-exponential function obtained in 7.2 and of the fit of the transporter current recorded in TBOA obtained in 7.1. Next, use the IFFT operation to compute the inverse discrete fast Fourier transform of the ratio of the two FFT functions. The resulting function I(t) can be described as follows:
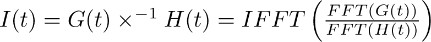
The step described in 7.3 allows deriving the filter of the transporter current in TBOA (10 μM) (Figure 3c right). The filter represents the distortion factors that convert the lifetime of glutamate at astrocytic membranes into the transporter current isolated in sections 4 - 6 (i.e. the fSTC or FTC). - Deconvolve the filter (Figure 3c right, Figure 3d middle) from the fit of the transporter current in control conditions (Figure 3a left, Figure 3d left).
Note: this step allows deriving the time course of glutamate clearance from astrocytes in control conditions (Figure 3d right). The assumption underlying this deconvolution step is that the temporal profile of the filter remains unaltered in control conditions and in TBOA (10 μM). - To obtain a quantitative estimate of the overall time course of glutamate clearance, calculate the centroid () of the waveform obtained in step 7.4. This is done by calculating as:
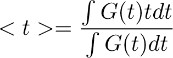
where G(t) is the waveform obtained in step 7.1. In the equation described above, the term t corresponds to the time window over which the integral is calculated.
Note: when the method was first established, was calculated over time windows that were left unconstrained 13 . This approach can be used as long as the integration window is set to be wider than the duration of glutamate clearance and the clearance waveform decays completely back to baseline. If this is not the case, however, this method of estimating encounters some limitations. For example, if the clearance waveform does not decay exactly back to baseline, then increases with the width of the integration window. To avoid any potential source of inaccuracy for the estimate of , we now calculate it over a time window corresponding to 10% of the peak of the transporter current, before and after its onset 14 . The latter approach improves the consistency with which is measured across cells. This comes in handy particularly when analyzing small effects of pharmacological treatments on the time course of clearance.
Results
The success of the analytical approach described here critically depends on obtaining high-quality electrophysiological recordings of transporter currents from astrocytes in any region of the central nervous system. In acute mouse hippocampal slices, astrocytes can be readily identified under Dodt illumination or IR-DIC because of their small cell body (Ø = 10 μm) and prominent nucleus (Figure 1). Their distinctive star-shaped morphology can be appreciated with epifluorescence, confocal, or two...
Discussion
Here we describe an experimental approach to obtain electrophysiological recordings from astrocytes, an analytical protocol to isolate glutamate transporter currents in astrocytes and a mathematical method to derive the time course of glutamate clearance from astrocytic transporter currents.
The success of the analysis relies on the ability to obtain high-quality patch clamp recordings from astrocytes and on the accuracy of the fitting algorithms used to describe the transporter currents. The ...
Disclosures
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program (NS002986). AS wrote the manuscript and implemented the deconvolution analysis. JSD developed the initial version of the deconvolution analysis and commented on the text.
Materials
| Name | Company | Catalog Number | Comments |
| CGP52432 | Tocris | 1246 | |
| (R,S)-CPP | Tocris | 173 | |
| DPCPX | Tocris | 439 | |
| LY341495 disodium salt | Tocris | 4062 | |
| MSOP | Tocris | 803 | |
| NBQX disodium salt | Tocris | 1044 | |
| D,L-TBOA | Tocis | 1223 | |
| Picrotoxin | Sigma | P1675 | |
| MNI-L-glutamate | Tocris | 1490 | |
| Alexa 594 | Life Technologies | A10438 | Optional |
| Matrix electrodes | Frederick Haer Company | MX21AES(JD3) | |
| Borosilicate glass capillaries | World Precision Instruments | PG10165-4 | |
| Dual-stage glass micro-pipette puller | Narishige | PC-10 | |
| Loctite 404 instant adhesive | Ted Pella | 46551 | |
| Xe lamp | Rapp OptoElectronic | FlashMic | |
| Igor Pro 6 | Wavemetrics |
References
- Ventura, R., Harris, K. M. Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 19, 6897-6906 (1999).
- Witcher, M. R., Kirov, S. A., Harris, K. M. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia. 55, 13-23 (2007).
- Danbolt, N. C. Glutamate uptake. Prog. Neurobiol. 65, 1-105 (2001).
- Herman, M. A., Jahr, C. E. Extracellular glutamate concentration in hippocampal slice. J. Neurosci. 27, 9736-9741 (2007).
- Arnth-Jensen, N., Jabaudon, D., Scanziani, M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat. Neurosci. 5, 325-331 (2002).
- Barbour, B. An evaluation of synapse independence. J. Neurosci. 21, 7969-7984 (2001).
- Rusakov, D. A., Kullmann, D. M. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J. Neurosci. 18, 3158-3170 (1998).
- Zerangue, N., Kavanaugh, M. P. Flux coupling in a neuronal glutamate transporter. Nature. 383, 634-637 (1038).
- Eliasof, S., Jahr, C. E. Retinal glial cell glutamate transporter is coupled to an anionic conductance. Proc. Natl. Acad. Sci. U.S.A. 93, 4153-4158 (1996).
- Wadiche, J. I., Amara, S. G., Kavanaugh, M. P. Ion fluxes associated with excitatory amino acid transport. Neuron. 15, 721-728 (1995).
- Wadiche, J. I., Kavanaugh, M. P. Macroscopic and microscopic properties of a cloned glutamate transporter/chloride channel. J. Neurosci. 18, 7650-7661 (1998).
- Bergles, D. E., Jahr, C. E. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 19, 1297-1308 (1997).
- Diamond, J. S. Deriving the glutamate clearance time course from transporter currents in CA1 hippocampal astrocytes: transmitter uptake gets faster during development. J. Neurosci. 25, 2906-2916 (2005).
- Scimemi, A., Tian, H. Neuronal transporters regulate glutamate clearance, NMDA receptor activation, and synaptic plasticity in the hippocampus. J. Neurosci. 29, 14581-14595 (2009).
- Zuo, Z. Isoflurane enhances glutamate uptake via glutamate transporters in rat glial cells. Neuroreport. 12, 1077-1080 (2001).
- Barbour, B., Brew, H., Attwell, D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander (Ambystoma) retina. J. Physiol. 436, 169-193 (1991).
- Bergles, D. E., Tzingounis, A. V., Jahr, C. E. Comparison of coupled and uncoupled currents during glutamate uptake by GLT-1 transporters. J. Neurosci. 22, 10153-10162 (2002).
- Diamond, J. S., Jahr, C. E. Synaptically released glutamate does not overwhelm transporters on hippocampal astrocytes during high-frequency stimulation. J. Neurophysiol. 83, 2835-2843 (2000).
- Benediktsson, A. M., et al. Neuronal activity regulates glutamate transporter dynamics in developing astrocytes. Glia. 60, 175-188 (2012).
- Hires, S. A., Zhu, Y., Tsien, R. Y. Optical measurement of synaptic glutamate spillover and reuptake by linker optimized glutamate-sensitive fluorescent reporters. Proc. Natl. Acad. Sci. U.S.A. 105, 4411-4416 (2008).
- Scimemi, A., Meabon, J., Woltjer, R. L., Sullivan, J. M., Diamond, J. S., Cook, D. G. Amyloidβ1-42 slows clearance of synaptically-released glutamate by mislocalizing astrocytic GLT-1. J. Neurosci. 33, 5312-5318 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved