A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Synthesis of Antiviral Tetrahydrocarbazole Derivatives by Photochemical and Acid-catalyzed C-H Functionalization via Intermediate Peroxides (CHIPS)
In This Article
Summary
A two-step procedure for the synthesis of pharmaceutically active indole-derivatives by C-H functionalization with anilines is described, using photo- and Brønsted acid catalysis.
Abstract
The direct functionalization of C-H bonds is an important and long standing goal in organic chemistry. Such transformations can be very powerful in order to streamline synthesis by saving steps, time and material compared to conventional methods that require the introduction and removal of activating or directing groups. Therefore, the functionalization of C-H bonds is also attractive for green chemistry. Under oxidative conditions, two C-H bonds or one C-H and one heteroatom-H bond can be transformed to C-C and C-heteroatom bonds, respectively. Often these oxidative coupling reactions require synthetic oxidants, expensive catalysts or high temperatures. Here, we describe a two-step procedure to functionalize indole derivatives, more specifically tetrahydrocarbazoles, by C-H amination using only elemental oxygen as oxidant. The reaction uses the principle of C-H functionalization via Intermediate PeroxideS (CHIPS). In the first step, a hydroperoxide is generated oxidatively using visible light, a photosensitizer and elemental oxygen. In the second step, the N-nucleophile, an aniline, is introduced by Brønsted-acid catalyzed activation of the hydroperoxide leaving group. The products of the first and second step often precipitate and can be conveniently filtered off. The synthesis of a biologically active compound is shown.
Introduction
The direct functionalization of C-H bonds is an important and long standing goal in organic chemistry1. Such transformations can be very powerful in order to streamline synthesis by saving steps, time and material compared to conventional methods that require the introduction and removal of activating or directing groups. Therefore, the functionalization of C-H bonds is also attractive for green chemistry2. Under oxidative conditions, two C-H bonds or one C-H and one heteroatom-H bond can be transformed to C-C and C-heteroatom bonds, respectively (Figure 1)3-9. Often these oxidative coupling reactions require synthetic oxidants, expensive catalysts or high temperatures. Therefore, many attempts are made to develop methods that use cheap catalysts, benign conditions and oxygen or air as terminal oxidant10.
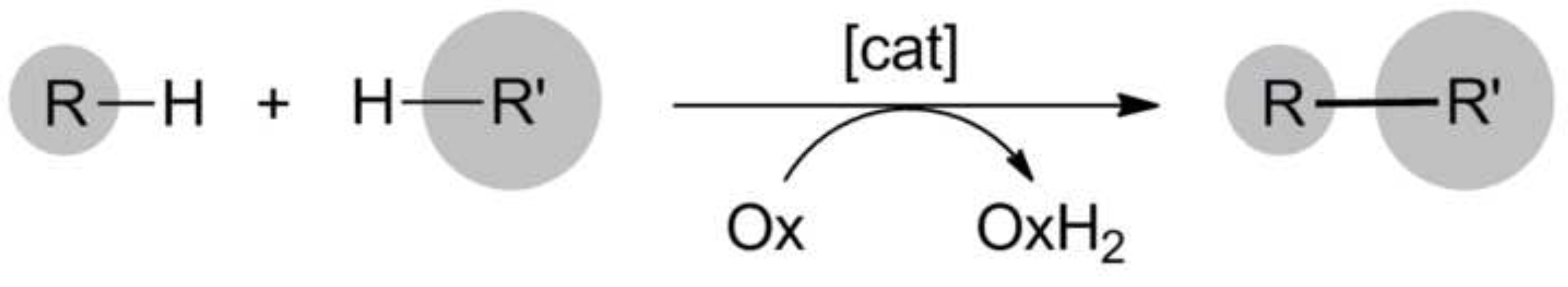
Figure 1. Oxidative coupling reactions. Please click here to view a larger version of this figure.
Many organic compounds react slowly with oxygen from air in autoxidation reactions which can functionalize C-H bonds by effectively inserting O2, forming a hydroperoxide moiety11,12. Autoxidation processes are used on industrial scale to generated oxygenated compounds from hydrocarbon feedstocks, but autoxidation is also an unwanted process if it leads to decomposition of valuable compounds or materials. In some cases, for example diethyl ether, hydroperoxides formed in air can also be explosive. Recently, we discovered a reaction that utilizes an autoxidation to form a new C-C bond from C-H bonds without need of a redox-active catalyst13,14. Simply stirring the substrates under oxygen in the presence of an acid catalyst leads to the formation of the new products. Key to the reaction is the facile formation of intermediate hydroperoxides, which are substituted with the second substrate by acid catalysis15. The reaction, however, is restricted to xanthene and a few related compounds that are easily oxidized under an atmosphere of oxygen and the products have so far not found applications. Nevertheless inspired by this discovery, we developed a related oxidative coupling method that utilizes the principle of C-H functionalization via Intermediate PeroxideS (CHIPS) to synthesize pharmaceutically active indole derivatives16.
Indoles, especially tetrahydrocarbazoles 1, can be easily oxidized to hydroperoxides 2 in the presence of singlet oxygen17-19, which can be generated using a sensitizer and visible light20. A hydroperoxide moiety can in principle act as a leaving group if activated by acid catalysis and allow for the introduction of a nucleophile21,22. Hydroperoxides are also known to undergo acid catalyzed rearrangement reactions as utilized in the industrial synthesis of phenol from cumene, the Hock process23. By careful optimization studies, we could find conditions to favor the desired substitution reaction with N-nucleophiles like anilines 3 over the unwanted decomposition pathways by rearrangement16. Here, we describe this two-step CHIPS procedure in detail, using only visible light, a sensitizer, oxygen and acid. Amongst the selected products are indole derivatives 4, which show high antiviral activity or inhibit the vascular endothelial growth factor (VGF), which can be important for tumor therapy24-26.
Protocol
1. Synthesis of Tetrahydrocarbazole Hydroperoxides
- The formation of the hydroperoxide is slowed down if the tetrahydrocarbazole is very colored. In this case, purify it by recrystallization using toluene/pentane or by column chromatography to get a colorless starting material. For purification by column chromatography, pack a column with a lower layer of silica gel and an upper layer of alumina. Put the tetrahydrocarbazole on top of the column and elute with toluene. All the unwanted yellow and black colored byproducts are adsorbed on the column and colorless tetrahydrocarbazole is eluting. Immediately evaporate the solvent and store the purified white product under an atmosphere of argon in the dark.
- Weigh out 1 g of tetrahydrocarbazole or of a substituted tetrahydrocarbazole (1, synthesized according to reported methods16) into a 250 ml flask. Add 100 ml toluene to this flask.
- Weigh out Rose Bengal (2 mg) and add it into the above reaction mixture.
- Add a stir bar and cover the flask with septa.
- Add an oxygen balloon through the septum; this keeps a positive pressure of oxygen atmosphere on the reaction.
- Irradiate the reaction mixture with a 23 watt lamp.
- Check the progress of the reaction by thin-layer chromatography (TLC, using a mixture of hexane/ethyl acetate in 70:30 ratio; the Rf value of the hydroperoxides described herein is between 0.2 and 0.3) or by 1H NMR from a sample taken (evaporate the solvent on a rotary evaporator and dissolve the residue in DMSO-d6). Reaction times can vary depending on the light source and the purity of the starting material, as mentioned in part 1.1. Generally, full conversion of tetrahydrocarbazoles 1 takes 3 hr.
- Filter the precipitated solid after full conversion of starting material. Washing of the solid can be done with pentane in order to remove most of the toluene, but is not necessary for purification.
- Dry the isolated solid under reduced pressure.
CAUTION: Although we never experienced any problem in working with or handling the compounds described in this work, precautions should be taken when working with peroxides. In particular, it should be avoided as much as possible to expose neat peroxides to heat or to mix them with metals or metal salts. Performing such reactions behind a blast shield is recommended.
2. Coupling Reaction – Method A Using 10 mol% Trifluoroacetic Acid in Methanol
- Weigh out the hydroperoxide (0.49 mmol, 1.0 equiv. from step 1) and the desired aniline nucleophile (0.49 mmol, 1.0 equiv.) into a 12 ml vial or a suitable round bottom flask.
- Add 10 ml MeOH and subsequently 3.74 µl trifluoroacetic acid (TFA, 0.049 mmol, 0.1 equiv.) to the vial or round bottom flask.
- Close the container with a cap and stir the reaction mixture at room temperature for 4 hr.
Workup variant A1 (for products which precipitate over the course of the reaction): - Filter the precipitated solid to get the desired product. Wash the product with methanol (3 x 0.5 ml).
- To obtain a second fraction of product, evaporate the methanol from the filtrate. Dissolve the crude product in 5 ml of ethyl acetate at 40 °C, then cool to room temperature and add 3-5 ml of pentane The pure product precipitates.
- Combine the different fractions of the product and dry them under high vacuum.
Workup variant A2 (for products which do not precipitate): - Evaporate the solvent directly after the reaction by using a rotary evaporator and purify the residue by column chromatography as specified (silica gel, hexane / ethyl acetate / triethylamine) to obtain the desired product.
3. Coupling Reaction – Method B Using Acetic Acid
- Weigh out the hydroperoxide (0.49 mmol, 1.0 equiv. from step 1) and the desired aniline nucleophile (0.49 mmol, 1.0 equiv.) into a 12 ml vial or a suitable round bottom flask.
- Add 10 ml acetic acid (AcOH) to the to the vial or round bottom flask.
- Close the container with a cap and stir the reaction mixture at room temperature for 4 hr.
Workup variant B1 (for products which precipitate over the course of the reaction): - Filter the precipitated solid to get the desired product. Wash the product with AcOH (3 x 0.5 ml).
- To obtain a second fraction of product, evaporate the acetic acid from the filtrate. Dissolve the crude product in 5 ml of ethyl acetate at 40 °C, then cool to room temperature and add 3-5 ml of pentane The pure product precipitates.
- Combine the different fractions of the product and dry them under high vacuum.
Workup variant B2 (for products which do not precipitate): - Evaporate the solvent directly after the reaction by using a rotary evaporator and purify the residue by column chromatography as specified (silica gel, hexane / ethyl acetate / triethylamine) to obtain the desired product.
Results
Synthesis of 1-(5-nitroindolin-1-yl)-2,3,4,9-tetrahydro-1H-carbazole (4a):
Synthesized according to Method A, Rf = 0.63 (hexane/ethyl acetate 70:30).
Purification: Purify the product by using Method A, workup variant A1 (steps 2.4, 2.5, 2.6). Orange solid, Yield: 95%.
1H NMR (500 MHz, DMSO-d6): δ 10.90 (s, 1H), 7.97 (dd...
Discussion
In summary, we could demonstrate that a C-H bond in tetrahydrocarbazoles can be conveniently functionalized to generate C-N-coupling products in a two-step procedure.
The first step is a well-known photocatalyzed oxidation of tetrahydrocarbazole (1) or its derivatives with elemental oxygen17,19, giving a hydroperoxide 2. If performed in toluene, the hydroperoxide products precipitate and can be conveniently isolated by filtration. Further purificati...
Disclosures
The authors have nothing to disclose.
Acknowledgements
Financial support from the DFG (Heisenberg scholarship to M.K., KL 2221/4-1; KL 2221/3-1) and the Max-Planck-Institut fuer Kohlenforschung is gratefully acknowledged.
Materials
| Name | Company | Catalog Number | Comments |
| 1,2,3,4-Tetrahydrocarbazole | Sigma Aldrich | T12408 | If coloured, purification may be necessary. See Protocol 1.1 |
| Methanol | Sigma Aldrich | 322415 | 99.8% purity |
| 4-Nitroaniline | Acros Organics | 128371000 | 99% purity |
| Trifluoroacetic acid | Sigma Aldrich | T6508 | 99% purity |
| Acetic acid | J. T. Baker | JTB RS 426960101 | 99-100% purity |
| Aniline | Merck | 8222560100 | |
| 4-Aminobenzonitrile | Sigma Aldrich | 147753 | 98% purity |
References
- Bergman, R. G. Organometallic chemistry - C-H activation. Nature. 446, 391-393 (2007).
- Anastas, P., Green Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 39, 301-312 (2010).
- Yeung, C. S., Dong, V. M. Catalytic Dehydrogenative Cross-Coupling: Forming Carbon−Carbon Bonds by Oxidizing Two Carbon−Hydrogen Bonds. Chem. Rev. 111, 1215-1292 (2011).
- Liu, C., Zhang, H., Shi, W., Lei, A. Bond Formations between Two Nucleophiles: Transition Metal Catalyzed Oxidative Cross-Coupling Reactions. Chem. Rev. 111, 1780-1824 (2011).
- Klussmann, M., Sureshkumar, D. Catalytic Oxidative Coupling Reactions for the Formation of C–C Bonds Without Carbon-Metal Intermediates. Synthesis. 3, 353-369 (2011).
- Yoo, W. -. J., Li, C. -. J. Cross-Dehydrogenative Coupling Reactions of sp3-Hybridized C–H Bonds. Top. Curr. Chem. 292, 281-302 (2010).
- Dick, A. R., Sanford, M. S. Transition metal catalyzed oxidative functionalization of carbon-hydrogen bonds. Tetrahedron. 62, 2439-2463 (2006).
- Collet, F., Dodd, R. H., Dauban, P. Catalytic C–H amination: recent progress and future directions. Chem. Commun. 34, 5061-5064 (2009).
- Rohlmann, R., Mancheño, O. G. Metal-Free Oxidative C(sp3)-H Bond Couplings as Valuable Synthetic Tools for C-C Bond Formations. Synlett. 24, 6-10 (2013).
- Wendlandt, A. E., Suess, A. M., Stahl, S. S. Copper-Catalyzed Aerobic Oxidative C-H Functionalizations: Trends and Mechanistic Insights. Angew. Chem. Int. Ed. 50, 11062-11087 (2011).
- Hermans, I., Peeters, J., Jacobs, P. A. Autoxidation of Hydrocarbons: From Chemistry to Catalysis. Top. Catal. 50, 124-132 (2008).
- Milas, N. A. Auto-oxidation. Chem. Rev. 10, 295-364 (1932).
- Pintér, &. #. 1. 9. 3. ;., Sud, A., Sureshkumar, D., Klussmann, M. Autoxidative Carbon-Carbon Bond Formation from Carbon-Hydrogen Bonds. Angew. Chem. Int. Ed. 49, 5004-5007 (2010).
- Pintér, &. #. 1. 9. 3. ;., Klussmann, M. Sulfonic Acid Catalyzed Autoxidative Carbon-Carbon Coupling Reaction under Elevated Partial Pressure of Oxygen. Adv. Synth. Catal. 354, 701-711 (2012).
- Schweitzer-Chaput, B., et al. Synergistic Effect of Ketone and Hydroperoxide in Brønsted Acid Catalyzed Oxidative Coupling Reactions. Angew. Chem. Int. Ed. 52, 13228-13232 (2013).
- Gulzar, N., Klussmann, M. Aerobic C-H Amination of Tetrahydrocarbazole Derivatives via Photochemically Generated Hydroperoxides. Org. Biomol. Chem. 11, 4516-4520 (2013).
- Beer, R. J. S., McGrath, L., Robertson, A., Woodier, A. B. Tetrahydrocarbazole Peroxides. Nature. 164, 362-363 (1949).
- Iesce, M. R., Cermola, F., Temussi, F. . Photooxygenation of Heterocycles. Curr. Org. Chem. 9, 109-139 (2005).
- Mateo, C. A., Urrutia, A., Rodríguez, J. G., Fonseca, I., Cano, F. H. Photooxygenation of 1,2,3,4-Tetrahydrocarbazole: Synthesis of Spiro[cyclopentane-1,2'-indolin-3'-one]. J. Org. Chem. 61, 810-812 (1996).
- Wasserman, H. H., Ives, J. L. Singlet oxygen in organic synthesis. Tetrahedron. 37, 1825-1852 (1981).
- Liguori, L., et al. Electrophilic Aromatic Alkylation by Hydroperoxides. Competition between Ionic and Radical Mechanisms with Phenols. J. Org. Chem. 64, 8812-8815 (1999).
- Dussault, P. H., Lee, H. -. J., Liu, X. Selectivity in Lewis acid-mediated fragmentations of peroxides and ozonides: application to the synthesis of alkenes, homoallyl ethers, and 1,2-dioxolanes. J. Chem. Soc., Perkin Trans. , 3006-3013 (2000).
- Hock, H., Lang, S. Autoxydation von Kohlenwasserstoffen IX. Mitteil.: Über Peroxyde von Benzol-Derivaten. Ber. 77, 257-264 (1944).
- Boggs, S. D., Gudmundsson, K. S., Richardson, L. D. A., Sebahar, P. R. Tetrahydrocarbazole derivatives and their pharmaceutical use. USA patent WO. 2004/110999 A1. , (2004).
- Gudmundsson, K. S. HCV Inhibitors. USA patent WO 2006/ 121467 A2. , (2006).
- Lennox, W. J., Qi, H., Lee, D. -. H., Choi, S., Moon, Y. -. C. Tetrahydrocarbazoles as active agents for inhibiting VEGF production by translational control. USA patent WO 2006/ 065480 A2. , (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved