A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Production and Use of Lentivirus to Selectively Transduce Primary Oligodendrocyte Precursor Cells for In Vitro Myelination Assays
In This Article
Summary
Here we present protocols that offer a flexible and strategic foundation for virally manipulating oligodendrocyte precursor cells to overexpress proteins of interest in order to specifically interrogate their role in oligodendrocytes via the in vitro model of central nervous system myelination.
Abstract
Myelination is a complex process that involves both neurons and the myelin forming glial cells, oligodendrocytes in the central nervous system (CNS) and Schwann cells in the peripheral nervous system (PNS). We use an in vitro myelination assay, an established model for studying CNS myelination in vitro. To do this, oligodendrocyte precursor cells (OPCs) are added to the purified primary rodent dorsal root ganglion (DRG) neurons to form myelinating co-cultures. In order to specifically interrogate the roles that particular proteins expressed by oligodendrocytes exert upon myelination we have developed protocols that selectively transduce OPCs using the lentivirus overexpressing wild type, constitutively active or dominant negative proteins before being seeded onto the DRG neurons. This allows us to specifically interrogate the roles of these oligodendroglial proteins in regulating myelination. The protocols can also be applied in the study of other cell types, thus providing an approach that allows selective manipulation of proteins expressed by a desired cell type, such as oligodendrocytes for the targeted study of signaling and compensation mechanisms. In conclusion, combining the in vitro myelination assay with lentiviral infected OPCs provides a strategic tool for the analysis of molecular mechanisms involved in myelination.
Introduction
Myelination of axons is crucial for the fast and efficient transmission of action potentials in both the central and peripheral nervous systems. Specialized cells, Schwann cells in the peripheral nervous system and oligodendrocytes in the central nervous system, wrap around and ensheathe axons in myelin, effectively insulating the nerve and facilitating saltatory conduction1. The process of myelination can be studied in vitro using retinal ganglion neurons2, engineered nanofibers3, or dorsal root ganglion neurons co-cultured with either Schwann cells4 or oligodendrocytes5-7. The in vitro myelination assay is an established model for studying nervous system myelination and it replicates many of the fundamental processes that occur during myelination in vivo5-8. The assay involves the coculture of purified populations of Dorsal Root Ganglion (DRG) neurons, with OPCs (for CNS myelination) or Schwann cells (for PNS myelination). Under specific conditions these myelinating cells ensheathe DRG axons in the ordered, ultra structurally verified, multi-lamellar sheet of insulating plasma membrane that express the same complement of myelin specific proteins present in vivo.
The most commonly used cell model of studying CNS myelination in vitro is the co-cultures of DRG neurons and OPCs, which have been successfully used to study the effect that exogenous factors such as the neurotrophins exert on CNS myelination in vitro5,6. Exogenous factors such as growth factors or small molecule pharmacological inhibitors have been widely used to study the role of signaling pathways in myelination using the DRG-OPC coculture model7,9. However, in the mixed co-culture settings that contain both the neurons and oligodendrocytes, it remained formally possible that either the growth factors or the pharmacological inhibitors could have exerted effects upon both the DRG neurons and oligodendrocytes (OL). This does offer the ability to specifically dissect the roles that the proteins expressed only by DRGs or oligodendroglia exerts upon myelination using this dual cell system. To unequivocally confirm that the signaling pathway in oligodendroglial directly regulates myelination, lentiviral transduction of OPCs, prior to seeding onto DRG neurons for the in vitro myelination assay, has proven to be an elegant way to overexpress both wild-type and mutant proteins, as well as knockdown expression of constitutively expressed proteins by oligodendrocytes. Thus this approach offers an avenue to specifically interrogate and manipulate signaling pathways within oligodendrocytes for studying myelination9,10.
In this paper, we report methods that we have developed to overexpress a protein of interest selectively in oligodendrocytes via a lentiviral approach for studying myelination in vitro. The technique begins with the generation of expression vectors containing the gene of interest, be it in a wild type, constitutively active or dominant negative form which are then subsequently cloned into the pENTR vector (pENTR L1-L2 pENTR4IRES2GFP). This vector (containing the gene of interest), the CMV promotor donor (pENTR L4-R1 pENTR-pDNOR-CMV) and the 2K7 lentivector are combined in an enzyme reaction to produce a 2K7 vector containing CMV promoter, the gene of interest, an internal ribosomal entry site and GFP (Figure 1). This Gateway cloned 2K7 construct combined with the PMD2.G virus envelope and the pBR8.91 virus package can be co-transfected into HEK293T cells to generate lentivirus that can subsequently be used to transduce OPCs. Once infected with the lentivirus the OPCs express a high level of the protein of interest. These OPCs can then be seeded onto DRG neuron cultures and the effect that expression of high levels of the desired protein exerts on myelination can be interrogated. The co-cultures are assessed for myelin protein expression by western blot analysis and visualized for the formation of myelinated axonal segments by immunocytochemistry.
Protocol
NOTE: All animals used for this study were of mixed sex and bred at the Animal Facilities of the Department of Anatomy & Pathology and The Florey Institute of Neuroscience and Mental Health Research at the University of Melbourne. All animal procedures were approved by Animal Experimentation Ethics Committees at the University of Melbourne.
1. Cloning of 2K7 Lentivector
- Before cloning the gene of interest into the 2K7 lentiviral vector, subclone the gene into the pENTR vector (3637 bp, Kanamycin resistant) using standard molecular techniques11. Use the EcoRI and SacII restriction sites for the subcloning.
- Amplification of the 2K7 Lentivector
- Transform 2K7 lentivector DNA by gently mixing 100 ng of plasmid DNA with competent cells and incubate on ice for 30 min.
- Heat shock DNA/ competent cells mix at 42 °C for 90 sec and plate them on LB-Agar plates containing both ampicillin (100 µg/ml) and chloramphenicol (15 µg/ml) at 37 °C for 16-18 hr.
NOTE: Chloramphenicol is used to prevent recombination between the long terminal repeats. - The next day, grow selected bacterial clones in LB media containing both ampicillin (100 µg/ml) and chloramphenicol (15 µg/ml) at 37 °C for 16-18 hr. Extract the DNA using a commercial plasmid DNA Maxiprep kit as per manufacturer’s instructions.
- Sub-cloning from pENTR vector to 2K7 Lentivector (Figure 1)
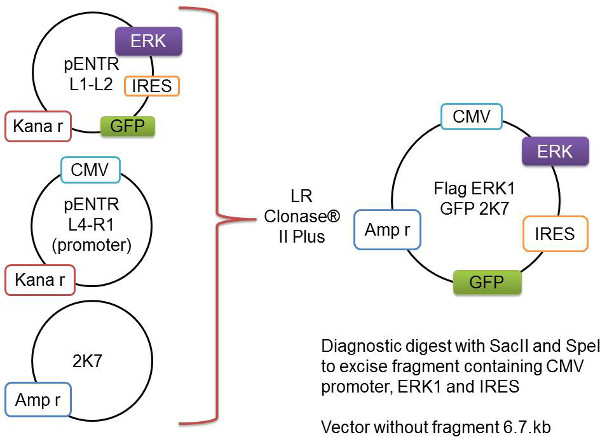
Figure 1: Schematic representation of the Gateway recombination process. The gene of interest, here represented as Flag-Erk1, is cloned into the pENTR L1-L2 vector. This is added to the pENTR L4-R1 vector containing the CMV promoter and the backbone 2K7 vector. These three vectors are recombined by the LR Clonase II Plus enzyme to insert the gene-of-interest and the promoter into the virus-ready 2K7 vector.- Add the following to a 1.5 ml tube and mix gently:
L4-R1 pENTR-pDNOR-CMV (promoter) (60 bng)1-2.5 µl
L1-L2 pENTR4IRES2GFP (with gene of interest) (80 bng)1-2.5 µl
K7 lentivector (80 ng/µl)1 µl
TE-buffer, pH 82-5 µl
Total 8 µl - Remove clonase enzyme mix from -80 °C and thaw on ice for 2 min. Ensure that this enzyme is a fresh aliquot from -80 °C as the enzyme has substantially reduced cloning efficiency with repeated freeze-thaw cycles
- Add 2 µl of enzyme per reaction and mix well via vortex, and incubate the clonase reaction mixture at 23-25 °C for 6-24 hr.
- Add 1 µl of proteinase K solution (supplied with enzyme kit) to the clonase reaction mixture and incubate for 10 min at 37 °C.
- Transform clonase reaction mixture into competent cells and grow the select colonies in LB media containing ampicillin (100 µg/ml) at 37 °C for 16-18 hr.
- Extract and purify DNA using a commercial plasmid DNA Miniprep kit as per manufacturer’s instructions.
- Confirm the DNA via digestion using restriction enzymes Spe I and Sac II and an appropriate buffer as per manufacturer’s instruction to remove the fragment containing the promoter and gene of interest.
NOTE: This step is critical to check if the subcloned DNA has the correct insert gene of interest with the right vector backbone. The size of the vector itself without inserted fragment is 6.7 kb. The size of the released inserted fragment includes both the promoter and the insert gene of interest. Calculate the expected fragment sizes from the digest for the specific gene via a DNA sequence editing program, e.g., APE – A Plasmid Editor. - Check the sizes of both DNA vector backbone and the released fragment by running a 1% agarose gel in 1x TAE buffer (see Table of stock solutions) at 100 V.
- Amplify the confirmed DNA by growing bacteria in 500 ml of LB media containing ampicillin (100 µg/ml) at 37 °C for 16-18 hr.
- Extract and purify DNA using a commercial plasmid DNA Maxiprep kit as per manufacturer’s instructions.
NOTE: Maxiprep typically generates sufficient amount of DNA required for viral production.
- Add the following to a 1.5 ml tube and mix gently:
- Repeat steps 1.3.9-1.3.10 to amplify the following DNAs for lentiviral preparations: Envelope vector (PMD2.G, 6.1 kb), Package vector (pBR8.91, 12.5 kb), and an empty lentiviral vector as a control (GFP-CMV-2K7, 8.7 kb).
2. 2K7 Virus Production
NOTE: Day 1:
- On the day of transfection, plate 32 million HEK293 T cells in T175 flask containing 25 ml HEK293 T cell media (see the Table of stock solutions). Alternatively, plate 16 million cells the day before the transfection if time runs tight on the day of transfection.
NOTE: Transfection can be equally successful by plating cells on the day of transfection or prior to the day. Using either of the two alternatives, the critical point here is to make sure cells be stuck down on culture dish surface prior to transfection.
NOTE: Day 2: - Transfection
- Prior to transfection, dilute DNA to 1 µg/µl in TE Buffer that contain 10 mM Tris pH 8, 1 mM EDTA pH 8 in deionized water.
- In a 50 ml tube, prepare a master mix (Table 1) for transfection in the T175 flask. Add DNA to pre-warmed Dulbecco’s Modified Eagle Medium (DMEM) and mix well by vortex, then add sterile polyethylenimine (PEI) (see the Table of stock solutions) to avoid premature precipitation.
Table 1: Preparing transfection mix for 2K7 Virus.Vector Concentration Volume pMDG.2 1 µg/µl 5 µl pBR8.91 1 µg/µl 15 µl 2K7 vector with GFP + gene of interest 1 µg/µl 22 µl Sterile Polyethylenimine (PEI) 1 g/L 500 µl DMEM 2,100 µl - Mix well by inverting the tube 3-4x and incubate for 15 min at room temperature (RT).
- Perform a full media change on HEK293T cells. Aspirate off the culture media from cells completely and feed with pre-warmed HEK293T cell culture media (25 ml per T175 flask).
- Add the transfection mixture (DNA/PEI mixture) drop wise to the monolayer cells. Move gently to mix well and incubate transfected cells at 37 °C, 5% CO2, overnight.
NOTE: Day 3: - To ensure the transfection is successful, check GFP expression 24 hr post-transfection via a fluorescent microscopy.
NOTE: Over 50% of cells expressing GFP typically indicates a good transfection.
NOTE: Day 4:
- At the 48 hr post-transfection, collect the viral supernatant and replace with fresh HEK293 T media (25 ml per T175 flask).
- Centrifuge the viral supernatant at 1,140 x g for 10 min at 4 °C to clear up cell debris from the supernatant. Transfer cleared supernatant to 50 ml tube and store at 4 °C.
NOTE: Day 5:
- Centrifuge the viral supernatant at 1,140 x g for 10 min at 4 °C to clear up cell debris from the supernatant. Transfer cleared supernatant to 50 ml tube and store at 4 °C.
- At 72 hr post-transfection, collect the second batch of viral supernatant. Repeat step 2.3.1 and pool 48 and 72 hr cleared supernatants.
- To concentrate the virus, centrifuge viral supernatant at 170,000 x g for 90 min at 4 °C using 30 ml ultracentrifuge tubes.
- Discard the supernatant and repeat step 2.6 until all the cleared supernatant has been centrifuged leaving an (invisible) pellet of virus plus precipitated PEI in the base of the tube.
- To resuspend virus, add 500 µl SATO media (see Table of stock solutions) to the ultracentrifuge tubes. Vortex for 30 sec and scrape the base of the tube with a pipette tip to mechanically loosen the virus. Repeat this step 6x in order to loose viral pellet.
- Pool the resuspended virus into microcentrifuge tubes and spin very briefly to remove insoluble PEI. Filter the supernatant through a 0.45 µm filter to remove proteins.
- Aliquot the virus into 20 µl, 50 µl, and 100 µl aliquots and store at -80 °C.
3. Viral Titer Determination in HEK293T Cells
- To check the expression of protein of interest and to determine the optimal viral concentration for experiments, add a series of serial dilutions of viral stock (e.g., 0, 5, 10, 20, 40, 80 µl) to transduce HEK293 T cells plated in 6-well plates, and culture for 24 hr at 37 °C, 5% CO2. This protocol typically generates virus that can be used at concentrations ranging from 1:50 to 1:200 that achieve robust expression of the gene of interest.
- 48 hr post viral transduction, lyse HEK293T cells in TNE buffer (see Table of stock solution) with protease inhibitors.
- Rinse wells twice with chilled DPBS and then add 150 µl TNE buffer to each well.
- Lyse cells by pipetting up and down 5-10x. Transfer whole cell lysates to 1.5 ml microcentrifuge tube and incubate on ice for 15-30 min.
- Centrifuge the lysates at 4 °C for 30 min at maximum speed (20,000 x g) and transfer cleared supernatant to a fresh tube for protein determination by Bradford and subsequent western blot analysis.
- Determine the level of expression of the protein of interest by standard western blot analysis while probing for antibodies against the protein itself and the fused tag (i.e., Flag). Use the viral dilution that yields >95% GFP+ cells and robust expression of protein of interest for experiments.
4. Isolation and Culture of DRGs (Figure 2 steps 1 & 2)
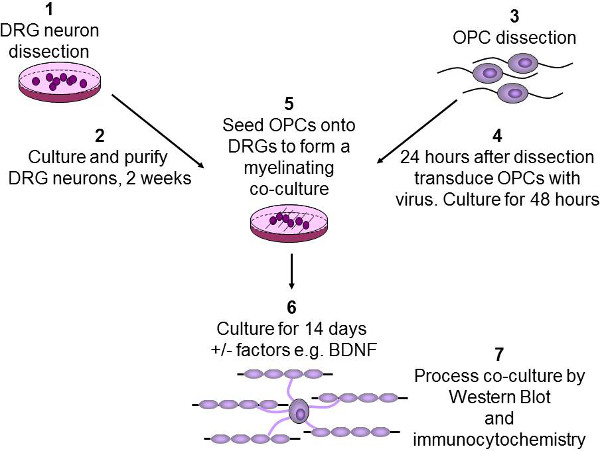
Figure 2: Schematic diagram of the in vitro myelination assay. DRG neurons are dissected from P2-3 rat pups, then purified and cultured over two weeks (1-2). OPCs are purified from P7-9 rat brains using immunopanning (3). OPCs are then infected with lentivirus and cultured for 48 hr (4). OPCs are then seeded onto DRGs, and any growth factors of interest such as BDNF are added (5). Co-cultures are then cultured for 2 weeks to allow OPCs to differentiate and myelinate the axons (6). Finally, co-cultures are either lysed for western blotting or fixed for immunocytochemistry (7).
NOTE: Day 1- 2 days prior to dissection:
- Coat autoclaved 22 mm x 22 mm coverslips with Poly-L-ornithine (0.5 mg/ml) in a 6-well plate, and incubate at 37 °C overnight.
- On the next day, coat coverslips again with Laminin (20 µg/ml in MEM) at 37 °C overnight, aspirate off excess and dry for 20 minutes in tissue culture hood.
NOTE: Day 2: Dissection and isolation of DRGs: - Sacrifice P2-P3 rat pups 12x by cervical transection.
- Remove the skin overlying the muscle from the back of the animal. Use vannas scissors to open vertebral foramen and use forceps to gently scoop out the spinal cord.
- Pluck out DRGs that lie in between vertebral columns and cut off attached spinal nerve fibres, then place DRGs in a 33 mm Petri dish containing 3 ml L-15 media. It is usually possible to gather between 8 and 12 DRG from each side of the spinal cord
- Transfer collected DRGs along with the L-15 media into a 15 ml tube, centrifuge at 180 x g for 5 min.
- Aspirate the supernatant, add 2 ml 0.25% trypsin to DRG pellets and incubate at 37 °C for 30 min.
- Add 5 ml of M1 medium (see Table of stock solution) to the DRG pellets to stop trypsin, and centrifuge at 180 x g for 3 min at RT.
- Aspirate supernatant, re-suspend the pellet in 2 ml pre-warmed M1 medium (not with growth factors). Triturate the ganglia pellet by pipetting 50x or until the ganglia are dispersed. Centrifuge cell suspension at 180 x g for 3 min.
- Resuspend dissociated neurons in M1 media and plate down in a 6-well plate at 5 ganglia per 100 µl per well.
- To remove proliferating non-neuronal cells, after a minimum of 4 hr incubation to facilitate attachment, remove M1 media and feed neurons with M2 media (see Table of stock solution). Culture DRG neurons in the presence of NGF (100 ng/ml) to purify a culture of NGF-dependent TrkA-expressing DRGs. Alternatively, use BDNF (100 ng/ml) to purify BDNF-dependent TrkB-expressing DRGs.
- Maintain neurons in M1 media (+NGF or BDNF at 100 ng/ml) alternating with antimitotic M2 media for 2 weeks as below.
- Maintain medium M1 (+NGF or BDNF at 100 ng/ml) on days: 4-6, 8-10, 12-14 and M2 media (+NGF or BDNF at 100 ng/ml) with FDU and uridine on days 1 to 4, 6-8, and 10-12. A minimum of 3 cycles of M2 media purification is required.
- Maintain DRGs in M1 alone for a further week after completing the 2 week antimitotic cycle. Change M1 media every 2-3 days.
5. Isolation and Culture of OPCs (Figure 2 step 3)
NOTE: Day 1- 2 days prior to dissection:
- Coat 10 cm tissue culture plates with Poly-D-Lysine (PDL, 10 µg/ml in sterilized deionized water) at 4 °C overnight.
NOTE: Day 2: 1 day prior to dissection: - Wash PDL plates with sterilized deionized water for 3x. Allow to dry for ~6 hr in tissue culture hood. If not being used straight away, wrap and store for up to 4 weeks at 4 °C.
- Prepare secondary antibody plates for immunopanning. For 1 brain dissection, prepare 2x IgG plates (for Ran2 antibody to remove astrocytes and O1 antibody to remove premyelinating oligodendrocytes), with 45 µl Goat α mouse IgG in DPBS (15 ml) per 10 cm Petri dish; 1x IgM plate for (O4 antibody for selecting oligodendrocyte precursor cells), 45 µl Goat α mouse IgM in DPBS (15 ml) per 10 cm Petri dish.
NOTE: Day 3: day of dissection: - Add 200 units of papain in 10 ml of papain buffer (Table 2), and warm up at 37 °C until the buffer turns clear.
Table 2: Preparation of papain buffer.Concentration for 250 ml Final concentration EBSS stock 10x 25 ml 1x MgSO4 100 mM 2.5 ml 1 mM Glucose 30% 3 ml 0.46% EGTA 0.5 M 1 ml 2 mM NaHCO3 1 M 6.5 ml 26 mM Bring volume up to 250 ml with deionized water and filter sterilize - Wash all secondary antibody plates with DPBS for 3x.
- Pour the Ran2 and O1 antibody onto the IgG plates and the O4 hybridoma to the IgM plate. Incubate all these primary antibody plates for over 2 hr at RT.
- Dissect one brain from a P7 rat. Decapitate a pup with sharpened scissors and remove the skin overlying the skull with scissors.
- Cut around the skull from the occipital lobe, the temporal lobe and to the frontal lobe. Remove brain from skull using a forceps and gently transfer it to a 35-mm Petri dish with 1 ml DPBS.
- Dice the brain into small pieces roughly with scissors or sterile blade.
- Filter pre-warmed papain buffer (10 ml) into a new 15 ml tube containing a few grains of L-cysteine, then add 200 µl DNAase (12,500 U/ml) to the filtered papain buffer.
- Pour the papain buffer onto the diced brain tissues; incubate at 37 °C for 90 min.
- Gentlly transfer dissociated brain tissues into 50 ml tube using a 25 ml pipette and allow to settle.
- Remove papain buffer, add 2 ml Lo Ovo (Ovomucoid) to brain tissues and titurate 5-10x by pipetting to break up chunks of brain tissues, allow to settle and remove the top 2 ml of supernatant to a new tube.
- Add another 2 ml Lo Ovo to brain tissues and repeat step 5.11 until no chunks of tissue remain. Tituration can get increasingly aggressive.
- Centrifuge dissociated cell suspension for 15 min at 200 x g. Aspirate off supernatant and resuspend cell pellet in 10 ml Hi Ovo and centrifuge for 15 min at 200 x g.
- Wash first immunopanning plate (Ran 2 plate) with DPBS for 3x. Aspirate supernatant, resuspend cells in 10 ml panning buffer (Table 2) and pour on to the first immunopanning plate (Ran 2 plate). Incubate cells for 15 min at RT.
- Wash the second immunopanning plate (O1 plate) with DPBS for 3x.
- After the incubation on the first immunopanning plate, tip the cell suspension onto second immunopanning plate (O1 plate), Rinse any loose cells off the surface of the plate with 1-3 ml of panning buffer and transfer by pipette to the O1 plate. Incubate cells for 15 min at RT.
- Wash the third immunopanning plate (O4 plate). This plate is the positive selection plate where the OPCs bind its surface. Wash the O4 plate with DPBS for 3x.
- After the incubation on the second immunopanning plate, transfer the cells to the final immunopanning plate (O4 plate), incubate cells for 45 min at RT. This step will select O4+ OPCs.
- Aspirate the supernatant from the last immunopanning plate (O4 plate) and rinse the plate with EBSS for 6x.
- To remove OPCs off the plate, incubate cells with 5 ml of warm 0.05% Trypsin-EDTA diluted 1:10 with EBSS at 37 °C for 8 min.
- Add 5 ml of 30% FBS (made in EBSS) to neutralize the trypsin. Remove cells off surface of the plate by pipetting for about 50x.
- Transfer all cell suspension to a new tube and centrifuge for 15 min at 200 x g.
- Discard supernatant and resuspend cell pellet in 1 ml pre-warmed SATO media, followed by cell counting. Dissection of one brain can yield 1.5-2 million OPCs.
- Plate cells onto dry PDL coated plates at a density between 1 x 105 and 5 x 105 per 10 cm plate with in SATO media (10 ml) that contain ciliary neurotrophic factor (CNTF, 10 ng/ml), platelet derived growth factor (PDGF, 10 ng/ml,), neurotrophin 3 (NT3, 1 ng/ml), and forskolin (4.2 µg/ml)2,12. Culture OPCs at 37 °C, 8% CO2.
6. Transducing OPCs
- Culture primary OPCs in SATO media (10ml/10cm plate) with CNTF (10 ng/ml), PDGF (10 ng/ml), NT3 (1 ng/ml), and forskolin (4.2 µg/ml) at 37 °C, 8% CO2 for 24 hr after dissection.
- Completely aspirate off the OPC culture media, feed cells with freshly made SATO media (10 ml) with growth factors (see above in step 6.1).
- Add virus to the OPCs to the optimal concentration determined by step 3 (Figure 2, step 4), culture OPCs for a further 48 hr.
7. OPC Seeding for Myelinating Co-cultures (Figure 2, steps 5 and 6)
- To remove OPCs off the surface, first rinse OPC plates with 8 ml EBSS twice, then incubate cells with 5 ml of warm 0.05% Trypsin-EDTA diluted 1:10 with EBSS at 37 °C for 2 min.
- Neutralize the trypsin with 30% FBS in EBSS (5 ml) and remove cells from plate by pipetting. Transfer cell suspension to a 15 ml tube, centrifuge at 180 x g for 15 min at RT.
- Aspirate off the supernatant and resuspend cell pellet in 1 ml pre-warmed SATO media followed by cell count.
- Prior to the OPC seeding, completely aspirate media from the DRG culture plate. Gently seed 200,000 OPCs drop wise on to the DRG neurons as previously described4-6.
NOTE: The total OPC seeding volume must be less than 200 µl per 22 mm coverslip. - Leave cells to settle without moving the plate for 10 min in the tissue culture hood, then gently top up with 1 ml pre-warmed SATO media per well.
- Replate the remaining sister OPCs with SATO media with growth factors (see step 6.1). Use these sister OPCs to verify expression of the protein of interest.
- After 24 hr, replace the SATO media with the co-culture media (2 ml/well) containing SATO media (no factors) and neurobasal (v/v) with 1% B27. Maintain the co-cultures for 14 days with media change every 2-3 days.
- Assess the co-cultures for myelin protein expression by western blot analysis and visualize for the formation of myelinated axonal segments by double-immunostaining with antibodies against myelin basic protein markers and neuronal markers4-6.
Results
The flag-tagged extracellular signal-related kinase 1(Flag-Erk1) construct used for lentivirus production is verified by restriction enzyme digest of the constructs used, including both the 2K7 constructs and the packaging and accessory constructs required for virus production (Figure 3).
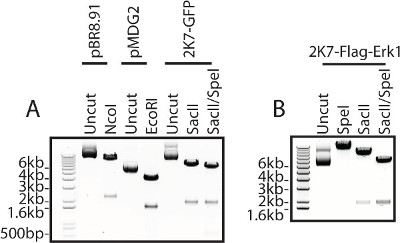
Figure 3: DNA construct verification. All DNA constructs...
Discussion
Myelination of axons is a crucial process for the optimal function of both the central and peripheral nervous systems of vertebrates. The generation and maintenance of myelinated axons is a complex and coordinated process involving molecular interactions between neuronal, glial (from Schwann cells or oligodendrocytes) and extra cellular matrix proteins. The significance and applicability of this protocol is that it allows manipulation of proteins in one specific cell type within the mixed co-culture settings. As multiple...
Disclosures
The authors declare that there is no conflict of interest regarding this research.
Acknowledgements
This work was supported by the Australian National Health and Medical Research Council (NHMRC fellowship #454330 to JX, project grant #628761 to SM and APP1058647 to JX), Multiple Sclerosis Research Australia (MSRA #12070 to JX), the University of Melbourne Research Grant Support Scheme and Melbourne Research CI Fellowship to JX as well as Australia Postgraduate Scholarships to HP and AF. We would like to acknowledge the Operational Infrastructure Scheme of the Department of Innovation, Industry and Regional Development, Victoria Australia.
Materials
| Name | Company | Catalog Number | Comments |
| 2K7 lentivector | Kind gift from Dr Suter9 | ||
| 5-Fluoro-2′-deoxyuridine | Sigma-Aldrich | F0503-100mg | |
| Alexa Fluor 488 Goat anti-mouse IgG | Jackson Immunoresearch | 115545205 | |
| Alexa Fluor 488 goat anti-rabbit IgG (H+L) | Life Technologies | A11008 | |
| Alexa Fluor 594 goat anti-mouse IgG (H+L) | Life Technologies | A11005 | |
| Alexa Fluor 594 goat anti-rabbit IgG (H+L) | Life Technologies | A11012 | |
| Ampicillin | Sigma-Aldrich | A9518-5G | |
| B27 - NeuroCul SM1 Neuronal Supplement | Stem Cell Technologies | 5711 | |
| BDNF (Human) | Peprotech | PT450021000 | |
| Biotin (d-Biotin) | Sigma Aldrich | B4639 | |
| Bradford Reagent | Sigma Aldrich | B6916-500ML | |
| BSA | Sigma Aldrich | A4161 | |
| Chloramphenicol | Sigma-Aldrich | C0378-100G | |
| CNTF | Peprotech | 450-13020 | |
| DAKO fluoresence mounting media | DAKO | S302380-2 | |
| DMEM, high glucose, pyruvate, no glutamine | Life Technologies | 10313039 | |
| DNase | Sigma-Aldrich | D5025-375KU | |
| DPBS | Life Technologies | 14190250 | |
| DPBS, calcium, magnesium | Life Technologies | 14040182 | |
| EBSS | Life Technologies | 14155063 | |
| EcoRI-HF | NEB | R3101 | |
| Entry vectors for promoter and gene of interest | Generate as per protocols 1-2 | ||
| Fetal Bovine Serum | Sigma-Aldrich | 12003C | |
| Forskolin | Sigma Aldrich | F6886-50MG | |
| Glucose (D-glucose) | Sigma-Aldrich | G7528 | |
| Glycerol | Chem Supply | GL010-500M | See stock solutions |
| Goat Anti-Mouse IgG | Jackson ImmunoResearch | 115005003 | |
| Goat Anti-Mouse IgM | Jackson ImmunoResearch | 115005020 | |
| Goat Anti-Rat IgG | Jackson ImmunoResearch | 112005167 | |
| Hoechst 33342 | Life Technologies | H3570 | |
| Igepal | Sigma Aldrich | I3021-100ML | |
| Insulin | Sigma Aldrich | I6634 | |
| Kanamycin | Sigma-Aldrich | 60615 | |
| Laminin | Life Technologies | 23017015 | |
| LB Medium | See stock solutions | ||
| LB-Agar | See stock solutions | ||
| L-Cysteine | Sigma-Aldrich | C-7477 | |
| Leibovitz's L-15 Medium | Life Technologies | 11415064 | |
| L-Glutamate | Sigma-Aldrich | G1626 | |
| L-Glutamine- 200 mM (100x) liquid | Life Technologies | 25030081 | |
| LR Clonase II Plus enzyme | Life Technologies | 12538-120 | |
| MEM, NEAA, no Glutamine | Life Technologies | 10370088 | |
| Mouse α βIII Tubulin | Promega | G7121 | |
| Mouse αMBP (monoclonal) | Millipore | MAB381 | |
| Na pyruvate | Life Technologies | 11360-070 | |
| NAC | Sigma Aldrich | A8199 | |
| NcoI-HF | NEB | R3193S | |
| NEBuffer 4 | NEB | B7004S | |
| Neurobasal medium | Life Technologies | 21103049 | |
| NGF (mouse) | Alomone Labs | N-100 | |
| NT-3 | Peprotech | 450-03 | |
| O1 antibody - Mouse anti-O1 | Millipore | MAB344 | Alternative if O1 hybridoma cells are unavailable |
| O1 hybridoma cells | Conditioned medium containing anti-O1 antibody to be used for immunopanning | ||
| O4 antibody - Mouse anti-O4 | Millipore | MAB345 | Alternative if O4 hybridoma cells are unavailable |
| O4 hybridoma cells | Conditioned medium containing anti-O4 antibody to be used for immunopanning | ||
| Competent cells | Life Technologies | A10460 | |
| One Shot Stbl competent cells | Life Technologies | C7373-03 | |
| Papain Suspension | Worthington/Cooper | LS003126 | |
| pBR8.91 | Kind gift from Dr Denham10 | ||
| PDGF-AA (Human) | Peprotech | PT10013A500 | |
| Penicillin-streptomycin 100x solution | Life Technologies | 15140122 | |
| pENTRY4IRES2GFP | Invitrogen | 11818-010 | |
| pMD2.G | Addgene | 12259 | |
| Poly-D-lysine | Sigma | P6407-5MG | |
| Polyethylenimine (PEI) | Sigma-Aldrich | 408727-100ML | |
| Poly-L-ornithine | Sigma Aldrich | P3655 | |
| Progesterone | Sigma Aldrich | P8783 | |
| Protease inhibitor tablet (Complete mini) | Roche | 11836153001 | |
| Proteinase K | Supplied with Clonase enzyme | ||
| Putrescine | Sigma Aldrich | P-5780 | |
| Rabbit α neurofilament | Millipore | AB1987 | |
| Rabbit αMBP (polyclonal) | Millipore | AB980 | |
| Ran2 hybridoma cells | ATCC | TIB-119 | Conditioned medium containing anti-Ran2 antibody to be used for immunopanning |
| Rat anti CD140A/PDGFRa antibody | BD Pharmingen | 558774 | |
| SacII | NEB | R0157 | |
| SOC medium | Supplied with competent bacteria | ||
| Sodium selenite | Sigma Aldrich | S5261 | |
| Spe I | NEB | R0133S | |
| T4 DNA Ligase | NEB | M0202S | |
| T4 DNA Ligase Buffer | NEB | B0202S | |
| TE buffer pH8 | See stock solutions | ||
| TNE lysis buffer | |||
| Trace Elements B | Cellgro | 99-175-CI | |
| Transferrin (apo-Transferrin human) | Sigma-Aldrich | T1147 | |
| Triton X-100 | Sigma-Aldrich | T9284 | |
| Trypsin | Sigma-Aldrich | T9201-1G | |
| Trypsin Inhibitor From Chicken Egg White | Roche | 10109878001 | |
| Trypsin-EDTA (1x), phenol red (0.05%) | Life Technologies | 25300-054 | |
| Unconjugated Griffonia Simplicifolia Lectin BSL-1 | Vector laboratories | L-1100 | |
| Uridine | Sigma-Aldrich | U3003-5G |
References
- Baumann, N., Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 81 (2), 871-927 (2001).
- Watkins, T. A., Emery, B., Mulinyawe, S., Barres, B. A. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 60 (4), 555-569 (2008).
- Lee, K., et al. MDGAs interact selectively with neuroligin-2 but not other neuroligins to regulate inhibitory synapse development. Proc Natl Acad Sci U S A. 110 (1), 336-341 (2013).
- Xiao, J., et al. BDNF exerts contrasting effects on peripheral myelination of NGF-dependent and BDNF-dependent DRG neurons. J Neurosci. 29 (13), 4016-4022 (2009).
- Chan, J. R., et al. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 43 (2), 183-191 (2004).
- Xiao, J., et al. Brain-Derived Neurotrophic Factor Promotes Central Nervous System Myelination via a Direct Effect upon Oligodendrocytes. Neurosignals. 18 (3), 186-202 (2010).
- Lundgaard, I., et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biology. 11 (12), e1001743 (2013).
- Kleitman, N., W, P. M., Bunge, R. P. . Tissue culture methodes for the study of myelination. , (1991).
- Xiao, J., et al. Extracellular signal-regulated kinase 1/2 signaling promotes oligodendrocyte myelination in vitro. J Neurochem. 122 (6), 1167-1180 (2012).
- Wong, A. W., Xiao, J., Kemper, D., Kilpatrick, T. J., Murray, S. S. Oligodendroglial expression of TrkB independently regulates myelination and progenitor cell proliferation. The Journal of Neuroscience. 33 (11), 4947-4957 (2013).
- Li, Z., et al. Molecular cloning, Characterization and Expression of miR-15a-3p and miR-15b-3p in Dairy Cattle. Molecular and Cellular Probes. , (2014).
- Emery, B., et al. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 138 (1), 172-185 (2009).
- Murai, K., et al. Nuclear receptor TLX stimulates hippocampal neurogenesis and enhances learning and memory in a transgenic mouse model. Proc Natl Acad Sci U S A. 111 (25), 9115-9120 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved