A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Development of an in vitro model system for studying the interaction of Equus caballus IgE with its high-affinity receptor FcεRI
In This Article
Summary
The current study describes the development and applications of a genetically engineered assay system based on the transfection of rat basophilic leukemia cells with the equine FcεRIα gene. Transfected cells express a functional receptor where the release of mediators of the allergic response can be activated by IgE and antigen.
Abstract
The interaction of IgE with its high-affinity Fc receptor (FcεRI) followed by an antigenic challenge is the principal pathway in IgE mediated allergic reactions. As a consequence of the high affinity binding between IgE and FcεRI, along with the continuous production of IgE by B cells, allergies usually persist throughout life, with currently no permanent cure available. Horses, especially race horses, which are commonly inbred, are a species of mammals that are very prone to the development of hypersensitivity responses, which can seriously affect their performance. Physiological responses to allergic sensitization in horses mirror that observed in humans and dogs. In this paper we describe the development of an in situ assay system for the quantitative assessment of the release of mediators of the allergic response pertaining to the equine system. To this end, the gene encoding equine FcεRIα was transfected into and expressed onto the surface of parental Rat Basophil Leukemia (RBL-2H3.1) cells. The gene product of the transfected equine α-chain formed a functional receptor complex with the endogenous rat β- and γ-chains 1. The resultant assay system facilitated an assessment of the quantity of mediator secreted from equine FcεRIα transfected RBL-2H3.1 cells following sensitization with equine IgE and antigenic challenge using β-hexosaminidase release as a readout 2, 3. Mediator release peaked at 36.68% ± 4.88% at 100 ng ml-1 of antigen. This assay was modified from previous assays used to study human and canine allergic responses 4, 5. We have also shown that this type of assay system has multiple applications for the development of diagnostic tools and the safety assessment of potential therapeutic intervention strategies in allergic disease 6, 2, 3.
Introduction
Allergy has been known for millennia. An asthma treatment was described in the Ancient Egyptian medical text known as the Ebers Papyrus (~1550 BCE) and discussed herbal remedies to treat it 7.
Today allergy is classified as a type I hypersensitivity response, where the T helper cell type 2 (TH2) arm of the immune system steers the production of immunoglobulin E (IgE) antibodies in response to environmental antigens called allergens. These are diverse substances that commonly interact with cells in the immune system and stimulate the synthesis and secretion of pro-inflammatory cytokines, including interleukin-4 and interleukin-13 8, 9 as do particles in cigarette smoke or diesel exhaust particles which enhance IgE synthesis 10.
The rise in allergic manifestations in industrialized countries in the last 50 years has been attributed to a combination of the effect of environmental pollutants and a trend to a more sanitized environment, which combine to shift the immune response towards a profile predominated by TH2 cytokines, as proposed by the ‘Hygiene Hypothesis’ 11.
As mentioned above, humans are not the only mammals afflicted by allergy. Notably horses and dogs can also develop classic allergic responses and a study by 12 has shown that, as in humans, equine allergy is attributed to genetic and environmental factors. As a consequence, these animals present good models for studying the interplay between genetic and environmental causes of allergy, its progression from sensitization to disease, and possible intervention strategies once clinical manifestations have set in
In 1887, Stömmer was the first person to describe the similarity between human and equine asthma 13, the effect of histamine on the equine cardiovascular system is very similar to that of humans 14. Horses are also the cornerstone of the horse racing industry, which is worth US$72 billion with a betting turnover of US$115 billion annually 15.
Most contemporary racehorses are descendants of the small number of Arabian horses bred by Lady Anne Blunt from 1878 onwards. Modern racehorses are commonly inbred to select for performance abilities. They are prone to genetic disorders, one of which is their susceptibility to mount allergic responses. They also have 1000 times higher serum IgE levels than even the most severely allergic humans 16. Horse allergic responses are usually manifested as insect bite hypersensitivity (IBH) 17, 18. IBH results in dermatitis due to bites form insects in the genus Culicoides. Another form of equine allergic disease is recurrent airway obstructions (RAO), this is manifested in the lungs and airways. It is characterized by wheezing and labored breathing. RAO commonly occurs in response to mould spores, and high allergen-specific IgE levels have been recorded in horses suffering from RAO in one study 19 although another investigation has not confirmed this 20.
Studies on equine allergy revolved around the attempt at monitoring and neutralizing equine IgE by developing anti-equine IgE monoclonal antibodies (mAbs) 21, 22. Furthermore the study by 23 discusses the production of the extracellular domains of the equine high-affinity Fc receptor’s α chain (FcεRIα) receptor in an attempt to detect and quantitate equine serum IgE. A related study by Ledin24 discusses a new approach aimed at neutralizing serum IgE by priming the immune system using a self/non-self immunogen. All these studies, however, lacked an effective assay to test the safety and efficacy of their protocols. In this article, we now present such an assay system applicable to the study of diagnostic and therapeutic strategies relevant to the equine system, where β-hexosaminidase release, as an indicator of cell mediator degranulation, was assessed on RBL-2H3.1 cells expressing equine FcεRIα. This protocol is based on previous publications 25, 4, 5, 2, 3 describing the engineering of RBL cells transfected with the gene encoding the IgE binding domain of the high-affinity receptor for IgE from different species. The protocol explains how to perform a β-hexosaminidase release assay, the results of which are presented as the mean ± standard deviation of triplicate experiments.
The release assay was first developed by Siraganian and Hook 25 to study human allergy. The lab group led by Dr. Reuben Siraganian also developed the RBL cell line. These RBL cells were developed to express the human FcεRIα and the protocol was published by 4. The final piece of the assay came with the development of the pSV plasmid in the paper by Neuberger 26 which described the production of an IgE antibodies by cloning its heavy chain gene downstream of a mouse gene for an IgE variable region that targets the hapten 4-hydroxy-3-nitro-phenacetyl (NP), the resulting chimeric antibody was fully functional. The ability to develop any IgE targeting the same hapten, while also cloning its receptor on the surface of RBL cells resulted in the standardization of the assay making it a useful protocol to measure the degranulation of basophil cells.
The assay does have pros and cons. The pros of the assay is its adaptability to be used in any mammalian system, our lab has thus used it to test for the degranulation in the human, canine and equine systems, and this is achievable simply by synthesizing the organism's IgE and cloning its receptor onto the surface of the RBL cells.
On the other hand, the cons of the assay is that the RBL cells are very sensitive to thermal, mechanical and PH changes, making them give a variation of degranulation levels within the same assay. It is thus strongly advised that the assays are always repeated in triplicates and then an average is taken from them. Furthermore, the RBL cells tend to shift toward a non-releasing phenotype if they are left in tissue culture for an extended times (>10 weeks) 27, making their maintenance cumbersome. They are also prone to infections by mycoplasma bacteria, which are not visible from a light microscope and do not change the cell's morphology, but would drastically change their degranulation levels. Thus regular mycoplasma tests are needed.
Protocol
1) Preparation of Cell Line:
- Developing the RBL-2H3.1 cell line expressing equine FcεRIα:
- Using basic tissue culture techniques for monolayer cell lines, transfect parental RBL-2H3.1 cells using the pEE6 plasmid, carrying the equine FcεRIα gene (GenBank: Y18204.1) 28. Add 2 μg μl-1 of the plasmid DNA to 0.8 ml of cells at a density of 1.2 x107 cells ml-1. Electroporate the cells at 250 V 960 μF using a 0.4 cm electrocuvette then immediately incubate on ice for 10 min.
- Select the transformed cells using media containing 0.4 g of geneticin G418 sulphate, then sort the remaining living cells through FACS by tagging them with a fluorescent IgE antibody. Use the resulting RBL-2H3.1 expressing equine FcεRIα cell line for the investigation 2, 3.
- Pre-assay antibody sensitization:
- Harvest the RBL-2H3.1 expressing equine FcεRIα cells from a confluent Petri dish. Wash then re-suspend the cells in culture media to a cell density of 5x105 cell ml-1.
- Add the IgE of interest to the suspended cells to a final concentration of 1 ng ml-1, then plate 100 μl of cells onto a 96 well plate at columns 1-6 and incubate at 37 °C + 5% CO2 + 90% relative humidity for 16 hr. After the incubation time, and before performing the release assay, check the wells under a microscope for well confluency and cell adherence.
2) Release Assay:
- Washing the cells:
- Warm release buffer (25 mM PIPES, 120 mM sodium chloride, 5 mM potassium chloride, 0.04 mM magnesium chloride, and 1 mM calcium chloride) at 37 °C to allow for gentle cell washing.
- Wash cells by flicking the plate to remove cell media and adding 100 μl warm, 37 °C, release buffer. Repeat twice.
- Antigen challenge:
- Prepare a serial dilution of the antigen (NIP-HSA or DNP-HSA) of 0 ng ml-1, 0.1 ng ml-1, 1 ng ml-1, 10 ng ml-1, 100 ng ml-1, 1,000 ng ml-1, 10,000 ng ml-1 in release buffer and warm at 37 °C.
- After the second cell wash, discard the media and replaced with 100 μl of the antigen solutions. Ensure that wells in the same row (A1-6 for example) have the same antigen concentration added to them.
- Set up a negative control in row A by adding 0 ng ml-1 antigen. Add increasing antigen concentration down the rows (B-G) followed by triton-x buffer (5% Triton X-100) in row H cells to lyse the cells to be used as a positive control. Incubate at 37 °C for 20 min to allow the cells to release its mediators.
- Setting up individual well controls:
- After the incubation, transfer 50 μl of cell supernatant to the other half of the plate (wells A1-6 to wells A7-12, etc.). Discard the remaining 50 μl of supernatant and replace with 50 μl of triton-x buffer to allow the measurement of the quantity of released mediators in each well in columns 7-12 as a percentage of the total mediators inside the cells in column 1-6.
- Enzyme substrate:
- Add 50 μl of β-hexosaminidase substrate (50 mM 4-nitrophenyl N-acetyl-β-D-glucosaminide prepared in DMSO diluted down to 2 mM by adding it to citrate buffer 0.2 M citric acid and 0.2 M sodium acetate, pH 4.5) to all the wells to facilitated the conversion of the substrate to 4-nitrophenol by the β-hexosaminidase enzyme. Incubate the plates at 37 °C for 2 hr.

- Terminating the reaction:
- Stop the reaction by the adding 150 μl Tris buffer (1 M Tris-HCl, pH 9) to each well as the high pH of the buffer stops the reaction and turns the 4-nitrophenol into a yellow color.
- Reading and analyzing the results:
- Read the plate using a plate spectrophotometer at 405 nm to measure the absorbance of the yellow color. Calculated the percentage of released β-hexosaminidase using the following formula:
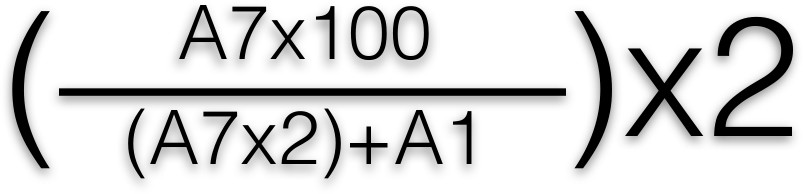
- Apply this formula to each well, after which an average is taken for each row. A1 and A7 represent the location of the wells in the 96 well plate. Plot a graph of percentage of β-hexosaminidase release (which corresponds to total mediator release) against antigen concentration 2, 3.
Results
The parental RBL-2H3.1 cells and those transfected with the equine FcεRIα receptor gene were first sensitized with mouse IgE anti DNP-HSA and challenged with the DNP-HSA antigen. Mouse IgE binds to the endogenous rat receptor in both cell lines and thus acts as a control to test the release viability of both cell lines to release mediators (Figure 1 A). This is an important check and should be carried out routinely since upon extended (> 10 weeks) passage in cell culture, RBL-2H3.1 cells dr...
Discussion
In summary the results of this investigation showed that when RBL-2H3.1 cells expressing equine FcεRIα are sensitized with equine IgE and challenged by an antigen, they give a peak mediator release of 36.68% ± 4.88% of the total amount of mediator inside the cells, compared to the RBL-2H3.1 parental cells not expressing equine FcεRIα.
Thus this assay provides a useful tool for investigating and studying equine allergic responses in vitro. Its allows the determ...
Disclosures
The authors declare that they have no competing financial interests in this paper.
Acknowledgements
The authors thank Dr. Lynda Partridge for the provision of advice and laboratory facilities.
Materials
| Name | Company | Catalog Number | Comments |
| RBL-2H3.1 Expressing Equine FcεRIα | - | - | Produced in the lab |
| Equine IgE anti NIP-HSA | - | - | Produced in the lab |
| 96 Well Plate | Sigma | CLS3595 | - |
| Multi Channel Pipette | Anachem | - | - |
| Incubator | Galaxy R | - | - |
| 4Hydroxy-5-iodo-3-nitrophenylacetic acid | Cambridge Research Biochemicals | N-1070-1 | NIP-OH was conjugated with Human Serum Albumin to make NIP-HSA in the lab |
| Dinitrophenyl Conjugated to Human Serum Albumin | Sigma | A6661 | Abbreviated DNP-HSA |
| Plate Spectrophotometer | Anthos Labtec HT2 | - | - |
| Pipes | Sigma | P1851 | - |
| Sodium Chloride | Sigma | S7653 | - |
| Potassium Chloride | Sigma | P9333 | - |
| Magnesium Chloride | Sigma | M2670 | - |
| Calcium Chloride | Sigma | C1016 | - |
| Triton x100 | Sigma | X100 | - |
| 4-nitrophenyl N-acetyl-β-D-glucosaminide | Sigma | N9376 | Stock solution called β-hexosaminidase substrate was 50mM prepared in DMSO |
| Dimethyl Sulfoxide | Sigma | D2650 | - |
| Citric Acid | Sigma | 251275 | - |
| Sodium Acetate | Sigma | S7670 | - |
| Tris | Sigma | T5941 | - |
References
- Taudou, G., et al. Expression of the Alpha Chain of Human FcεRI in Transfected Rat Basophilic Leukemia Cells: Functional Activation after Sensitization with Human Mite-Specific IgE. Int Arch Allergy Immunol. 100 (4), 344-350 (1993).
- Sabban, S. . Development of an in Vitro Model System for Studying the Interaction of EquuscaballusIgE with Its High-Affinity FcεRI Receptor. , (2011).
- Sabban, S., Ye, H., Helm, B. A. Development of an in Vitro Model System for Studying the Interaction of EquuscaballusIgE with Its High-Affinity Receptor FcεRI. Vet ImmunolImmunopathol. 153 (1-2), 6-10 (2013).
- Wilson, A. P. M., Pullar, C. E., Camp, A. M., Helm, B. A. Human IgE mediates stimulus secretion coupling in rat basophilic leukemia cells transfected with the a chain of the human high-affinity receptor. Eur J Immunol. 23, 240-244 (1993).
- Hunter, M. J., Vratimos, A. P., Housden, J. E. M., Helm, B. A. Generation of canine-human Fc IgE chimeric antibodies for the determination of the canine IgE domain of interaction with FcεRIα. MolImmunol. 45 (8), 2262-2268 (2008).
- Rashid, A., et al. Review: Diagnostic and therapeutic applications of rat basophilic leukemia cells. MolImmunol. 52 (3-4), 224-228 (2012).
- Cohen, S. G. Asthma in antiquity: the Ebers Papyrus. Allergy Proc. 13 (3), 147-154 (1992).
- Dudler, T., et al. A link between catalytic activity, IgE-independent mast cell activation, and allergenicity of bee venom phospholipase A2. J. Immunol. 155, 2605-2613 (1995).
- Machado, D. C., Horton, D., Harrop, R., Peachell, P. T., Helm, B. A. Potential allergens stimulate the release of mediators of the allergic response from cells of mast cell lineage in the absence of sensitization with antigen-specific IgE. Eur J Immunol. 26 (12), 2972-2980 (1996).
- Smyth, L. J., et al. Assessment of the molecular basis of pro-allergenic effects of cigarette smoke. Environ Sci Technol. 34 (7), 1370-1374 (2000).
- Okada, H., Kuhn, C., Feillet, H., Bach, J. F. The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. ClinExpImmunol. 160 (1), 1-9 (2010).
- Eder, C., et al. Influence of environmental and genetic factors on allergen-specific immunoglobulin-E levels in sera from Lipizzan horses. Equine Vet J. 33 (7), 714-720 (2001).
- Cook, W. R., Rossdale, P. D. The syndrome of 'Broken Wind' in the horse. Proceedings of the Royal Society of Medicine. 56, 972-977 (1963).
- Eyre, P., Lewis, A. J. Acute systemic anaphylaxis in the horse. Br. J. Pharmacol. 48 (3), 426-437 (1973).
- Wagner, B. IgE in horses: occurrence in health and disease. Vet ImmunolImmunopathol. 132 (1), 21-23 (2009).
- Hellberg, W., et al. Equine insect bite hypersensitivity: immunoblot analysis of IgE and IgG subclass responses to Culicoidesnubeculosus salivary gland extract. Vet. Immunol. Immunopathol. 113 (1-2), 99-112 (2006).
- Schaffartzik, A., et al. Equine insect bite hypersensitivity: what do we know. Vet ImmunolImmunopathol. 147 (3-4), 113-126 (2012).
- Künzle, F., et al. IgE-bearing cells in bronchoalveolar lavage fluid and allergen-specific IgE levels in sera from RAO-affected horses. J Vet Med A PhysiolPatholClin Med. 54 (1), 40-47 (2007).
- Tahon, L., et al. In vitro allergy tests compared to intradermal testing in horses with recurrent airway obstruction. Vet ImmunolImmunopathol. (1-2), 85-93 (2009).
- Wagner, B., Radbruch, A., Rohwer, J., Leibold, W. Monoclonal anti-equine IgE antibodies with specificity for different epitopes on the immunoglobulin heavy chain of native IgE. Vet ImmunolImmunopathol. 92 (1-2), 45-60 (2003).
- Wilson, A. D., Harwood, L., Torsteinsdottir, S., Marti, E. Production of monoclonal antibodies specific for native equine IgE and their application to monitor total serum IgE responses in Icelandic and non-Icelandic horses with insect bite dermal hypersensitivity. Vet ImmunolImmunopathol. 112 (3-4), 156-170 (2006).
- McAleese, S. M., et al. Cloning and expression of the extra-cellular part of the alpha chain of the equine high-affinity IgE receptor and its use in the detection of IgE. Vet ImmunolImmunopathol. 110 (1-2), 187-191 (2006).
- Ledin, A., et al. Generation of therapeutic antibody responses against IgE in dogs, an animal species with exceptionally high plasma IgE levels. Vaccine. 24 (1), 66-74 (2006).
- Siraganian, R. P., Hook, W. A. Histamine release and assay methods for the study of human allergy. Manual of Clinical Immunology. , (1980).
- Neuberger, M. S., et al. A hapten-specific chimaericIgE antibody with human physiological effector function. Nature. 314 (6008), 268-270 (1985).
- Bingham, B. R., Monk, P. N., Helm, B. A. Defective Protein Phosphorylation and Ca2+ Mobilization in a low secreting variant of the rat basophilic leukemia cell line. The Journal of Biological Chemistry. 269 (30), 19300-19306 (1994).
- McAleese, S. M., Halliwell, R. E., Miller, H. R. Cloning and sequencing of the horse and sheep high-affinity IgE receptor alpha chain cDNA. Immunogenetics. 1 (51), 878-881 (2000).
- Hongtu, Y. Study of the structure/function relationship in canine and human IgE as the basis for the development of rational therapeutic intervention strategies in allergic disease. , (2010).
- Sabban, S., et al. Towards a pan-anti-allergy vaccine. JIBTVA. 2 (2), 15-27 (2013).
- Moran, G., Burgos, R., Araya, O., Folch, H. In vitro bioassay to detect reaginic antibodies from the serum of horses affected with recurrent airway obstruction. Vet Res Commun. 34, 91-99 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved