A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Chemical Vapor Deposition of an Organic Magnet, Vanadium Tetracyanoethylene
In This Article
Summary
We present the synthesis of the organic-based ferrimagnet vanadium tetracyanoethylene (V[TCNE]x, x~2) via low temperature chemical vapor deposition (CVD). This optimized recipe yields an increase in Curie temperature from 400 K to over 600 K and a dramatic improvement in magnetic resonance properties.
Abstract
Recent progress in the field of organic materials has yielded devices such as organic light emitting diodes (OLEDs) which have advantages not found in traditional materials, including low cost and mechanical flexibility. In a similar vein, it would be advantageous to expand the use of organics into high frequency electronics and spin-based electronics. This work presents a synthetic process for the growth of thin films of the room temperature organic ferrimagnet, vanadium tetracyanoethylene (V[TCNE]x, x~2) by low temperature chemical vapor deposition (CVD). The thin film is grown at <60 °C, and can accommodate a wide variety of substrates including, but not limited to, silicon, glass, Teflon and flexible substrates. The conformal deposition is conducive to pre-patterned and three-dimensional structures as well. Additionally this technique can yield films with thicknesses ranging from 30 nm to several microns. Recent progress in optimization of film growth creates a film whose qualities, such as higher Curie temperature (600 K), improved magnetic homogeneity, and narrow ferromagnetic resonance line-width (1.5 G) show promise for a variety of applications in spintronics and microwave electronics.
Introduction
The organic-based ferrimagnetic semiconductor vanadium tetracyanoethylene (V[TCNE]x, x~2) exhibits room temperature magnetic ordering and promises the advantages of organic materials for magnetoelectronic applications, such as flexibility, low cost production, and chemical tunability. Previous studies have demonstrated functionality in spintronic devices, including hybrid organic/inorganic1,2 and all-organic spin valves3, and as a spin polarizer in an active organic/inorganic semiconductor heterostructure4. In addition, V[TCNE]x~2 has demonstrated promise for inclusion in high frequency electronics due to its extremely narrow ferromagnetic resonance linewidth5.
There are four different methods which have been established for synthesizing V[TCNE]x~26-9. V[TCNE]x~2 was first synthesized as powder in dichloromethane via reaction of TCNE and V(C6H6)6. These powders exhibited the first room temperature magnetic ordering observed in an organic-based material. However, the powder form of this material is extremely air sensitive, limiting its application in thin film devices. In 2000, a chemical vapor deposition (CVD) method was established for creating V[TCNE]x~2 thin films7. More recently physical vapor deposition (PVD)8 and molecular layer deposition (MLD)9 have also been used to fabricate thin films. The PVD method requires an ultra-high vacuum (UHV) system and both PVD and mLD methods require extremely long times to grow films thicker than 100 nm, whereas the CVD films can easily be deposited in thicknesses ranging from 30 nm to several microns. In addition to the variety of thicknesses available with the CVD method, extensive studies have yielded optimized films that consistently show high quality magnetic properties including: narrow ferromagnetic resonance (FMR) linewidth (1.5 G), high Curie temperature (600 K), and sharp magnetic switching5.
Magnetic ordering in V[TCNE]x~2 thin films proceeds via an unconventional route. SQUID magnetometry measurements show strong local magnetic ordering, but the absence of X-ray diffraction peaks and featureless transmission electron microscopy (TEM)10 morphology reveal a lack of long-range structural order. However, extended X-ray absorption fine-structure (EXAFS) studies11 show that each vanadium ion is octahedrally coordinated with six different TCNE molecules, indicating a robust local structural order with a vanadium-nitrogen bond length of 2.084(5) Å. Magnetism arises from an antiferromagnetic exchange coupling between the unpaired spins of the TCNE- radical anions, which are distributed across the entire TCNE- molecule , and the spins on the V2+ ions, leading to a local ferrimagnetic ordering with TC ~ 600 K for optimized films5. In addition to exhibiting room temperature magnetic ordering, V[TCNE]x~2 films are semiconducting with 0.5 eV bandgap12. Other properties of note include possible sperimagnetism below a freezing temperature of ~150 K13,14, anomalous positive magnetoresistance12,15,16, and photo-induced magnetism13,17,18.
The CVD method for synthesizing V[TCNE]x~2 thin films is compatible with a variety of substrates due to low temperature (<60 °C) and conformal deposition. Previous studies have shown successful deposition of V[TCNE]x~2 on both rigid and flexible substrates7. Further, this deposition technique lends itself to tuning through modification of precursors and growth parameters.19-22 While the protocol shown here yields the most optimized films to date, significant progress has been made in improving some of the film properties since the discovery of this method and further gains may be possible.
Protocol
1. Synthesis and Preparation of Precursors
- Preparation of [Et4N][V(CO)6]23
- In a nitrogen glovebox, cut 1.88 g of sodium metal into ~40 pieces and mix with 14.84 g of anthracene in 320 ml of anhydrous tetrahydrofuran (THF) in a 1 L three-neck round bottom flask.
CAUTION: Both sodium metal and tetrahydrofuran are highly flammable. - Stir the solution for 4.5 hr at RT under a nitrogen atmosphere until a deep blue solution of NaC14H10 is formed.
- Cool the solution to 0 °C.
- In a nitrogen glovebox, prepare a pink-red solution of VCL3(THF)3 by adding 400 ml of anhydrous THF into 7.48 g of VCl3(THF)3 in a 500 ml round bottom flask and stir at RT for 1 hr.
- Remove the pink-red solution VCl3(THF)3 from the glovebox and cool to 0 °C for 20 min. Transfer to the previous solution of NaC14H10 via cannula under nitrogen atmosphere. A homogeneous deep purple solution is formed immediately after the addition is completed.
- Remove from the nitrogen and stir for 15 hr. Slowly warm to RT by placing flask in ice bucket allowing the ice to melt O/N.
- Cool the solution again to 0 °C and fill the reaction flask with carbon monoxide. The solution will change from deep purple to yellow-brown in a matter of minutes.
CAUTION: Carbon monoxide is highly toxic. This step should not be performed alone and a carbon monoxide alarm should be installed in the lab. - Stir the solution under a carbon monoxide atmosphere at 0 °C for 15 hr and then slowly warm to RT.
- Remove all but 200 ml of THF under vacuum. Add 500 ml of O2 free water while stirring the solution. V(CO)6 is easily oxidized and the presence of O2 will result in a low yield.
- Filter the resulting yellow slurry into a solution composed of 20.8 g of tetraethylammonium bromide (Et4NBr) in 200 ml of H2O.
- Wash the filter cake with O2 free water until it is colorless.
- Filter the resulting slurry of [Et4N][V(CO)6] by vacuum filtration and dry under vacuum.
- Store [Et4N][V(CO)6] in a glovebox freezer for future use.
- In a nitrogen glovebox, cut 1.88 g of sodium metal into ~40 pieces and mix with 14.84 g of anthracene in 320 ml of anhydrous tetrahydrofuran (THF) in a 1 L three-neck round bottom flask.
- Preparation of V(CO)623
- Grease the connection points for a vacuum adaptor with stopcock, glass two-way connecting tube, and cold-finger. Place a cold finger in the center neck and a vacuum adaptor with stopcock in the third opening.
- In an argon glovebox, mix 100 mg of [Et4N][V(CO)6] with 1 g of phosphoric acid in a round bottom flask containing a magnetic stirring bar.
- Connect the round bottom flask to a three-neck round bottom flask via glass two-way connecting tube in the argon glovebox.
- Remove the sealed flask system from the glovebox and set up in chemical hood.
- Add methanol to the cold finger and stir with a spatula-while adding liquid nitrogen until methanol is frozen. Pump down the system by opening the stopcock to a vacuum line until the pressure reaches 5 x 10-2 Torr.
- Submerge the round bottom flask in an oil bath set to 45 °C and turn on the magnetic stirring. Once the reaction starts, the phosphoric acid will melt and a black-blue powder condenses on the cold finger.
- Open the vacuum line when a black powder condenses on the round bottom flask instead of the cold finger because the pressure is too high. Pump the system back to 5 x 10-2 Torr before closing again.
- Rotate the reaction flask as necessary to mix all of the reactants.
- Allow the reaction to continue until the remaining residue in round bottom flask is white-grey and no longer bubbling.
- Pour copper pellets into a cold safe container and cool with liquid nitrogen.
- Remove the methanol from the cold finger with a micropipette. Pour chilled copper pellets into the cold finger to keep it cold during transfer to glovebox.
- Wipe oil and condensed water off the flask system before transferring into an argon glovebox.
- Inside the glovebox, remove the cold finger from the flask system and use a spatula to scrape the black V(CO)6 powder onto a piece of weighing paper.
- Store V(CO)6 in a bottle under an argon atmosphere and keep below RT.
- Purification of TCNE by sublimation
- Purchase commercially available tetracyanoethylene (TCNE) and store in a chemical refrigerator.
- Mix ~5 g of TCNE with ~0.5 g of activated carbon and grind with a mortar and pestle.
- Place TCNE/carbon mixture into a glass boat or wrap in delicate task wipes and put in the bottom of a flask with a vacuum line.
- Place a cold finger into the top of the flask and seal the two parts together with a clamp.
- Add methanol to the cold finger and stir with a spatula-while adding liquid nitrogen until methanol is frozen. Place the bottom of the flask containing the TCNE in an oil bath heated to 70 °C.
- Open the vacuum line to reach a pressure of 10-4 Torr and then close the vacuum line.
- Occasionally open the vacuum line to maintain the pressure. TCNE condenses on the cold finger as sublimation begins. Once no more TCNE accumulates on the cold finger the sublimation is finished.
- Remove the methanol from the cold finger with a micropipette.
- Wipe oil and condensed water off the flask system before transferring into an argon glovebox.
- Inside the glovebox, remove the cold finger from the flask system and use a spatula to scrape the TCNE powder onto a piece of weighing paper.
- Store purified TCNE in a refrigerator below RT under inert atmosphere.
2. Set up Deposition System inside an Argon Glovebox
- Assemble the reactor inside an argon glovebox as shown in Figure 1A.
- Set up a connection to a vacuum pump.
- Set up the gas flow connections by connecting a 3-way stopcock between a flow meter and two lines connected to micrometer valves.
- Slide the glass heater coil around the reactor (part A, Figure 1B).
- Wrap a glass slide with polytetrafluoroethylene (PTFE) thread seal tape.
- Push the glass slide approximately 10 cm from the right side of the reactor, part A.
- Place an O-ring on Part B and slide into the right side of the reactor. Join the two pieces together with a clamp.
- Attach a vacuum line to the bottom connection on part A and attach the gauge to the top connection.
- Place a boat filled with purified TCNE into part C near end so that the TCNE will sit in the hottest part of the reactor.
- Grease the connection of part C and slide it into the left side of the reactor.
- Grease both sides of the T-boat filled with V(CO)6 and slide into the right end of part B.
- Connect each micrometer valve. One should be connected to the right side of the T-boat and the other to the left side of part C and clamp both in place.
- Run a test deposition to determine where the reaction zone is located.
- Deposit V[TCNE]x~2 onto substrates
- Set the temperature of the reaction heating coil so that the reaction zone is set to a value near 46 °C when measured on the bottom of the reactor and the area of the TCNE boat is near 75 °C. Set the temperature of a silicone oil bath to 10 °C. Allow the temperatures to stabilize for at least 30 min.
- Slide the glass heater coil around the reactor (part A, Figure 1A).
- Wrap a glass slide with polytetrafluoroethylene (PTFE) thread seal tape. Arrange samples on top of covered slide within a two-inch space.
- Push the glass slide into the reactor so the samples are located in the reaction zone. Alternately samples can be placed directly on the bottom of the reactor, although the reaction zone may be shifted without a glass slide.
- Place an O-ring on Part B and slide into the right side of the reactor. Join the two pieces together with a clamp.
- Attach a vacuum line to the bottom connection on part A and attach the gauge to the top connection.
- Put 50 mg of TCNE into the TCNE boat and 5 mg of V(CO)6 into the T-boat (these quantities are appropriate for an 75-90 min deposition).
- Slide the TCNE boat into part C near the end so that the TCNE will sit in the hottest part of the reactor which should be about 75 °C.
- Grease the connection of part C and slide it into the left side of the reactor.
- Grease both sides of the T-boat and slide into the right end of part B.
- Slide the flow line onto the right side of the T-boat and left sides of part C and clamp in place. The assembled set-up should resemble Figure 1A.
- Raise the oil bath to cover the entire bottom of the T-boat.
- Open the vacuum line to reach a pressure of 30-35 mmHg.
- Set the flow rate to 56 sccm for the V(CO)6 and to 84 sccm for the TCNE. The reaction should begin immediately with a greenish material condensing on the wall of reaction zone.
- Allow reaction to proceed for the desired length of time. The thickness of the thin film is based on reaction time and location inside the reactor, as shown in Figure 2.
- To stop the reaction, close vacuum line and turn off the heater and oil bath.
3. Clean up
- Take apart the system in any order.
- Soak all the glassware except the heater coil in a base bath solution for at least 1-2 hr.
- Rinse glassware with water and dry in an oven.

Figure 1. (A) Fully assembled custom chemical vapor deposition (CVD) system. (B) Expanded view of the components for the CVD system. Please click here to view a larger version of this figure.
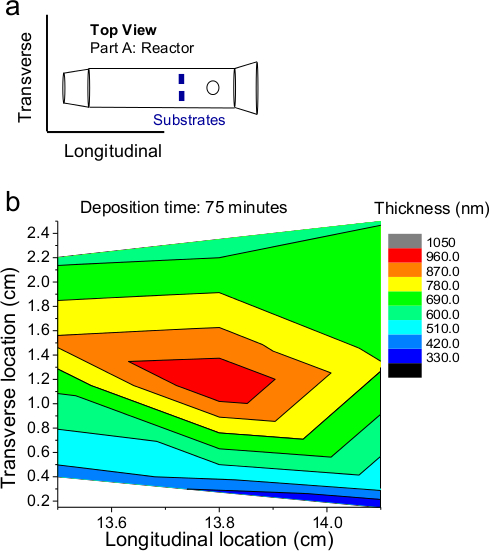
Figure 2. (A) A top view of the substrates in the reactor showing their location. (B) Approximate film thickness as function of position inside the reactor tube, Part A from Figure 1B for a deposition of 75 min. Please click here to view a larger version of this figure.
Results
The first and easiest method for determining if a deposition is successful is to do a visual inspection of the films. The film should appear dark purple with a mirror finish that is uniform across the substrates. If there are spots on the surface of the substrate where there is no V[TCNE]x~2 or it is lighter in color, then this is likely due to the presence of solvents or other impurities on the substrate surface. Additionally the film should be opaque. Unless a thin film was deposited over a short ti...
Discussion
The key parameters for V[TCNE]x~2 deposition include temperature, carrier gas flow, pressure, and ratio of precursors. Because the chemical vapor deposition set-up is not commercially available these parameters will need to be optimized for each system. A previous study by Shima et al. revealed that the temperature has the largest impact on the sublimation rate of the TCNE precursor26. The temperature can be modified both by the value set on the temperature controller and also by m...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by NSF Grant No. DMR-1207243, the NSF MRSEC program (DMR-0820414), DOE Grant No. DE-FG02-03ER46054, and the OSU-Institute for Materials Research. The authors acknowledge the NanoSystems Laboratory at Ohio State University, and technical assistance from C. Y. Kao and C.Y. Chen.
Materials
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Nitrogen Glovebox | Vacuum Atmospheres | Omni | steps done in nitrogen glovebox can also be done in an argon glovebox |
| 1 L three-neck round bottom flask | Corning | 4965A-1L | |
| 500 ml round bottom flask | Sigma Aldrich | 64678 | |
| Turbo vacuum pumping station | Agilent Varian | G8701A-011-037 | |
| Glass Stopcock | Kontes | 185000-2440 | |
| Glass two way connecting tube | Corning | 8940-24 | Corning Pyrex(R) 105 degree Angled Tube Adapter with Two-Way 24/40 Standard Taper Joint |
| Coldfinger | Custom part made by OSU chemistry glass shop | ||
| Argon Glovebox | Vacuum Atmospheres | Nexus I | |
| Hot plate stirrer | Corning | 6795 | |
| Thermoeletric cooler | Advanced Thermoelectric | TCP-50 | |
| Temperature controller | Advanced Thermoelectric | TLZ10 | for TE cooler |
| Power supply | Advanced Thermoelectric | PS-145W-12V | for TE cooler and temperature controller |
| Temperature controller | J-Kem Scientific | Model 150 | For heating coil |
| Heating wire | Pelican Wire Company | Nichrome 60 | |
| Custom glassware pieces | Made by OSU Chemistry glass shop | ||
| Vacuum pump | BOC Edwards | XDS-5 | Connected to the CVD set-up |
| Flow meter | Gilmont | GF-2260 | |
| Micrometer valve | Gilmont | 7300 | Controls flow of argon over TCNE |
| Micrometer valve | Gilmont | 7100 | Controls flow of argon over V(CO)6 |
| Tubing | Tygon | R3603 | 1/8 in walls, connected between valves and meter |
| 3-way Stopcock | Nalgene | 6470 | used to adjust the flow rates |
| Pressure gauge | Matheson | 63-4105 | connects to the top of Figure 1 part A |
| SQUID magnetometer | Quantum Design | MPMS-XL | |
| EPR | Bruker | Elexsys | |
| PPMS | Quantum Design | 14T PPMS | |
| Sourcemeter | Keithely | 2400 | |
| Materials | |||
| Sodium metal | Sigma Aldrich | 262714 | |
| Anthracene | Sigma Aldrich | 141062 | |
| Anhydrous tetrahydrofuran | Sigma Aldrich | 186562 | |
| Vanadium(III) chloride tetrahydrofuran complex | Sigma Aldrich | 395382 | |
| Carbon monoxide gas | OSU stores | 98610 | |
| Tetraethylammonium bromide | Sigma Aldrich | 241059 | |
| Phosphoric acid | Sigma Aldrich | 79622 | |
| Methanol | Sigma Aldrich | 14262 | |
| Silcone oil | Sigma Aldrich | 146153 | |
| Copper pellets | Cut from spare copper wire | ||
| Tetracyanoethylene | Sigma Aldrich | T8809 | |
| Glass slides | Gold Seal | 3010 | |
| Activated Charcoal | Sigma Aldrich | 242276 | |
References
- Yoo, J. W., et al. Spin injection/detection using an organic-based magnetic semiconductor. Nat. Mater. 9, 638-642 (2010).
- Li, B., et al. Room-temperature organic-based spin polarizer. Appl. Phys. Lett. 99, 153503 (2011).
- Li, B., Kao, C. Y., Yoo, J. W., Prigodin, V. N., Epstein, A. J. Magnetoresistance in an All-Organic-Based Spin Valve. Adv. Mater. 23, 3382-3386 (2011).
- Fang, L., et al. Electrical Spin Injection from an Organic-Based Ferrimagnet in a Hybrid Organic-Inorganic Heterostructure. Phys. Rev. Lett. 106, 156602 (2011).
- Yu, H., et al. Ultra-narrow ferromagnetic resonance in organic-based thin films grown via low temperature chemical vapor deposition. Appl. Phys. Lett. 105, 012407 (2014).
- Manriquez, J. M., Yee, G. T., McLean, R. S., Epstein, A. J., Miller, J. S. A Room-Temperature Molecular Organic Based Magnet. Science. 252, 1415-1417 (1991).
- Pokhodnya, K. I., Epstein, A. J., Miller, J. S. . Thin-film V TCNE (x) magnets. Adv. Mater. 12, 410-413 (2000).
- Carlegrim, E., Kanciurzewska, A., Nordblad, P., Fahlman, M. Air-stable organic-based semiconducting room temperature thin film magnet for spintronics applications. Appl. Phys. Lett. 92, 163308 (2008).
- Kao, C. Y., Yoo, J. W., Min, Y., Epstein, A. J. Molecular Layer Deposition of an Organic-Based Magnetic Semiconducting Laminate. ACS Appl. Mater. Interfaces. 4, 137-141 (2012).
- Miller, J. S. Oliver Kahn Lecture: Composition and structure of the V TCNE (x) (TCNE = tetracyanoethylene) room-temperature, organic-based magnet - A personal perspective. Polyhedron. 28, 1596-1605 (2009).
- Haskel, D., et al. Local structural order in the disordered vanadium tetracyanoethylene room-temperature molecule-based magnet. Phys. Rev. B. 70, 054422 (2004).
- Prigodin, V. N., Raju, N. P., Pokhodnya, K. I., Miller, J. S., Epstein, A. J. Spin-Driven Resistance in Organic-Based Magnetic Semiconductor V[TCNE]x. Adv. Mater. 14, 1230-1233 (2002).
- Yoo, J. W., Edelstein, R. S., Lincoln, D. M., Raju, N. P., Epstein, A. J. Photoinduced magnetism and random magnetic anisotropy in organic-based magnetic semiconductor V(TCNE)(x) films, for x similar to 2. Phys. Rev. Lett. 99 (15), 157205 (2007).
- Cimpoesu, F., Frecus, B., Oprea, C. I., Panait, P., Gîrţu, M. A. Disorder, exchange and magnetic anisotropy in the room-temperature molecular magnet V[TCNE]x – A theoretical study. Computational Materials Science. 91, 320-328 (2014).
- Raju, N. P., Prigodin, V. N., Pokhodnya, K. I., Miller, J. S., Epstein, A. J. High field linear magnetoresistance in fully spin-polarized high-temperature organic-based ferrimagnetic semiconductor V(TCNE)(x) films, x similar to 2. Synth. Met. 160, 307-310 (2010).
- Raju, N. P., et al. Anomalous magnetoresistance in high-temperature organic-based magnetic semiconducting V(TCNE)(x) films. J. Appl. Phys. 93, 6799-6801 (2003).
- Yoo, J. W., et al. Multiple photonic responses in films of organic-based magnetic semiconductor V(TCNE)(x), x similar to 2. Phys. Rev. Lett. 97, 247205 (2006).
- Yoo, J. W., Edelstein, R. S., Raju, N. P., Lincoln, D. M., Epstein, A. J. Novel mechanism of photoinduced magnetism in organic-based magnetic semiconductor V(TCNE)(x), x similar to 2. J. Appl. Phys. 103, 07B912 (2008).
- Caro, D., et al. CVD-grown thin films of molecule-based magnets. Chem. Mat. 12, 587-589 (2000).
- Erickson, P. K., Miller, J. S. Thin film Co TCNE (2) and VyCo1-y TCNE (2) magnetic materials. J. Magn. Magn. Mater. 324 (2), 2218-2223 (2012).
- Valade, L., et al. Thin films of molecular materials grown on silicon substrates by chemical vapor deposition and electrodeposition. J. Low Temp. Phys. 142, 393-396 (2006).
- Casellas, H., de Caro, D., Valade, L., Cassoux, P. A new chromium-based molecular magnet grown as a thin film by CVD. Chem. Vapor Depos. 8, 145-147 (2002).
- Barybin, M. V., Pomije, M. K., Ellis, J. E. Highly reduced organometallics - 42. A new method for the syntheses of V(CO)(6) (-) and V(PF3)(6) (-) involving anthracenide mediated reductions of VCl3(THF)(3). Inorg. Chim. Acta. 269, 58-62 (1998).
- Froning, I. H. M., Lu, Y., Epstein, A. J., Johnston-Halperin, E. Thin-film Encapsulation of the Air-Sensitive Organic Ferrimagnet Vanadium Tetracyanoethylene. Appl. Phys. Lett. 106, 122403 (2015).
- Pokhodnya, K. I., Bonner, M., Miller, J. S. Parylene protection coatings for thin film V TCNE (x) room temperature magnets. Chem. Mat. 16, 5114-5119 (2004).
- Shima Edelstein, R., Yoo, J. -. W., Raju, N. P., Bergeson, J. D., Pokhodnya, K. I., Miller, J. S., Epstein, A. J., Tessler, N., Arias, A. C., Burgi, L., Emerson, J. A. . Materials Research Society. , (2005).
- Katz, H. E. Recent advances in semiconductor performance and printing processes for organic transistor-based electronics). Chem. Mat. 16, 4748-4756 (2004).
- Subbarao, S. P., Bahlke, M. E., Kymissis, I. Laboratory Thin-Film Encapsulation of Air-Sensitive Organic Semiconductor Devices. IEEE Trans. Electron Devices. 57, 153-156 (2010).
- Lungenschmied, C., et al. Flexible, long-lived, large-area, organic solar cells. Solar Energy Materials and Solar Cells. 91, 379-384 (2007).
- Lu, Y., et al. Thin-Film Deposition of an Organic Magnet Based on Vanadium Methyl Tricyanoethylenecarboxylate. Adv. Mater. 26, 7632-7636 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved