A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
In Vitro Model of Physiological and Pathological Blood Flow with Application to Investigations of Vascular Cell Remodeling
In This Article
Summary
This protocol replicates physiological or pathological blood flow in vitro to aid in determining cell response in disease pathologies. By introducing a pressure damping chamber downstream of a blood pump, blood flow across the vasculature can be recapitulated and imposed on a monolayer of vascular endothelium or a mimetic co-culture.
Abstract
Vascular disease is a common cause of death within the United States. Herein, we present a method to examine the contribution of flow dynamics towards vascular disease pathologies. Unhealthy arteries often present with wall stiffening, scarring, or partial stenosis which may all affect fluid flow rates, and the magnitude of pulsatile flow, or pulsatility index. Replication of various flow conditions is the result of tuning a flow pressure damping chamber downstream of a blood pump. Introduction of air within a closed flow system allows for a compressible medium to absorb pulsatile pressure from the pump, and therefore vary the pulsatility index. The method described herein is simply reproduced, with highly controllable input, and easily measurable results. Some limitations are recreation of the complex physiological pulse waveform, which is only approximated by the system. Endothelial cells, smooth muscle cells, and fibroblasts are affected by the blood flow through the artery. The dynamic component of blood flow is determined by the cardiac output and arterial wall compliance. Vascular cell mechano-transduction of flow dynamics may trigger cytokine release and cross-talk between cell types within the artery. Co-culture of vascular cells is a more accurate picture reflecting cell-cell interaction on the blood vessel wall and vascular response to mechanical signaling. Contribution of flow dynamics, including the cell response to the dynamic and mean (or steady) components of flow, is therefore an important metric in determining disease pathology and treatment efficacy. Through introducing an in vitro co-culture model and pressure damping downstream of blood pump which produces simulated cardiac output, various arterial disease pathologies may be investigated.
Introduction
Morbidity rates for cardiovascular diseases are the largest in America, with many resulting from unhealthy vasculature. Healthy arteries consist of elastic tissue, with soft luminal surface coated with an endothelial cell (EC) monolayer. Arterial flow may be modeled as an oscillating wave function with positive mean flow rate. The pulsatility index (PI) is the quotient of oscillation magnitude and mean flow (PI = (Max. - Min.) / Mean),1 and has been modeled in vitro with variable vessel elasticity.2 Arterial elasticity is important in storage of flow energy from heart contractions, dilating under systolic pressure, and plays a significant role in modulating blood flow PI. Because the heart maintains a consistent, pulsatile, volumetric flow, arterial expansion increases cross-sectional area, enhancing flow stability by reducing flow velocity, shear stress, and PI. Frequently, unhealthy arteries present changes to elasticity or compliance, displaying stiffening from vascular remodeling, scar tissue or calcification3,4. Additionally, other vascular disorders, such as neointimal hyperplasia (NIH),5 aneurysm and hypertension6 and vascular fibrosis4, may constrict vessel diameter. However, current drug treatment and device treatment of vascular diseases often neglect the importance of vessel wall compliance or blood flow dynamics in vascular disease which is often complicated by changes in vessel morphology and properties. Neither balloon angioplasty nor stenting answer the complication of wall elasticity7. Therefore, in vitro modeling of blood flows resulting from arterial disease and treatments is important in investigating disease pathologies and future efficacy of treatment. Herein, we describe a method of replicating physiological and pathological blood flow designed to determine cell response in vascular disease pathologies. Fluid flow causes shear stress at the vessel wall, which is an important mechanical signal in vessel health, affecting all cells within the vasculature. Several mechanical sensors on the vascular endothelium for fluid shear have been identified, including primary cilium shown in recent studies for endothelial mechanosensing8. Endothelial cell activity and morphology are affected by flow velocity, direction, and pulsatility. Additionally, smooth muscle cell (SMC) migration can be affected by mechano-signals of low velocity flow through interstitial fluid9, and can also be through the paracrine signaling from endothelial cells through their response to flow and mechano-transduction of flow signals via cytokine release10. The “dose” dependence of mean shear, PI, and paracrine signaling may also be interdependent. To this end, the determination of vascular cell response to fluid shear with varied “dosing” in monolayer culture or co-culture in vitro could provide mechanistic insights into vascular remodeling and improve disease and treatment prediction. The flow system used in this experiment consists of a blood pump, an upstream flow damping air reservoir, a downstream flow meter only used during experimental setup, a downstream cell culture, parallel-plate flow chamber, and media reservoir. Control of vascular flow variables such as mean flow rate, beats per minute, and PI may be achieved through controlling flow rate, pulse frequency, and introduction of pressure damping. Pulsatile blood pumps are available with variable stroke displacement, at controlled stroke frequency, relating directly to mean volumetric flow rate, and pulse frequency. Introduction of an air reservoir within the flow circuit allows for pressure damping, reducing flow oscillation magnitude. Media is an incompressible fluid, while air within the damping chamber is compressible, allowing excess pressure from the flow wave to be absorbed by air compression. The air to media ratio allows for control over how much damping occurs. A custom cell culture flow chamber 75 mm in length by 50 mm in width was created from acrylic. Flow enters through the inlet port, and expands through the inlet manifold, providing consistent flow across the entirety of the flow chamber. Similar flow and structures are present at chamber outlet. Cells are seeded onto functionalized slides, and subsequently attached to the flow chamber. This allows for large populations, easily retrieved after the study. Co-culture experiments may use a porous polycarbonate membrane to eliminate cell-to-cell contact between cultures while allowing cytokine/flow transport. This system has previously been used to model high PI flow and its effect on endothelial monolayer culture and EC/SMC co-culture1,10, to investigate cell response to pathologically high PI disease. By describing the protocol used to model these flow conditions, we hope to aid others in determining flow signal contribution to cell response.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Silanization and Biomolecule Functionalization of Slide or Polycarbonate Membrane
Note: Many of the chemicals and solutions within this protocol have high evaporation rates (ethanol (EtOH), acetone, etc.). Other steps entail long incubation times for low evaporation rates. Paraffin film is recommended to seal containers. Caution: Many of the chemicals (including: sulfuric acid, acetone, (3-aminopropyl)triethoxysilane, glutaraldehyde, EtOH) are considered hazardous, or volatile. Consult material safety data sheet (MSDS) of each material for proper storage, handling, and disposal before use.
- Place new glass slide measuring 75 mm by 50 mm by 1 mm, in clean, glass slide staining dish without regard to orientation, ensuring full immersion. Use container for steps 1.1-1.12.
Note: For co-culture, include polycarbonate (PC) membrane with 0.4 micron pore size, cut to size of 75 mm by 50 mm, for all subsequent steps for PC functionalization and EC seeding. - Immerse slide in sulfuric acid (20%), O/N.
Caution: Consult MSDS for sulfuric acid before use. - Wash slide by immersing in deionized water (DI) for 5 min, three times, changing DI each time.
- Immerse slide in acetone for 30 min.
Caution: Consult MSDS for acetone before use. - Immerse slide in 6% (3-aminopropyl)triethoxysilane / acetone solution O/N.
Caution: Consult MSDS for (3-aminopropyl)triethoxysilane and acetone before use. - Immerse slide in acetone for 5 min, three times, changing acetone each time.
Caution: Consult MSDS for acetone before use. - Wash slide by immersing in DI for 5 min, three times, changing DI each time.
- Immerse slide in 1.5% glutaraldehyde / DI solution for 60 min.
Caution: Consult MSDS for glutaraldehyde before use. - Wash slide by immersing in DI for 5 min, two times, changing DI each time.
- Place slides into 70% ethanol (EtOH) for 30 min in sterile, laminar flow hood.
- Remove EtOH and allow remaining residue to evaporate. Immerse slides in sterile DI and drain in order to rehydrate the slides.
Note: Slides or PC membrane may be sealed in glass slide holder and stored at 4 °C up to one week. Upon use for cell culture, repeat steps 1.10 and 1.11. - Remove the slide from the slide holder and place it into a 100 mm by 100 mm square petri dish in the laminar flow hood.
Note: This container will be used for all remaining steps.- Prepare slide or PC for endothelial cell seeding for monoculture experiment.
- Coat the functionalized slide or PC (step 1.11) with 1 ml fibronectin solution (25 μg/ml), on one side, covering the approximate area exposed to cell culture chamber.
- Incubate slides or PC in sterile incubator set at 37 °C for 1-2 hr.
- After incubation, aspirate the remaining solution using a glass aspirator pipette connected to a vacuum.
Note: Slides may now be stored O/N at 4 °C for future use.
- Follow 1.12.2 steps only for preparing EC/SMC co-culture. Prepare and seed smooth muscle cell (SMC) culture for co-culture experiment.
- Place silicone gasket on top of the slide (step 1.11), with dimensions of 75 mm by 50 mm outer diameter, inner diameter of 50 mm by 30 mm, thickness of 0.5 mm, and perforations which align with flow chamber vacuum.
- Prepare 1 ml SMC in 10% FBS/DMEM suspension. Neutralize 1 ml solution of 2 mg/ml collagen type-I with 7% NaHCO3 and 0.1 M NaOH solution to pH of 7.4, and mix with SMC suspension with final cell density of 2 x 106 cells/ml.
- Spread SMC solution within gasket and incubate slides at 37 °C and 5% CO2 for 30 min.
- After incubation, cover the slide with 25 ml 0.1% fetal bovine serum (FBS) mixed in Dulbecco’s Modified Eagle Medium (DMEM) for 72 hr.
- Prepare slide or PC for endothelial cell seeding for monoculture experiment.
- Culture endothelial cells (ECs) on glass slide/polycarbonate membrane.
- Seed 1 ml ECs in 10% FBS/DMEM, with initial concentration of 6.0 x 105 cells/ml, on fibronectin surfaces of glass slide or polycarbonate membrane.
- Incubate cells in an incubator at 37 °C, 5% CO2, and 10% FBS for 120 min.
- Cover slides containing cells with additional 10% FBS/DMEM, and place in incubator in 37 °C, 5% CO2 O/N, until 70%-80% confluent.
2. Determination of Fluid Viscosity and Volumetric Flow Rate
Note: Rotating viscometers are sensitive equipment, and the viscometer user manual should be consulted before calibrating, zeroing, or performing measurements.
- Determine desired fluid shear (τ) from literature of the experiment’s targeted vasculature.
- Measure 1% FBS/DMEM viscosity (μ), using a rotational viscometer.
- Determine required volumetric flow rate (Q) from Poiseuille equation:
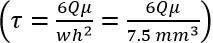 ,
,
where τ = fluid shear, μ = viscosity, Q = vol. flow rate, w = chamber width, and h = chamber height). - Set pump to flow rate derived in step 2.3, and use for all subsequent steps.
3. Determination of Pulsatility Index
Note: All connection ports within the system should be connected with appropriately sized lock-ring-to-barb, or female luer-to-barb connections. Connecting PVC tubing may then be connected to barb fittings, and circuit completed.
- Connect flow circuit as in Figure 2, with damping chamber (Figure 3) and ultrasonic flow meter in correct flow direction.
Note: Ultrasonic flow meter is a sensitive piece of equipment, and the user manual should be consulted before use. - Fill flow circuit and reservoir with DI water, by ensuring reservoir outlet tube (to pump) is submerged within reservoir volume. Visualize flow waveform using a flow meter.
- Open air release valve on the damping chamber to change fluid/air volume ratio. Close air release valve at different fluid level intervals and calculate pulsatility index (PI = (VMax - VMin) / VMean) by using flow waveform peak (VMax), trough (VMin), and mean (VMean). Record PI values in notebook.
- Mark resulting fluid level and PI on the damping chamber, using a felt tipped, permanent ink pen for future use. Determine desired PI levels from pathology literature11.
- Silicon Tube Formation- Optional, more advanced method of controlling pulsatility
- Mix the silicone elastomer base and curing agent at different ratios (e.g., a base-to-crosslinker ratio from 10:1 to 36:1) for varied targeted elastic moduli, as illustrated previously.2
- Fabricate silicone tubes by repeated dip-cure process, briefly dipping steel cannula (14 G) into silicone prepolymer mixture with a predetermined base-to-crosslinker ratio.
- Place the prepolymer-coated cannula into an oven set at 60 °C for 4 hr to cure the polymer coating on the cannula, switching the direction in the middle of curing process.
- Repeat the dipping process with the same silicone elastomer mixture and place back in the oven for additional 4 hr, which results in ~0.3 mm thick tubes.
- Remove the ultrathin silicone tube from the stainless steel cannula.
- Connect tubes to flow circuit instead of damping chamber, and test PI for each as in 3.3.
4. Pump Sterilization
- Immerse the flow chamber (Figure 2), PVC tubing, and gaskets in 10% hydrogen peroxide (H2O2). Wash with sterile DI before step 4.
- Place the blood pump on a spare incubator shelf.
Note: Incubator shelves should be stainless steel, and be able to support the weight of the entire flow circuit. - Fill the flow circuit and reservoir with 10% H2O2 by ensuring reservoir outlet tube (to pump) is submerged within reservoir volume. Circulate H2O2 by pumping through circuit for 20 min in the laminar flow hood with UV light on.
- Aspirate all H2O2 from tubing, and wash with sterile PBS by pumping through flow circuit.
5. Flow Chamber Assembly
Note: The flow chamber consists of an acrylic, custom made plate, with vacuum ports and inlet and outlet flow ports (see Figure 2). Chamber assembly consists of placing the flow chamber and gaskets on top of culture slides, properly aligned, and is described below.
- Warm up 1% FBS/DMEM in a 37 °C water bath and prepare flow chamber.
- Take EC slide out of media (from step 1.13) and place gasket on top with cell side up.
- (Optional) Take PC membrane out of media (from step 1.13) and place gasket on top with cell side up. Place co-culture slide with seeded SMC (step 1.12.2) underneath PC membrane.
- Align vacuum channel of the flow chamber with the holes in the gasket (Figure 2).
- Attach vacuum tube to vacuum ports (Figure 2) ensuring no media leaks to vacuum.
- Place polycarbonate sheet underneath glass slide and clamp flow chamber assembly with spring clamps, one on each side of flow chamber (Figures 2 and 5).
- Place flow system in incubator at 37 °C and 5% CO2, and fill circuit with media by pumping from filled reservoir at low pulsatility. Maintain this flow for 4 hr for preconditioning cells.
- Adjust fluid volume in damping chamber to marked PI level, and culture for desired time.
Access restricted. Please log in or start a trial to view this content.
Results
Maintenance of flow conditions is reliant on correct assembly of the flow circuit (Figure 1). Tubing diameter is an important selection in assembly, with larger diameters reducing flow resistance and subsequent pressure drop before and after the culture chamber. To ensure intended pressure and flow velocity, assemble the system with flow meter before experiment with intended tubing. Alignment of culture chamber vacuum channel (Figure 2), with perforations on silicon gasket (not pictured)...
Access restricted. Please log in or start a trial to view this content.
Discussion
This protocol describes a method of reproducing pulsatile flow in vitro, and may be instrumental first step in determining contribution of flow conditions to disease pathologies. Previous studies using this protocol have found flow conditions contribute to vascular inflammatory response.1,10 Additionally, this protocol is intended for experienced laboratories. As such, neither in depth fluid mechanics, nor biochemical analysis is described herein. For more advanced fluid dynamics,1
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors wish to acknowledge funding sources, including AHA (13GRNT16990019 to W.T) and NHLBI (HL097246 and HL119371 to W.T.).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Acetone | Sigma-Aldrich | 34850 | |
| Sulfuric Acid | Sigma-Aldrich | 320501 | |
| (3-Aminopropyl)triethoxysilane | Sigma-Aldrich | 440140 | |
| Glutaraldehyde Solution | Sigma-Aldrich | G5882 | |
| Ethanol | Sigma-Aldrich | 459844 | |
| Glass Slide (70 mm x 50 mm) | Sigma-Aldrich | CLS294775X50 | |
| Polycarbonate Membrane | Millipore Corp. | HTTP09030 | |
| Silicone Gasket | Grace Bio-Labs | RD 475464 | |
| Fibronectin (25 μg/ml) | Sigma-Aldrich | F1141 | |
| Collagen Type-I | Sigma-Aldrich | C3867 | |
| NaHCO3 | Fluka | 36486 | |
| NaOH | Sigma-Aldrich | S5881 | |
| Damping Chamber | This chamber is custom made, and may be requested using the engineering drawing of Figure 3. | ||
| Blood Pump | Harvard Apparatus | 529552 | |
| Poly-Vinyl Carbonate Tubing | US Plastic | 65066, 65063, 65062 | Various sizes may be required |
| Luer Connections | Nordson Medical | Various | Various sizes will be required, and a number of parts should be purchased for replacement use. |
| Culture Chamber | Machined in-house | Custom | Acrylic may be purchased in sheets and machined for intended use. The engineering drawing shown in Figure 2 may be used to recreate this chamber. |
| Square Petri Dish | Cole-Parmer | EW-14007-10 | |
| Glass Slide Holder | Capitol Scientific | WHE-900303 | |
| Fetal Bovine Serum | Mediatech, Inc. | 35-010-CV | |
| Dulbecco's Modified Eagle Medium | Mediatech, Inc. | 10-013-CV | |
| Flow Meter | Sonotec, GmbH | Sonoflow co.55/060 | |
| Sylgard Elastomer Kit | Sigma-Aldrich | 761036-5EA | |
| 14 G Steel Cannula | General Laboratory Supply | S8365-1 |
References
- Scott-Drechsel, D., Su, Z., Hunter, K., Li, M., Shandas, R., Tan, W. A new flow co-culture system for studying mechanobiology effects of pulse flow waves. Cytotechnology. 64 (6), 649-666 (2012).
- Tan, Y., et al. Stiffening-Induced High Pulsatility Flow Activates Endothelial Inflammation via a TLR2/NF-κB Pathway. PLoS ONE. 9 (7), e102195(2014).
- Wexler, L., et al. Coronary Artery Calcification: Pathophysiology, Epidemiology, Imaging Methods, and Clinical Implications A Statement for Health Professionals From the American Heart Association. Circulation. 94 (5), 1175-1192 (1996).
- Lan, T. -H., Huang, X. -Q., Tan, H. -M. Vascular fibrosis in atherosclerosis. Cardiovasc Pathol. 22 (5), 401-407 (2013).
- Lee, C. H., et al. Promoting endothelial recovery and reducing neointimal hyperplasia using sequential-like release of acetylsalicylic acid and paclitaxel-loaded biodegradable stents. Int J Nanomedicine. 9, 4117-4133 (2014).
- Intengan, H. D., Schiffrin, E. L. Vascular Remodeling in Hypertension Roles of Apoptosis Inflammation, and Fibrosis. Hypertension. 38 (3), 581-587 (2001).
- Greil, O., et al. Changes in carotid artery flow velocities after stent implantation: a fluid dynamics study with laser Doppler anemometry. J Endovasc Ther. 10 (2), 275-284 (2003).
- Egorova, A. D., van der Heiden, K., Poelmann, R. E., Hierck, B. P. Primary cilia as biomechanical sensors in regulating endothelial function. Differentiation. 83 (2), S56-S61 (2012).
- Liu, S. Q., Goldman, J. Role of blood shear stress in the regulation of vascular smooth muscle cell migration. IEEE T Bio-Med Eng. 48 (4), 474-483 (2001).
- Scott, D., Tan, Y., Shandas, R., Stenmark, K. R., Tan, W. High pulsatility flow stimulates smooth muscle cell hypertrophy and contractile protein expression. AJP: Lung C. 304 (1), L70-L81 (2013).
- Panaritis, V., et al. Pulsatility Index of Temporal and Renal Arteries as an Early Finding of Arteriopathy in Diabetic Patients. Ann Vasc Surg. 19 (1), 80-83 (2005).
- Miao, H., et al. Effects of Flow Patterns on the Localization and Expression of VE-Cadherin at Vascular Endothelial Cell Junctions: In vivo and in vitro Investigations. J Vasc Res. 42 (1), 77-89 (2005).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved