A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Controlled Synthesis and Fluorescence Tracking of Highly Uniform Poly(N-isopropylacrylamide) Microgels
In This Article
Summary
Non-stirred precipitation polymerization provides a rapid, reproducible prototyping approach to the synthesis of stimuli-sensitive poly(N-isopropylacrylamide) microgels of narrow size distribution. In this protocol synthesis, light scattering characterization and single particle fluorescence tracking of these microgels in a wide-field microscopy setup are demonstrated.
Abstract
Stimuli-sensitive poly(N-isopropylacrylamide) (PNIPAM) microgels have various prospective practical applications and uses in fundamental research. In this work, we use single particle tracking of fluorescently labeled PNIPAM microgels as a showcase for tuning microgel size by a rapid non-stirred precipitation polymerization procedure. This approach is well suited for prototyping new reaction compositions and conditions or for applications that do not require large amounts of product. Microgel synthesis, particle size and structure determination by dynamic and static light scattering are detailed in the protocol. It is shown that the addition of functional comonomers can have a large influence on the particle nucleation and structure. Single particle tracking by wide-field fluorescence microscopy allows for an investigation of the diffusion of labeled tracer microgels in a concentrated matrix of non-labeled microgels, a system not easily investigated by other methods such as dynamic light scattering.
Introduction
Stimuli-sensitive poly(N-isopropylacrylamide) (PNIPAM) microgels 1,2 have attracted continuous interest over the past two decades due to their potential in various smart applications. Demonstrated use cases include switchable emulsion stabilizers 3-8, microlenses 9, cell culture substrates for easy cell harvesting 10,11, and smart carriers for low molecular weight compounds and other biomedical uses 12. From a fundamental research point of view these particles have been proven to be useful for investigating subjects such as colloidal interactions 13-15 and polymer-solvent interactions 16-18.
Successful use of PNIPAM microgels and their derivatives in any given application typically requires knowledge on the mean particle size and width of the particle size distribution. For the correct interpretation of the experimental results involving PNIPAM microgels, the particle structure, which can be affected by functional comonomers, has to be known. Dynamic and static light scattering (DLS and SLS, respectively) are uniquely suited for acquiring this information because these methods are fast and relatively easy to use; and they probe the particle properties non-invasively in their native environment (dispersion). DLS and SLS also collect data from vast number of particles avoiding the bias arising from small sample sizes, typical for microscopy methods. Therefore, the first aim of this work is to introduce good practice regarding light scattering for practitioners new to colloidal characterization.
Typically, precipitation polymerization is carried out in laboratory scale and finding the right reaction conditions for specific particle properties can be laborious and require many repetitions of the synthesis. In contrast to large batch synthesis, non-stirred precipitation polymerization 19,20 is a rapid procedure in which batches of different reactant composition can be polymerized simultaneously yielding particles of narrow size distribution. Simultaneous polymerization minimizes experimental variation and large output means that right reaction conditions can be found fast for upscaling the reaction. Hence, our second aim is to demonstrate the usefulness of non-stirred precipitation polymerization in prototyping and in applications that do not require a large amount of product.
Different aspects of synthesis and characterization come together in the example of application of fluorescent labeled PNIPAM microgels in colloidal interaction research. Here we use highly accurate single particle tracking to investigate the diffusion of labeled tracer microgels in dispersion of unlabeled matrix microgels over a wide matrix concentration range and resolve the cage effect in concentrated colloidal dispersion. Wide-field fluorescence microscopy is well suited for this purpose as it can characterize the specific behavior of a few tracer molecules among a large number of potentially different matrix species. This is in contrast to techniques such as DLS, SLS and rheology, which measure the ensemble average properties of systems and therefore cannot resolve behavior of small number of probe particles in a large system. Furthermore, in this specific example conventional light scattering methods cannot be utilized also due to high particle concentration, which leads to strong multiple scattering invalidating any standard analysis. Use of automated data processing and statistical methods enable analysis of overall system behavior also for single particle tracking, when averaged over large sample sizes.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Microgel Synthesis
NOTE: N-isopropylacrylamide (NIPAM) was recrystallized from n-hexane. Other reagents were used as received.
- Conventional Batch Synthesis of Poly(NIPAM) Matrix Microgels
- Dissolve 1.8 g NIPAM and 24 mg N,N'-bisacrylamide (BIS) in 245 ml filtered (0.2 µm regenerated cellulose (RC) membrane filter) double distilled water in a 500 ml three-neck round bottom flask equipped with a reflux condenser, a stirrer and a rubber septum.
- Insert a thermometer and a 120 mm needle for the nitrogen input through the septum.
- Heat the solution to 60 °C, while stirring. Deoxygenate the solution by purging with nitrogen for 40 min.
- Simultaneously prepare an initiator solution of 155 mg potassium persulfate (KPS) in 5 ml filtered double-distilled water and bubble the solution with nitrogen to remove oxygen.
- Transfer the complete 5 ml KPS solution in a nitrogen-washed syringe equipped with a 120 mm needle.
- Lift the nitrogen needle above the solution level in the three-neck flask and add the KPS solution rapidly through the rubber septum into the reactor.
- Let the polymerization proceed for 1 hr under nitrogen flow and slow stirring at 60 °C.
- Use a Buchner funnel and filter paper to filter the hot reaction solution in order to discard big aggregates. Let the dispersion to cool down.
- Centrifuge and redisperse the dispersion three times for 40 min at 257,000 x g and finally redisperse the sediment in a minimum viable amount of double distilled water. Typically this is 2-4 ml.
- Lyophilize the dispersion for storing.
- Non-stirred Synthesis of Fluorescently Labeled Poly(NIPAM) Microgels
- Weigh 257.7 mg NIPAM, 3.5 mg BIS, and 1.5 mg methacryloxyethyl thiocarbamoyl rhodamine B (dye) in glass vessel and add 10 ml of filtered double distilled water.
- Ultrasonicate the dye–monomer solution for 15 min to dissolve the dye in water.
- Prepare the same solution without the dye into a separate glass vessel.
- Prepare various dilutions of the monomer solution with the dye using the monomer solution without the dye to obtain a concentration series with various dye concentrations. In this work, use dye in the concentration range of 0.02-0.1 mmol/L.
- Dissolve 8.4 mg KPS in 10 ml filtered double distilled water in order to get the initiator solution.
- Transfer 0.5 ml of the concentration series and 0.5 ml of the KPS solution to test tubes with 10 mm diameter to obtain the final reaction solutions and seal them with rubber septa.
- Preheat an oil bath in a double-walled glass vessel connected to a heating circulator to 63 °C.
- Deoxygenize the reaction solutions by purging with nitrogen through 120 mm needles for 20 min.
- Insert the tubes into a floating platform and immerse the platform into the preheated oil bath. Set the temperature to 60 °C. Initially higher temperature in the bath is necessary as the room temperature solutions cool down the bath. For high precision particle size tuning the temperature control during the initial reaction has to be rigorous, typically ±0.1 °C.
- Let the reaction proceed for an appropriate time. Typically 1 hr is enough.
- Transfer the reaction tubes rapidly to an oven at 60 °C and put one drop of the hot dispersion to 10 ml filtered double distilled water preheated over PNIPAM volume phase transition temperature (VPTT, 32 -34 °C) 1, for DLS characterization in the collapsed state.
- Let the rest of the dispersions cool down to room temperature and transfer them into centrifuge tubes.
- Centrifuge the solution three times for 40 min at 257,000 x g and dilute the microgels finally in 2 ml filtered double distilled water for use as tracer particles.
2. Light Scattering Characterization
- Hydrodynamic Radius Determination in Collapsed State by Dynamic Light Scattering
- Wash cuvettes and glassware with acetone vapor.
- Heat 10 ml of filtered (e.g., 200 nm or smaller RC filter) double distilled water over PNIPAM VPTT.
- Transfer a drop of hot dispersion to the filtered water using a pre-heated needle (0.9 x 40 mm) and syringe (1 ml).
- Temper the DLS goniometer index match bath to 50 °C and transfer the sample to the instrument without letting it cool down.
- Find the largest scattering angle where the scattered intensity is sufficient to acquire a correlogram by performing test measurements.
- Insert sample cuvette (10 mm diameter glass tube with 1 ml of particle dispersion). Move the detector arm to small scattering angle (here 30°).
- Check the beam profile for multiple scattering: no glow around the primary beam, no multiple scattering, etc. Check that the count range is suitable for measurement at the lowest scattering angle (approx. between 30 and 600 kHz; top right corner of the software window.)
- Move the goniometer arm to highest scattering angle (choose 120° here). Check that the count rate is still high enough for the measurement (between 30 and 600 kHz). If the intensity is too low, move the arm to lower scattering angle.
- Check the beam visually through the toluene bath glass at the lowest scattering angle, if glow around the incident beam is observed multiple scattering takes place. In this case, reduce the laser intensity or use a higher dilution.
- Acquire 20 correlograms between the minimum and maximum scattering angle (e.g., 30° - 140°) with minimum acquisition time of 60 sec. Increase the acquisition time for weak intensity large scattering angles if necessary.
- Data Analysis 37
- Calculate scattering vector magnitudes for the scattering angle according to
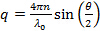 , where n is the refractive index of the dispersion,
, where n is the refractive index of the dispersion, 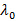 the wavelength of the laser in vacuum and
the wavelength of the laser in vacuum and 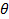 the scattering angle.
the scattering angle. - In the case the measurement software provides the intensity correlation function
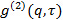 , transform it to electric field correlation function
, transform it to electric field correlation function 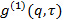 according to
according to  . Parameter
. Parameter 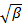 is an uninteresting instrumental parameter related to the degree of spatial coherence of the scattered light over the detector area.
is an uninteresting instrumental parameter related to the degree of spatial coherence of the scattered light over the detector area. - Perform cumulant analysis on correlograms, i.e., fit second order polynomial to the logarithm of each electric field correlation function
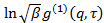 by linear least squares.
by linear least squares. 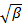 appears as the intercept of the fit and its exact value is unimportant in respect to the data analysis. Restrict the fit to a meaningful lag time τ value, e.g., so that the correlation amplitude is 10 - 20 % of the maximum amplitude. The coefficient of the first order term is the mean decay rate of the correlation function,
appears as the intercept of the fit and its exact value is unimportant in respect to the data analysis. Restrict the fit to a meaningful lag time τ value, e.g., so that the correlation amplitude is 10 - 20 % of the maximum amplitude. The coefficient of the first order term is the mean decay rate of the correlation function, 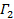 .
. - Find the most likely value for the mean diffusion coefficient
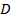 of the particles by linear least squares fit on
of the particles by linear least squares fit on  . If
. If 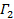 against
against 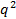 does not appear linear and go through the origin within the error, the particle size distribution is broad and hydrodynamic radius will be poorly defined.
does not appear linear and go through the origin within the error, the particle size distribution is broad and hydrodynamic radius will be poorly defined. - Calculate the mean hydrodynamic radius from the Stokes-Einstein relation
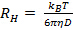 , where
, where 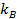 is the Boltzmann coefficient,
is the Boltzmann coefficient, 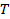 the absolute temperature and
the absolute temperature and 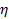 the viscosity of the dispersion at
the viscosity of the dispersion at 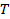 . Propagate the standard deviation of
. Propagate the standard deviation of 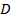 to
to 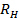 .
.
- Calculate scattering vector magnitudes for the scattering angle according to
- Particle Structure Determination by Static Light Scattering
- Wash cuvettes and glassware with acetone vapor. Use 20 mm diameter or larger cuvettes to minimize the cylindrical lens effect.
- Filter (200 nm RC filter or smaller) approximately 20 ml of double distilled water to a glass vial and transfer a drop of purified dispersion to the vial. Wash the filter with 10 ml water before using it for sample preparation to remove impurities remaining from the manufacturing process.
- Check sample against any ambient light source. If blue hue is observed, the sample is likely to be too concentrated. Dilute accordingly.
- Prepare background water sample by flushing the cuvette multiple times with filtered water and then fill up to appropriate sample volume, depending on the cuvette and the laser position in the instrument. The laser must pass through the sample without being refracted from the meniscus.
- Calibrate the instrument using a toluene sample.
- Measure water scattering (background) throughout the available angular range.
- Measure the scattering intensity from the sample throughout the available angular range preferably at several wavelengths. The scattering pattern normalized to the forward scattering intensity is known as the form factor.
- If particle structure is known, use the appropriate model expression to calculate global fit on the datasets measured at different wavelengths.
- For unknown particle structure use regularized direct (such as FitIt! 33) or a more general indirect inverse Fourier transform 21,22 routine in conjunction with deconvolution of the pair distance distribution function (only for spherical particles) 23,24 for approximate classification of particle type.
- In case the fitting or inversion routine provides an estimate of the particle radius distribution function, calculate the polydispersity index (standard deviation of the distribution divided by its mean).
3. Particle Tracking by Wide-field Fluorescence Microscopy
NOTE: Tracer and matrix particles of 465 ± 7 nm and 405 ± 7 nm hydrodynamic radii at 20 °C, respectively, were used for particle tracking.
- Sample Preparation
- Prepare concentrated matrix microgel dispersion by redispersing known amount of lyophilized unlabeled microgel to known amount of double distilled water. Add a small volume of labeled tracer particles.
- Confirm the appropriate tracer microgel concentration in the microscope. The optimal concentration is a compromise between simultaneous acquisitions of maximum number of tracks, while having the tracer concentration low enough so that the probability that the tracer particle tracks cross during the acquisition is negligible.
- Prepare concentrated dispersions by evaporating water in an oven. Determine weight concentration by comparing the weight of the dispersion to the original weight of the sample before evaporation.
- Data Acquisition and Analysis
- Use an appropriate objective lens of the desired magnification and aperture for excitation of the tracers and simultaneous fluorescence collection from the sample. In this work, use a 100X/1.3 NA oil immersion objective lens.
- Place the moisture chamber onto a xyz-piezo table, which fits into a commercial microscope.
- To prevent the sample from drying, place a plasma cleaned cover slip into the moisture chamber and pipette 10 µl of poly(NIPAM) dispersion of the desired concentration onto the slip.
- Depending on the excitation and emission spectra of the fluorescent dye, use a suitable laser for excitation and adjust the laser power appropriately. The intensity should be sufficiently low to avoid fast photobleaching of the dyes, but at the same time strong enough for accurate single particle positioning (see below). In this work, use a 561 nm diode-pumped solid-state laser and keep the laser power constant at 16 mW (ca. 0.5 kW cm-2 at the sample) for all the measurements.
- To obtain homogeneous sample illumination, use the critical illumination setup described here. For this, couple the laser into a multimode fiber (NA 0.22 ± 0.02, 0.6 mm core diameter), shake the fiber using a vortexer in order to temporally average out laser speckles, and project the fiber end into the sample plane.
- Calibrate the z distance from the back reflection of the cover slip and focus several micrometers into the sample by moving the objective slightly up and fix the z-position using a z-compensator. This avoids any interface effects with the coverslip.
- Adjust the detector parameters, such as exposure time, to the strength of the fluorescence signal. In this case, use an EMCCD camera with exposure time of 0.1 sec, electron multiplying mode and gain of 50.
- Acquire several movies with the appropriate number of frames to obtain adequate lag time to calculate the mean square displacement of the microgels in different regions of the sample. In this work, use acquisition frame numbers of 500 or 1,000 frames.
- Analyze the data by positioning the particles in each frame using Gaussian fitting 25 and use an appropriate particle tracking algorithm 26 to obtain the mean square displacement. 27 Calculate mean values and standard deviation by averaging over all the tracks in all the movies. Calculate the long lag time diffusion coefficients by linear regression from
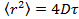 , where
, where 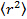 is the mean square displacement, D the mean diffusion coefficient and τ the lag time.
is the mean square displacement, D the mean diffusion coefficient and τ the lag time. - Estimate the anomaly parameter γ from the anomalous diffusion equation
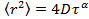 by transforming the data to logarithmic scale, yielding
by transforming the data to logarithmic scale, yielding 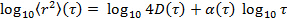 . The anomaly parameter
. The anomaly parameter 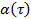 is given by the derivative of the plot. The derivative can be estimated by the finite differences of the data points, or fitting the data points by polynomial functions and differentiating analytically. Determine the sufficient degree of the polynomial fit functions by plotting the fit residuals and residual norm for increasing polynomial order.
is given by the derivative of the plot. The derivative can be estimated by the finite differences of the data points, or fitting the data points by polynomial functions and differentiating analytically. Determine the sufficient degree of the polynomial fit functions by plotting the fit residuals and residual norm for increasing polynomial order. - Repeat the same procedure for different concentrations of the microgel matrices.
Access restricted. Please log in or start a trial to view this content.
Results
The number of PNIPAM microgel particles in the batch, and thus the final particle volume, is determined early in the reaction during the nucleation phase 20 Hydrophobic co-monomer dye methacryloxyethyl thiocarbamoyl rhodamine B influences the nucleation by reducing the particle number density in the batch. The decrease in particle concentration for two different initial NIPAM concentrations can be seen as increase in...
Access restricted. Please log in or start a trial to view this content.
Discussion
Addition of small amounts of functional comonomer can have a significant effect on the particle size and structure of the PNIPAM derived microgels. Simultaneous small-scale test tube polymerization is a good method to account for such changes, and helps to rapidly find the right reactant compositions for target particle size for upscaling the reaction as needed. The mass of the particles is approximately exponentially dependent on the ...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The Deutsche Forschungsgemeinschaft (DFG) is acknowledged for financial support within the Sonderforschungsbereich SFB 985 "Functional Microgels and Microgel Systems".
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Acetone | VWR Chemicals | KRAF13455 | |
| Bisacrylamid | AppliChem | A3636 | |
| n-Hexane | Merck | 104374 | |
| N-Isopropylacrylamide | Fisher Scientific | AC412785000 | recrystallized from n-hexane |
| Methacryloxyethyl thiocarbamoyl rhodamine B | Polysciences | 23591 | |
| Potassium peroxodisulfate | Merck | 105091 | |
| Silicone oil 47 V 350 | VWR Chemicals | 83851 | |
| Toluene | Sigma Aldrich | 244511 | |
| F12 Refrigerated/heating circulator | Julabo | 9116612 | |
| Microscope | Olympus | IX83 | |
| XY(Z) Piezo System | Physik Instrumente | P-545.3R7 | |
| 100X Oil immersion objective | Olympus | UPLSAPO | |
| QuadLine Beamsplitter | AHF Analysentechnik | F68-556T | |
| Cobolt Jive 150 laser | Cobolt | 0561-04-01-0150-300 | |
| Multimode Fiber | Thorlabs | UM22-600 | |
| iXON Ultra 897 EMCCD camera | Andor | DU-897U-CS0-BV | |
| Laser goniometer | SLS Systemtechnik | Mark III | |
| CF40 Cryo-compact circulator | Julabo | 9400340 | |
| Laser goniometer system | ALV GmbH | ALV / CGS-8F | |
| Multi-tau corretator | ALV GmbH | ALV-7004 | |
| Light scattering electronics | ALV GmbH | ALV / LSE 5004 | |
| Photon counting module | PerkinElmer | SPCM-CD2969 | 2 units in pseudo cross-correlation mode |
| 633 nm HeNe Laser | JDS Uniphase | 1145P | |
| F32 Refrigerated/heating circulator | Julabo | 9312632 |
References
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interfac. 85, 1-33 (2000).
- Pich, A., Richtering, W. Microgels by Precipitation Polymerization: Synthesis, Characterization and Functionalization. Adv. Polym. Sci. 234, 1-37 (2010).
- Richtering, W. Responsive Emulsions Stabilized by Stimuli-Sensitive Microgels: Emulsions with Special Non-Pickering Properties. Langmuir. 28 (50), 17218-17229 (2012).
- Wiese, S., Spiess, A. C., Richtering, W. Microgel-Stabilized Smart Emulsions for Biocatalysis. Angew. Chem. Int. Edit. 52 (2), 576-579 (2012).
- Schmitt, V., Ravaine, V. Surface compaction versus stretching in Pickering emulsions stabilised by microgels. Curr. Opin. Colloid In. 18 (6), 532-541 (2013).
- Wellert, S., Richter, M., Hellweg, T., von Klitzing,, R,, Hertle, Y. Responsive Microgels at Surfaces and Interfaces. Z. Phys. Chem. 229 (7-8), 1-26 (2015).
- Li, Z., Harbottle, D., Pensini, E., Ngai, T., Richtering, W., Xu, Z. Fundamental Study of Emulsions Stabilized by Soft and Rigid Particles. Langmuir. 31 (23), 6282-6288 (2015).
- Deshmukh, O. S., van den Ende, D., Stuart, M. C., Mugele, F., Duits, M. H. G. Hard and soft colloids at fluid interfaces: Adsorption, interactions, assembly & rheology. Adv. Colloid Interfac. 222, 215-227 (2015).
- Serpe, M. J., Kim, J., Lyon, L. A. Colloidal Hydrogel Microlenses. Adv. Mater. 16 (2), 184-187 (2004).
- Schmidt, S., Zeiser, M., Hellweg, T., Duschl, C., Fery, A., Möhwald, H. Adhesion and Mechanical Properties of PNIPAM Microgel Films and Their Potential Use as Switchable Cell Culture Substrates. Adv. Func. Mater. 20 (19), 3235-3243 (2010).
- Xia, Y., He, X., et al. Thermoresponsive Microgel Films for Harvesting Cells and Cell Sheets. Biomacromolecules. 14 (10), 3615-3625 (2013).
- Guan, Y., Zhang, Y. PNIPAM microgels for biomedical applications: from dispersed particles to 3D assemblies. Soft Matter. 7 (14), 6375(2011).
- Yunker, P. J., Chen, K., Gratale, M. D., Lohr, M. A., Still, T., Yodh, A. G. Physics in ordered and disordered colloidal matter composed of poly(N-isopropylacrylamide) microgel particles. Rep. Prog. Phys. 77 (5), 056601-056629 (2014).
- Lohr, M. A., Still, T., et al. Vibrational and structural signatures of the crossover between dense glassy and sparse gel-like attractive colloidal packings. Phys. Rev. E. 90 (6), 062305(2014).
- Dreyfus, R., Xu, Y., Still, T., Hough, L. A., Yodh, A. G., Torquato, S. Diagnosing hyperuniformity in two-dimensional, disordered, jammed packings of soft spheres. Phys. Rev. E. 91 (1), 012302-012312 (2015).
- Kojima, H., Tanaka, F. Reentrant volume phase transition of cross-linked poly(N-isopropylacrylamide) gels in mixed solvents of water/methanol. Soft Matter. 8 (10), 3010-3011 (2012).
- Hofmann, C. H., Plamper, F. A., Scherzinger, C., Hietala, S., Richtering, W. Cononsolvency Revisited: Solvent Entrapment by N-Isopropylacrylamide and N, N-Diethylacrylamide Microgels in Different Water/Methanol Mixtures. Macromolecules. 46 (2), 523-532 (2013).
- Bischofberger, I., Calzolari, D. C. E., Trappe, V. Co-nonsolvency of PNiPAM at the transition between solvation mechanisms. Soft Matter. 10 (41), 8288-8295 (2014).
- Virtanen, O. L. J., Richtering, W. Kinetics and particle size control in non-stirred precipitation polymerization of N-isopropylacrylamide. Colloid Polym. Sci. 292 (8), 1743-1756 (2014).
- Virtanen, O. L. J., Ala-Mutka, H. M., Richtering, W. Can the Reaction Mechanism of Radical Solution Polymerization Explain the Microgel Final Particle Volume in Precipitation Polymerization of N-Isopropylacrylamide? Macromol. Chem. Phys. 216 (13), 1431-1440 (2015).
- Glatter, O. A new method for the evaluation of small-angle scattering data. J. Appl. Crystallogr. 10 (5), 415-421 (1977).
- Svergun, D. I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 25 (4), 495-503 (1992).
- Glatter, O. Convolution Square Root of Band-Limited Symmetrical Functions and Its Application to Small-Angle Scattering Data. J. Appl. Crystallogr. 14, 101-108 (1981).
- Glatter, O., Hainisch, B. Improvements in Real-Space Deconvolution of Small-Angle Scattering Data. J. Appl. Crystallogr. 17, 435-441 (1984).
- Cheezum, M. K., Walker, W. F., Guilford, W. H. Quantitative Comparison of Algorithms for Tracking Single Fluorescent Particles. Biophys. J. 81 (4), 2378-2388 (2001).
- Wöll, D., Kölbl, C., Stempfle, B., Karrenbauer, A. A novel method for automatic single molecule tracking of blinking molecules at low intensities. Phys. Chem. Chem. Phys. 15 (17), 6196-6205 (2013).
- Saxton, M. J., Jacobson, K. Single-particle tracking: Applications to membrane dynamics. Annu. Rev. Bioph. Biom. 26, 373-399 (1997).
- Pusey, P. N., van Megen, W. Detection of small polydispersities by photon correlation spectroscopy. J. Chem. Phys. 80 (8), 3513(1984).
- Stieger, M., Pedersen, J. S., Richtering, W., Lindner, P. Small-angle neutron scattering study of structural changes in temperature sensitive microgel colloids. J. Chem. Phys. 120 (13), 6197-6206 (2004).
- Wu, X., Pelton, R. H., Hamielec, A. E., Woods, D. R., McPhee, W. The kinetics of poly(N-isopropylacrylamide) microgel latex formation. Colloid Polym. Sci. 272, 467-477 (1994).
- Weeks, E. R., Weitz, D. A. Subdiffusion and the cage effect studied near the colloidal glass transition. Chem. Phys. 284 (1-2), 361-367 (2002).
- Ernst, D., Köhler, J., Weiss, M. Probing the type of anomalous diffusion with single-particle tracking. Phys. Chem. Chem. Phys. 16 (17), 7686-7691 (2014).
- Virtanen, O. L. J. FitIt! (Version 1.1.4). , Available from: https://www.github.com/ovirtanen/fitit (2015).
- Provencher, S. W. A Constrained Regularization Method For Inverting Data Represented By A Linear Algebraic or Integral Equations. Comput. Phys. Commun. 27 (3), 213-227 (1982).
- Holtzer, L., Meckel, T., Schmidt, T. Nanometric three-dimensional tracking of individual quantum dots in cells. Appl. Phys. Lett. 90 (5), 053902-053904 (2007).
- Diezmann, A. V., Lee, M. Y., Lew, M. D., Moerner, W. E. Correcting field-dependent aberrations with nanoscale accuracy in three-dimensional single-molecule localization microscopy. Optica. 2 (11), 985-989 (2015).
- Neutrons, X-rays and Light: Scattering Methods Applied to Soft Condensed Matter. Lindner, P., Zemb, T. , North Holland Delta Series. Amsterdam. (2002).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved