A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Extraction of Organochlorine Pesticides from Plastic Pellets and Plastic Type Analysis
In This Article
Summary
Microplastics act as vector of potentially toxic organic contaminants with unpredictable effects. This protocol describes an alternative methodology for assessing the levels of organochlorine pesticides adsorbed on plastic pellets and identifying the polymer chemical structure. The focus is on pressurized fluid extraction and attenuated total reflectance Fourier transform infrared spectroscopy.
Abstract
Plastic resin pellets, categorized as microplastics (≤5 mm in diameter), are small granules that can be unintentionally released to the environment during manufacturing and transport. Because of their environmental persistence, they are widely distributed in the oceans and on beaches all over the world. They can act as a vector of potentially toxic organic compounds (e.g., polychlorinated biphenyls) and might consequently negatively affect marine organisms. Their possible impacts along the food chain are not yet well understood. In order to assess the hazards associated with the occurrence of plastic pellets in the marine environment, it is necessary to develop methodologies that allow for rapid determination of associated organic contaminant levels. The present protocol describes the different steps required for sampling resin pellets, analyzing adsorbed organochlorine pesticides (OCPs) and identifying the plastic type. The focus is on the extraction of OCPs from plastic pellets by means of a pressurized fluid extractor (PFE) and on the polymer chemical analysis applying Fourier Transform-InfraRed (FT-IR) spectroscopy. The developed methodology focuses on 11 OCPs and related compounds, including dichlorodiphenyltrichloroethane (DDT) and its two main metabolites, lindane and two production isomers, as well as the two biologically active isomers of technical endosulfan. This protocol constitutes a simple and rapid alternative to existing methodology for evaluating the concentration of organic contaminants adsorbed on plastic pieces.
Introduction
Global production of plastics is continuously rising since the 1950's to reach 311 million tons in 2014 with about 40% used in packaging1. In parallel, increasing quantities of these materials are accumulating in the environment, which might pose a serious threat to the ecosystems2. Although already reported in the 1970's, the occurrence of plastic debris in the marine environment has only received a greater attention in the past decade. Especially microplastics, plastic fragments with a diameter of ≤ 5 mm, are now recognized as one of the main marine water quality issues3.
Plastic resin pellets are small granules generally in the shape of a cylinder or a disk and with a diameter of a few mm (e.g., 2 to 5 mm)4,5. They fall in the category of microplastics. These plastic granules are industrial raw material from which final plastic products are manufactured through re-melting and molding at high temperature6. They can be unintentionally released to the environment during manufacturing and transport. For instance, they can be directly introduced to the ocean through accidental spills during shipping4,7,8. They can be carried from land to oceans by surface run-off, streams and rivers. Because of their environmental persistence, plastic pellets are widely distributed in the oceans and found on beaches all over the world4. They can negatively affect marine organisms and can enter the food chain, where their effects are unpredictable6,7. Furthermore, several studies have revealed the presence of environmental contaminants adsorbed onto plastic pellets collected in a coastal environment, which act as vector of these potentially toxic chemicals4,9,10. In fact, there is laboratory evidence suggesting that these chemicals can bioaccumulate in tissues of organisms after being released from ingested plastic fragments11,12.
In order to better assess the hazards associated with the occurrence of plastic pellets in the marine environment, it is necessary to develop methodologies that can determine sorbed organic contaminants. An important step is the extraction of the chemicals from the plastic matrices, which can present heterogeneous physical-chemical characteristics depending on the polymer type, its degradation stage, and pre-treatments. Most of the investigations reported in the literature use maceration or Soxhlet techniques4,5,6,9,13,14,15,16,17,18, which are solvent and/or time consuming. Regarding the growing interest for this issue, alternatives should be developed, for a faster evaluation of organic contaminants adsorbed on plastic pieces. In addition, plastic chemical analysis provides information about the chemical structure of the microplastics. As a result, the predominant types of polymers and copolymers present in the environment can be evaluated. Although plastic fragments are usually made of polyethylene (PE) and polypropylene (PP)5, some sampling locations can present a particular profile where other categories are significantly represented (e.g., ethylene/vinyl acetate copolymer and polystyrene (PS)). FT-IR spectroscopy is a reliable and user-friendly technique for polymer identification commonly used to identify microplastics19,20.
The main aim of the present work is to offer a rapid and simple option for extracting OCPs and related compounds from plastic pellets by means of a PFE. However, the design of the protocol includes all steps leading to the determination of sorbed OCPs, from the sampling of the resin pellets to the analysis of the compounds. The method of identifying the plastic type is also described. The developed methodology focuses on 11 OCPs and related compounds: i) DDT (2,4'- and 4,4'-dichlorodiphenyltrichloroethane) and its two main metabolites DDE (2,4'- and 4,4'-dichlorodiphenyldichloroethylene) and DDD (2,4'- and 4,4'-dichlorodiphenyldichloroethane); ii) the isomer gamma-hexachlorocyclohexane (γ-HCH) as the main ingredient of the pesticide lindane and the two isomers α-HCH and β-HCH released during its production15; iii) and the two biologically active isomers endosulfan I (Endo I) and II (Endo II) present in the technical endosulfan. The studied pesticides are broad-spectrum insecticides, chemically stable, hydrophobic, and classified as persistent organic pollutants (POPs) by the Stockholm Convention21.
Protocol
1. Plastic Pellet Sampling
- Before going to the field, triple rinse all required sampling materials (e.g., tweezers and aluminum foil) with acetone or ethanol (99%). In case the material cannot be solvent-rinsed, heat it at 450 °C overnight in an oven (e.g., glassware).
NOTE: In tourist areas, obtain information about possible beach cleaning activities that would remove most of the marine litter including microplastics. If possible, plan the sampling ahead of this operation. If sampling during the clean-up season, specify the details of this activity in the identity form (e.g., dates, clean-up method used, etc.) - Wearing gloves, collect plastic pellets from the beach with solvent-rinsed stainless steel tweezers.
- Sample 50 to 100 pellets per location, which corresponds to 5 to 10 replicates per location (10 pellets per replicate). If the required number of pellets cannot be obtained, collect the maximum pellets possible and specify it in the identity beach form.
- At the end of the sampling, wrap the collected pellets in solvent-rinsed aluminum foil. Glass bottles can be used as an alternative or even paper bags.
- Fill in the identity form of the selected beach with the missing information (i.e., beach location, weather conditions, details on pellets, etc.).
- Transport the samples to the laboratory in an icebox if the ambient temperature exceeds 25 °C. This step can be skipped in the case of short trips (e.g., <1 h).
- Once in the laboratory, gently wipe off removable particles (e.g., sand) of the pellets. Dry the samples if necessary in a desiccator prior to storage (darkness, T <25 °C). Avoid rooms where OCPs might be in use (e.g., storage of standard solutions).
- Store the pellets in the fridge (4 °C) for short periods (i.e., few days) or in the freezer (-18 °C) for longer periods in solvent-rinsed aluminum foil.
- Avoid exposure of samples to artificial light or sunlight. Handle the samples as little as possible before analysis to decrease the risk of contamination.
2. Extraction of OCPs from Plastic Pellets
- To reduce the risk of contamination, work in a clean laboratory using carefully washed glassware as follows: 2 rinses with analytical-grade acetone, dichloromethane and n-hexane. Dry the glassware under nitrogen flow and protect from contact with ambient air (e.g., cover with cleaned aluminum foil). Apply this cleaning procedure in the further steps of the protocol (i.e., sections 3 and 4).
- Using solvent-rinsed tweezers, sort the pellets by color in the following categories: white/transparent, whitish/yellowish, yellow/orange, amber/brown, and pigmented (e.g., red, green, blue, etc.)
- Gather 10 pellets of similar color randomly (i.e., plastic type not considered), which will constitute one replicate.
- Weigh the sample on an analytical balance and record the mass. At this stage, the samples can be put back in the fridge or freezer.
- To take into account the background contamination, perform a blank sample with each set of replicates (e.g., 1 blank for 5 replicates). To this end, apply the same protocol as described above, but do not add plastic pellets in the extraction cell. This blank sample will undergo the further steps of the protocol and be analyzed together with the samples.
- Switch on the PFE. Download the extraction method and warm-up the instrument to 60 °C. The details of the method are as follows:
- Set the temperature to 60 °C and the pressure to 100 bar.
- Select one cycle with heat-up time of 1 min, a hold time of 25 min, and a discharge time of 2 min.
- Set the solvent and gas (N2) flush times to 3 min each.
- Select n-hexane as the extraction solvent.
- While the instrument is warming up, prepare the extraction cell as described below. If necessary, adapt the protocol to the supplier's instructions of your instrument:
- Place the bottom filter and the frit in the extraction cell. Close it and turn it over.
- Fill approximately half the cell with cleaned quartz sand using a funnel.
- Add the weighed sample (i.e., one replicate of 10 pellets). Frozen plastic pellets should be placed in the fridge overnight prior to extraction.
- Add quartz sand up to 1 cm from the top of the cell. Take special care to use ultra clean quartz sand (or alternatively glass beads) since it is exposed to the same extraction conditions as the samples. To clean the sand, successively extract it in the PFE in analytical-grade dichloromethane and n-hexane, applying 2 or more cycles per solvent (e.g., 30 min at 100 °C under 100 bar). Alternatively, use an ultrasonic bath and/or rotating evaporator. Repeat the cleaning procedure, if necessary.
- Insert the top filter in the cell and place the cell in the instrument.
- Place the collecting vessels in the instrument and start the extraction method (total run of about 35 min).
- When the method is completed, empty the extraction cell in a cleaned glass vessel (e.g., beaker, glass cell-culture dish) and retrieve the 10 pellets in the sand. Store them in a container until further analysis for plastic identification (e.g., zip bag or glass vial).
3. Concentration and Clean-up of the Extract
- Transfer the obtained extract (about 40 mL) from the collecting vessel to a glass tube and evaporate it to 1 mL in a rotating concentrator set to 35 °C for 20 min. Alternative methods could be used such as evaporation under nitrogen flow or rotating evaporator. The temperature and duration should be optimized accordingly.
- In the meantime, prepare the solid-phase extractor (SPE) by placing a waste tube in the rack and a cartridge filled with activated magnesium silicate sorbent (1 g) on the manifold in the close-valve position. The clean-up is based on the EPA method 3620C22 as follows:
- Turn the vacuum on at the source and add 4 mL of hexane in the cartridge to activate the sorbent.
- Open the valve and let the solvent pass through the entire sorbent bed. Then, close the valve and allow the sorbent to soak in hexane for 5 min.
- Open the valve and let the solvent pass through, but close the valve before the sorbent dries off.
- When the sample is concentrated, transfer it to the cartridge with a glass Pasteur pipette. Gently open the valve and let it pass through slowly. 1-2 drops per second is an appropriate speed.
- Rinse the glass tube containing the extract with 0.5 mL of hexane and add it to the cartridge when the extract has passed through.
- When the entire solvent has passed through, close the valve and turn off the vacuum.
- Replace the waste tube with a collecting tube and use a clean solvent guide needle.
- Add 9 mL of acetone/hexane (10/90, v/v) to the cartridge and turn on the vacuum at the source. Allow the sorbent to soak in the solvent for 1 min.
- Open the valve and collect the entire eluate in the collecting tube.
- Place the collecting tube in the concentrator and evaporate the solvent for 9 min at 35 °C in order to reach 1 mL of eluate.
- Transfer the concentrated eluate into an amber autosampler vial with a glass Pasteur pipette. At this stage, the samples can be stored in the freezer prior to analysis.
4. Analysis of the Cleaned and Concentrated Extract
- Download the analytical method on the control software of the GC-μECD instrument (gas chromatograph equipped with a micro electron capture detector). The details of the method are as follows:
- Set the injector to splitless mode, its temperature to 250 °C, and the purge time to 1 min.
- Set the flow of the carrier gas (He) to 1.5 mL min-1.
- Program the column oven with the following temperature gradient: 60 °C hold for 1 min, ramp of 30 °C min-1 to 200 °C, ramp of 5 °C min-1 to 230 °C, ramp of 3 °C min-1 to reach 250 °C, hold this temperature for 5 min.
- Set the detector temperature to 300 °C and the back-up gas flow (N2) to 60 mL min-1.
- Place the vial containing the sample (cleaned and concentrated) in the autosampler rack and run the method (run time of 23.3 min). Inject 2 µL of sample.
- After the analysis, identify the different compounds on the chromatogram by their retention times and record the corresponding peak areas.
- Taking into account the recoveries (R) and the peak areas (A1), calculate the concentration (C1) of each OCP in the extract using the equations of the calibration curves as follows:
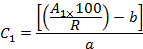
where b is the intercept at the origin and a is the slope of the calibration equation,
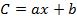
- Taking into account the mass (m) of the replicate (i.e., 10 pellets; see section 2.4) and the volume (V) of the final extract (i.e., 1 mL), calculate the concentration (C2) of each OCP adsorbed on the plastic pellets (i.e., ng of OCP per g of plastic pellet):
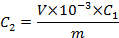
5. Plastic Type Identification
- Transfer the pellets in a glass Petri dish and place it in a plastic bag.
- Hold one pellet with tweezers and cut a slice of the pellet with a scalpel. The plastic bag prevents the loss of pellets during the cutting process.
- Clean the attenuated total reflectance (ATR) crystal of the FT-IR instrument with ethanol.
- Record a background spectrum.
- Place the fragment on the ATR crystal and screw the sample holder. The inner side of the piece must be in contact with the crystal.
- Scan the sample and record the spectrum.
- Identify the polymer constituting the plastic pellet by comparing the obtained spectrum to a spectra library. Although more time-consuming, the interpretation of the obtained spectra could be carried out manually as well, but most probably without reaching the degree of specificity achieved with a library search.
Results
Plastic pellets are usually found along the high and low tide lines of sandy beaches (Figure 1A). They can also stick to seagrass freshly stranded on beaches, after a storm for instance. They can occasionally be found on pebble and stony beaches in accumulation areas of stranded material.
Plastic pellets are usually easily recognizable by their shape, size and color as shown in ...
Discussion
Most studies focusing on organic contaminants associated to plastic pellets have relied on classical extraction methods of the adsorbed chemicals. The Soxhlet apparatus is the most widely used technique with typical extraction times ranging from 12 to 24 h and with high consumption of organic solvents (i.e., from 100 to 250 mL per extraction)23. Maceration extractions require a long contact time between the sample and the organic solvent (e.g., 6 days)4 an...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by IPA Adriatic Cross-border Cooperation Program 2007-2013, within the DeFishGear project (1°str/00010).
Materials
| Name | Company | Catalog Number | Comments |
| Alpha–HCH | Dr. Ehrenstorfer, Augsburg, Germany | DRE-C14071000 | H301, H351, H400, H410, H312 |
| Beta–HCH | Fluka, Sigma-Aldrich, St. Louis, USA | 33376-100MG | H301, H312, H351, H410 |
| Lindane | Fluka, Sigma-Aldrich, St. Louis, USA | 45548-250MG | H301, H312, H332, H362, H410 |
| Endosufan I | Supleco, Sigma-Aldrich Bellefonte, PA, USA | 48576-25MG | H301, H410 |
| Endosulfan II | Supleco, Sigma-Aldrich, Bellefonte, PA, USA | 48578-25MG | H301, H410 |
| 2,4'–DDD | Fluka, Sigma-Aldrich, St. Louis, USA | 35485-250MG | H351 |
| 4,4’–DDD | Dr. Ehrenstorfer, Augsburg, Germany | DRE-C12031000 | H301, H351, H400, H410, H312 |
| 2,4’–DDE | Dr. Ehrenstorfer, Augsburg, Germany | DRE-C12040000 | H351, H400, H410, H302 |
| 4,4’-DDE | Fluka , Sigma-Aldrich, St. Louis, USA | 35487-250MG | H302, H351, H410 |
| 2,4’–DDT | Dr. Ehrenstorfer, Augsburg, Germany | DRE-C12081000 | H301, H311, H330, H351, H400, H410 |
| 4,4’–DDT | National Institute of Standards and Technology, Gaithersburg, USA | RM8469-4,4'-DDT | H301, H311, H351, H372, H410 |
| n-Hexane | VWR International GmbH, Graumanngasse, Viena, Austria | 83992.320 | H225, H315, H336, H373, H304, H411 |
| Acetone for HPLC | J.T.Baker, Avantor performance Materials B.V., Teugseweg, Netherlands | 8142 | H225, H319, H 336 |
| FL-PR Florisil 1000mg/6mL | Phenomenex, Torrance, CA, USA | 8B-S013-JCH | |
| Fat free quartz sand 0.3-0.9 mm | Buchi, Flawil, Switzerland | 37689 | |
| Gas chromatograph Hawlett Packard HP 6890 Series gas chromatograph with GERSTEL MultiPurpose Sampler MPS 2XL with ECD and FID detector | Agilent technologies, Santa Clara USA | ||
| Presure fluid extractor, Speed Extractor E-916 | Buchi, Flawil, Switzerland | ||
| Solid phase extractor | Supleco, Sigma-Aldrich Bellefonte, PA, USA | ||
| Concentrator miVac DUO | Genevac SP Scientific, Suffolk UK | ||
| GC capillary column Zebron ZB-XLB (30 x 0.25 x 0.25) | Phenomenex, Torrance, CA, USA | 122-1232 | |
| ATR FT-IR Spectrometer, Spectrum-Two | Perkin Elmer |
References
- Plastic Europe. . Plastics - the Facts 2015. An analysis of European plastics production, demand and waste data. , (2017).
- Wang, J., Tan, Z., Peng, J., Qiu, Q., Li, M. The behaviors of microplastics in the marine environment. Mar Environ Res. 113, 7-17 (2016).
- UNEP. . Marine plastic debris and microplastics - Global lessons and research to inspire action and guide policy change. , (2016).
- Ogata, Y., et al. International Pellet Watch: Global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar Pollut Bull. 58 (10), 1437-1446 (2009).
- Andrady, A. L. Microplastics in the marine environment. Mar Pollut Bull. 62 (8), 1596-1605 (2011).
- Antunes, J. C., Frias, J. G. L., Micaelo, A. C., Sobral, P. Resin pellets from beaches of the Portuguese coast and adsorbed persistent organic pollutants. Estuarine Coastal Shelf Sci. 130, 62-69 (2013).
- Cole, M., Lindeque, P., Halsband, C., Galloway, T. S. Microplastics as contaminants in the marine environment: A review. Mar Pollut Bull. 62 (12), 2588-2597 (2011).
- Takada, H. Call for pellets! International Pellet Watch Global Monitoring of POPs using beached plastic resin pellets. Mar Pollut Bull. 52 (12), 1547-1548 (2006).
- Teuten, E. L. Transport and release of chemicals from plastics to the environment and to wildlife. Phil Trans R Soc B. 364, 2027-2045 (2009).
- Heskett, M., et al. Measurement of persistent organic pollutants (POPs) in plastic resin pellets from remote islands: Toward establishment of background concentrations for International Pellet Watch. Mar Pollut Bull. 64 (2), 445-448 (2012).
- Besseling, E., Wegner, A., Foekema, E., Van Den Heuvel-Greve, M., Koelmans, A. A. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ Sci Technol. 47 (1), 593-600 (2013).
- Rochman, C. M., Hoh, E., Kurobe, T. The SJ Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci Rep. 3, 3263 (2013).
- Endo, S., et al. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Mar Pollut Bull. 50 (10), 1103-1114 (2005).
- Frias, J. P. G. L., Sobral, P., Ferreira, A. M. Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar Pollut Bull. 60 (11), 1988-1992 (2010).
- Karapanagioti, H. K., Endo, S., Ogata, Y., Takada, H. Diffuse pollution by persistent organic pollutants as measured in plastic pellets sampled from various beaches in Greece. Mar Pollut Bull. 62 (2), 312-317 (2011).
- Mizukawa, K., et al. Monitoring of a wide range of organic micropollutants on the Portuguese coast using plastic resin pellets. Mar Pollut Bull. 70 (1-2), 296-302 (2013).
- Gauquie, J., Devriese, L., Robbens, J., De Witte, B. A qualitative screening and quantitative measurement of organic contaminants on different types of marine plastic debris. Chemosphere. 138, 348-356 (2015).
- Yeo, B. G., et al. POPs monitoring in Australia and New-Zealand using plastic resin pellets, and International Pellet Watch as a tool for education and raising public awareness on plastic debris and POPs. Mar Pollut Bull. 101 (1), 137-145 (2015).
- Kovač Viršek, M., Palatinus, A., Koren, &. #. 3. 5. 2. ;., Peterlin, M., Horvat, P., Kržan, A. Protocol for microplastics sampling on the sea surface and sample analysis. J Vis Exp. (118), e55161 (2016).
- Löder, M. G. J., Kuczera, M., Mintenig, S., Lorenz, C., Gerdts, G. Focal plane array detector- based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ Chem. 12 (5), 563-581 (2015).
- . . Stockholm Convention on Persistent Organic Pollutants (POPs) as amended in 2009 . , (2017).
- EPA - Environmental protection Agency. . Method 3620C: Florisil Cleanup, part of Test Methods for Evaluating Solid Waste, Physical/Chemical Methods (2014). , (2017).
- Hirai, H., et al. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar Pollut Bull. 62 (8), 1683-1692 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved