A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Hydrodynamic Renal Pelvis Injection for Non-viral Expression of Proteins in the Kidney
In This Article
Summary
This protocol describes a method to inject plasmid DNA into the mouse kidney via the renal pelvis to produce transgene expression specifically in the kidney.
Abstract
Hydrodynamic injection creates a local, high-pressure environment to transfect various tissues with plasmid DNA and other substances. Hydrodynamic tail vein injection, for example, is a well-established method by which the liver can be transfected. This manuscript describes an application of hydrodynamic principles by injection of the mouse kidney directly with plasmid DNA for kidney-specific gene expression. Mice are anesthetized and the kidney is exposed by a flank incision followed by a fast injection of a plasmid DNA-containing solution directly into the renal pelvis. The needle is kept in place for ten seconds and the incision site is sutured. The following day, live animal imaging, Western blot, or immunohistochemistry may be used to assay gene expression, or other assays suited to the transgene of choice are used for detection of the protein of interest. Published methods to prolong gene expression include transposon-mediated transgene integration and cyclophosphamide treatment to inhibit the immune response to the transgene.
Introduction
The hydrodynamic tail vein injection technique has become a commonly used way to achieve high levels of gene expression in mouse liver1,2. The kidneys are also transfected by this technique at a much lower level, approximately 100-fold less3. The hydrodynamic renal pelvis injection described here provides a simple way to control the specificity of organ expression through physical means using the same hydrodynamic principles that have been established previously in liver4,5, muscle6, and other organs7,8. This method transfects cells in live animals in vivo by using pressure and speed to force fluid containing DNA into the cells, simultaneously inducing damage to the organ that is quickly resolved9. Using well-established surgical techniques to visualize the kidney via a flank incision10 along with a single injection by insulin syringe, we have found successful transfection of various types of kidney cells, mainly interstitial fibroblasts, tubules, and collecting duct11. Dissection of these mice has shown that other organs are not transfected at levels high enough to visualize by luciferase imaging techniques11. Since the technique is non-viral, use of plasmid DNA for transfection permits fast and easy preparation of the reagents required for injection.
We have used localized hydrodynamic injections to express the antioxidant glutathione S-transferase A4, the insulin-like growth factor-1 receptor, and the hormone erythropoietin in the kidney, all with the expected biological effects11,12,13. Detailed evaluation of route of administration, injection volume, DNA dosage, and promoter choice has been performed11. Additionally, both the piggyBac transposon system and/or cyclophosphamide treatment to suppress the immune reaction to the transgene have been shown to improve long-term gene expression outcomes11. Other investigators have used a renal vein approach in rat with success, achieving high transfection efficiency for time periods of greater than one month14. However, genetic correction of phenotypes mimicking human disease are usually performed in mice first as a proof-of-concept since most mammalian genetic models are mouse models. We compared renal vein injection to renal pelvis injection and found that injection into the renal pelvis was superior to the renal vein for gene expression (approximately ten-fold higher) and survival11. The renal pelvis is an ideal route of entry into the kidney because it is flexible enough to tolerate fluctuations in urine production and is often able to maintain its structural integrity even when dilated during hydronephrosis. Additionally, injection into the renal pelvis allowed access to the kidney without piercing the kidney capsule, allowing the injected fluid to be visibly retained by the kidney better than intraparenchymal injection. Other mouse organs do not have a route of entry other than the vasculature, but the urinary space of the kidney is an ideal injection site. Additionally, injection into the renal vein resulted in leakage of blood into the abdominal cavity. The total kidney volume of wild-type mouse kidneys has been estimated by magnetic resonance imaging to be approximately 0.2 cm3, so the volume of a single kidney is approximately equal to the amount of fluid injected by renal pelvis hydrodynamic injection (100 µL)15. Herein, we have made available all of the detailed nuances of the hydrodynamic renal pelvis injection protocol to achieve reproducible transfection of the kidney.
Protocol
All methods described here have been approved by the Institutional Animal Care and Use Committees (IACUCs) of Baylor College of Medicine and Vanderbilt University Medical Center.
1. Prepare the DNA solution for injection
- Select the plasmid(s) to express the transgene(s) carefully to maximize the desirable characteristics to improve transfection efficiency and transgene expression.
NOTE: Hydrodynamic renal pelvis injection of plasmids to express the fluorescent marker TdTomato, the hormone erythropoietin (Epo), and firefly luciferase have been previously described11. If there is not a well-developed assay for transfection efficiency for the transgene, perform live animal imaging of luciferase by introducing 10 µg of luciferase-expressing plasmid such as enhanced firefly luciferase to the diluted DNA for injection.- Choose the smallest plasmid that is practical to the application.
NOTE: Small plasmids generally transfect cells better than large plasmids. For example, the zeomycin resistance gene and R6K origin of replication are small, so utilizing these elements in the plasmid backbone will decrease plasmid size. - Express the transgene from a mammalian promoter.
NOTE: The kidney cell type transfected, length of gene expression, and strength of gene expression will all be affected by the promoter choice. See Woodard et al. for a comparison of cytomegalovirus (CMV; constitutive viral), elongation factor-1 alpha (EF-1α; constitutive endogenous), gamma-glutamyl transferase (γGT; tubule-specific), and podocin (NPHS2; podocyte-specific)11. - Employ a non-viral integrating system such as transposons if higher long-term gene expression levels are desirable11,16. For studies with early readouts less than 5 days post-injection, integration of the transgene is unnecessary.
- Choose the smallest plasmid that is practical to the application.
- Prepare plasmid DNA for injection using a commercial method such as an endotoxin-free maxiprep kit. Carefully avoid introducing endotoxins via labware.
NOTE: Presence of endotoxins in the DNA solution will elicit a severe immune response in the subject animal, compromising the experiment and possibly killing the animal.- After the last DNA isolation wash step is complete, make sure no ethanol is left by visual inspection and odor. Use gel-loading tips to remove visible drops. Dry the tubes containing the DNA pellet upside down on laboratory delicate task wiper tissues at room temperature or in a 37-60 °C oven with constant monitoring. Resuspend the pellet immediately after residual ethanol has evaporated to prevent overdrying of the pellet.
NOTE: Residual ethanol interferes with the spectrophotometer reading of the DNA concentration and introduction to the kidney is not desirable. - Resuspend the DNA pellet in the same buffer as will be used for injection (hydrodynamic delivery buffer; 100-300 µL) to limit variation in the chemical composition of the DNA buffer solution between groups.
NOTE: Do not resuspend the DNA pellet in the elution buffer solution that comes with the kit. The chelating agent EDTA commonly found in "TE" buffer preparations may affect kidney and cardiovascular function. Depending on pellet size, elute in 100-300 µL of hydrodynamic delivery buffer to achieve a high concentration. The final concentration of the fully diluted DNA will be between 100 ng/µL (10 µg/mouse dose) and 500 ng/µL (50 µg/mouse dose) so the stock DNA concentration should exceed this. - Fully resuspend the DNA pellet in buffer. Leave the pellet in hydrodynamic delivery buffer in the original tube at room temperature for at least one hour or overnight at 4 °C prior to transferring to a sterile microfuge tube to ensure that the DNA is completely dissolved.
- Read the plasmid DNA concentration on a spectrophotometer or by another established method.
NOTE: Readings should be done in duplicate with additional replicates if necessary. If the 260/280 ratio is below 1.8, the preparation is contaminated with RNA or another substance so discard it and prepare the DNA again. - Store plasmid DNA resuspended in injection buffer at -20 °C inside a box in a manual defrost freezer to avoid degradation of the DNA.
NOTE: Stored this way, the DNA preparation will last for many years. Check the integrity of the DNA by restriction digest and agarose gel electrophoresis. DNA that is smeared down the lane has degraded and cannot be used while DNA that produces crisp bands of the correct size can be used for injection.
- After the last DNA isolation wash step is complete, make sure no ethanol is left by visual inspection and odor. Use gel-loading tips to remove visible drops. Dry the tubes containing the DNA pellet upside down on laboratory delicate task wiper tissues at room temperature or in a 37-60 °C oven with constant monitoring. Resuspend the pellet immediately after residual ethanol has evaporated to prevent overdrying of the pellet.
- Prepare the diluted plasmid DNA solution for injection into the renal pelvis.
NOTE: Consider control groups carefully. For many studies, naïve and buffer injected-only controls are included because the injection itself causes damage and may affect the experimental outcome11.- Defrost the endotoxin-free DNA eluted in hydrodynamic delivery buffer at room temperature on the bench.
- Calculate the amount of DNA needed for the injections and prepare the diluted DNA for each group. Administer 10-50 µg of plasmid DNA brought to a total volume of 100 µL with hydrodynamic delivery buffer per mouse.
NOTE: For an example, the calculations to inject 3 mice with 10 µg/mouse of a DNA plasmid that has a concentration of 0.5 µg/µL are as follows.- Prepare enough DNA solution to have approximately 20% extra for loading the syringes and dead space in the needle. For 3 mice, prepare a mix at 3.5x. Calculate the mass of DNA in µg that should be in the solution. Here it would be 10 µg x 3.5 = 35 µg.
- Calculate the total volume of solution desired for injecting 100 µL per mouse. In this case, it would be 100 µL x 3.5 = 350 µL total volume.
- Calculate the volume of stock plasmid DNA to add to the solution. In this case, it would be 35 µg / 0.5 µg/µL = 70 µL of stock plasmid DNA.
- Calculate the volume of buffer to add to the diluted DNA solution by subtracting the volume of stock DNA added (step 1.3.2.3) from the total volume of diluted DNA that is desired (step 1.3.2.2). In this case, it would be 350 µL - 70 µL = 280 µL hydrodynamic delivery buffer.
- Prepare solutions in sterile microfuge tubes using commercially available filter pipette tips with sterile technique on the lab bench.
NOTE: DNA solutions may be prepared and stored at room temperature for injections set to occur that day. Do not inject mice with a cold solution as this will lower their body temperature, but warming is unnecessary.
2. Perform the hydrodynamic renal pelvis injection surgery
- Select mice carefully for surgery.
NOTE: Strain-specific differences have not yet been observed but may be possible. Most injections have been on the C57BL/6 or FVB backgrounds. Renal pelvis injections work best in mice that are 6-12 weeks old. In mice greater than 16 weeks, up to a 50% failure rate by luciferase imaging is possible for unclear reasons. The same age-related failure rate has been observed for hydrodynamic tail vein injections to the liver so this may be a general limitation relating to the principle of hydrodynamic injection. Along the same lines, others have shown that hydrodynamic tail vein injection into fibrotic rat liver is not as effective as healthy liver, so it may be possible that in settings of renal fibrosis the renal pelvis hydrodynamic injection will not be as effective, but this has not been tested directly17. - Prepare mice and DNA syringes for surgery.
- Anesthetize the mice with ketamine and xylazine.
- Put on the correct personal protective equipment required by the animal facility, such as disposable lab apron, surgical face mask, and nitrile gloves.
- Working with 2-4 mice at one time, weigh each mouse in a 500 mL plastic beaker on a scale that is accurate to 0.1 g. Calculate the correct amount of 24 mg/mL ketamine and 2 mg/mL xylazine diluted in normal 0.9% saline to administer to each mouse by intraperitoneal injection (see referenced video for more on intraperitoneal injection)18. Use the formula (50 µL + ((5 µL) x (weight (g))), or alternatively calculate according to another formula after consultation with the local veterinarian team and IACUC.
- Inject the mouse by intraperitoneal injection by standard techniques. Place the mouse in a paper bucket until the mouse is immobile.
NOTE: Mice are ready for surgery when they no longer respond to the toe-pinch test. Give mice that continue to respond to the toe-pinch test 20-30 min after the initial injection 20-60 µL more anesthetic, depending on the strength of the response. - Place the mouse on a water-circulated heat pad covered with a blue pad and place vet ointment in both eyes to prevent corneal desiccation.
- Shave the left side of the back of the mouse from shoulder to rump and flank to spine with a shaver designed for pet grooming. Remove loose hair and debris.
- Prepare a separate syringe containing 100 µL of diluted DNA for each anesthetized mouse, making sure there are no air bubbles.
- Use a sterile 29G x ½" 0.5 mL U-100 insulin syringe with a permanently attached needle without a safety.
NOTE: The syringe type is of critical importance. This gauge allows for a fast injection. The permanently attached needle prevents the solution from leaking out. Presence of a safety will impede access to the renal pelvis. The syringes specified in the Materials Table glide more evenly during injection than other brands tested. - Load the syringe to ~120 µL and pull the plunger down to create a space at the top. Tap with a pen until all air bubbles rise to the top. Holding the needle up, carefully depress the plunger to remove all air until a droplet forms at the tip of the needle. There should not be any visible bubbles present, as these may cause an air embolism that will kill the animal.
- Finish depressing the plunger until there is 100 µL in the syringe by emptying excess DNA solution into the original microfuge tube. Carefully label if necessary and place the loaded syringes on a sterile drape to maintain sterility if the facility where work is being performed does not allow recapping of needles.
- Use a sterile 29G x ½" 0.5 mL U-100 insulin syringe with a permanently attached needle without a safety.
- Anesthetize the mice with ketamine and xylazine.
- Perform injection surgery.
- Prepare the site for surgery. Place a sterile drape over the heat pad and empty sterile surgical tools onto the sterile drape without touching them. Pick up the mouse and place it in the field of view. Adjust lighting to illuminate the area.
- Remove three 3.15% chlorhexidine gluconate and 70% isopropyl alcohol skin antiseptic swabs from the packages and place near the animal.
- Change into sterile surgical gloves. Working in a circular motion beginning at the incision site, swab the animal with a new chlorhexidine/alcohol swab three times.
- Locate the incision site as shown in Figure 1A. Pinching the skin with tweezers, use scissors to make a cut to the skin layer approximately 1 cm from the spine and below the ribcage. Once the cut site is approximately 1 cm long, make a similar cut site below, in the muscle layer.
NOTE: Make the incision the right length to allow the kidney to just barely come through the incision and then be kept in place by the incision itself. Too small an incision and the kidney cannot be exposed; too large and the kidney will continually slip back into the abdominal cavity. - Locate the kidney.
NOTE: It may be visible amongst white adipose tissue. The spleen is also located on the left side of the animal. The color of these organs can be visually differentiated, as the spleen is a dark maroon while the kidney is a dark red-orange. It is not desirable to manipulate the spleen as it can be easily ruptured. - Without touching the kidney, gently expose it from the abdominal cavity by putting steady, gentle pressure on the abdomen with the fingers (Figure 2C). Use closed tweezers to gently push undesired organs back into the abdomen if necessary. Do not use open forceps as this may damage the kidney or other organs.
- Once the kidney is out of the abdomen, gently separate it from the surrounding fat just enough to visualize the renal pelvis, a small white dot (Figure 1B). Push excess fat to one side or remove if necessary. Be sure not to remove the adrenal gland, located near the top pole of the kidney, or the kidney capsule.
- Using closed forceps to push down on the right side of the kidney so that the renal pelvis remains in view, grasp the loaded insulin syringe with the right hand and hold it parallel to the working surface with the needle pointed at the renal pelvis (Figure 2E). Insert the needle carefully into the renal pelvis of the immobilized kidney as shown in Figure 1B.
- Inject the solution quickly within three s. About one-third of the kidney may clear and change color to white.
NOTE: It is common to observe fluid buildup in the kidney capsule following injection as well as formation of a hematoma. Some damage is necessary to achieve a sufficient level of DNA transfection for detection. - Keep the needle in place for approximately 10 s to prevent backflow of the solution. Then carefully and slowly remove the needle. Return the organ to the abdominal cavity by gently stretching the incision site and using closed forceps.
- Suture the muscle layer of the animal with 5-0 absorbable sutures, placing 2-4 independent knots.
- Suture the skin layer of the animal with 5-0 or 6-0 non-absorbable nylon sutures, placing 2-4 independent knots.
NOTE: Surgical tools may be reused after placing in a sterile bead bath and cooled down.
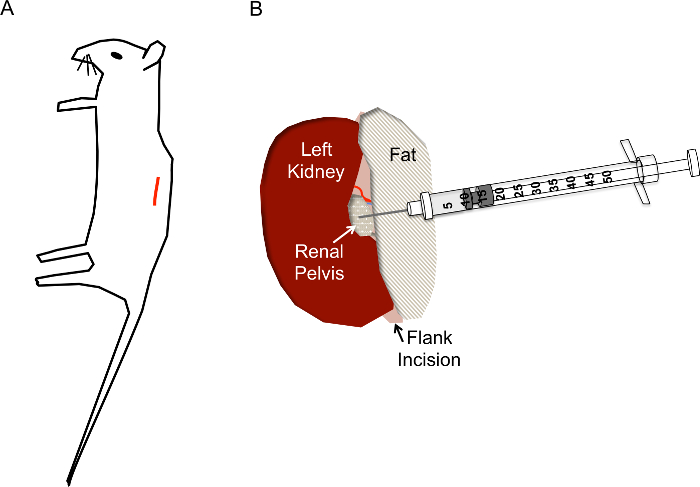
Figure 1. Correct incision site and needle placement for hydrodynamic renal pelvis injections. A) The incision (red line) should be located approximately 1 cm from the spine and approximately 1 cm below the ribcage of the mouse. B) After the kidney is exposed via the flank incision, the renal pelvis should be located as a small yellowish clear/white dot midway down the kidney. The injection should not disturb the renal vein, renal artery, or ureter. The needle of the insulin syringe is inserted directly into the renal pelvis as shown to a depth of approximately 0.5 cm and quickly depressed in 2-3 s. Please click here to view a larger version of this figure.
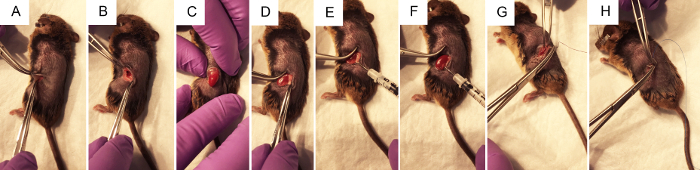
Figure 2. The surgical steps to perform renal pelvis hydrodynamic injection of plasmid DNA. A) Forceps pinch the skin to allow the surgeon to make a ~1 cm flank incision with a scalpel, first through the skin layer, then through the muscle layer. B) Using two pairs of closed forceps to open the surgical wound, the kidney is visualized within the abdomen if possible. C) With gentle pressure on the abdomen, without touching any organs directly, the kidney is exposed through the flank incision. D) Fat is gently dissected from the kidney, disturbing it as little as possible to achieve access to the renal pelvis. E) Pressing on the right side of the left kidney to better visualize the renal pelvis, the syringe is held with the thumb on the depressor and the needle is carefully but firmly placed into the renal pelvis. F) Following the <3 s injection, clearing may be observed in the areas of the kidney that received the bulk of the injection. G) Sterile purple vicryl absorbable sutures are used to make 2-4 independent knots in the muscle layer. H) Sterile blue nylon non-absorbable sutures are used to make 2-4 independent knots in the skin layer. Please click here to view a larger version of this figure.
- Recovery from surgery and post-operative monitoring.
- Provide mice with analgesic according to the institution's IACUC requirements for pain control. For example, administer buprenorphine by subcutaneous injection every 8-12 h for 48 h post-operatively if NSAID-related kidney damage is to be avoided for the study.
- Keep the mice on a heat pad and attended by the investigator until fully mobile with sternal recumbency. When fully recovered, return the mice to a clean cage containing a small amount of bedding from their home cage to reduce stress. Do not house mice subjected to surgery with naïve mice.
NOTE: The condition of the mice will improve quickly, appearing to have normal behavior within 24-48 h. - Give saline fluids and increased analgesia to mice that appear stressed to improve their condition. If mice do not quickly recover, measure the mouse weight, and euthanize mice that have lost greater than 20% of their initial body weight. Euthanize mice appearing uremic or otherwise moribund. Possible health problems are detailed in Table 1.
NOTE: Mice sometimes remove their outer sutures or the sutures come loose prior to wound closure. Poor suturing of the muscle layer with an intact skin layer can result in a hernia at the incision site, indicated by bulging. In such cases, place the mouse under anesthesia (isoflurane is faster than ketamine/xylazine) and repair the surgical site with new sutures as soon as possible. If non-absorbable skin sutures remain after 10-14 days, remove them. Occasionally mice may remove their cage-mates' sutures or fight. Remove aggressor mice to their own cage with enrichment to prevent them from hurting the other mice as soon as such behavior is identified. - When health or experimental endpoints are reached, euthanize the mice by carbon dioxide inhalation or isoflurane overdose followed by a secondary method of euthanasia such as cervical dislocation according to the Standard Operating Procedure for euthanasia in place at the institution
| Cause | Onset | Number of mice affected | Symptoms | Immediate action | Long-term solution | ||
| DNA contained endotoxins | 6-40 h post-injection. | Likely to affect every mouse given the contaminated DNA preparation. | Breathing problems, signs of severe pain, organ failure, death. | Euthanize the affected mice. Test each injected component for endotoxins with Limulus Amebocyte Lysate. | Use endotoxin-free maxiprep kits and only sterile and new or NaOH-treated labware. Use a commercially purchased, endotoxin-tested buffer to dilute the DNA. | ||
| Anesthesia overdose | During surgery, either before or after DNA injection. | May affect only the younger, smaller, or leaner mice. | Cease respiration while on heating pad, urination. | Decrease anesthesia for remaining mice. | Double-check preparation and protocol for dosage accuracy. Exclude “runts.” Consult veterinarian if appropriate dose was used. | ||
| Air bubble in the syringe or needle used for injection | Immediately following renal pelvis injection. | Unless all syringes were loaded carelessly, this only affects one mouse. | Gasping for breath. | Check remaining syringes for signs of air bubbles. | Prepare syringes carefully, tapping the syringe to remove bubbles at the bottom. Improve lighting conditions to better visualize bubbles. | ||
| Opening of surgical site | 12-72 h post-injection | One or more mice. Sometimes the entire cage. | Gaping wound, usually no other distress | Repeat suturing to repair the wound carefully under isoflurane anesthesia with sterile technique. May need to irrigate with saline or remove wound borders with scissors. | If all mice have very short or missing sutures, there may be one mouse removing them, so mice may be separated. Improve suturing technique. Use independent knots. | ||
| Hernia at surgical site | 48+ h post-injection | One or more mice. | Mound is visible at surgical site. | Under isoflurane anesthesia with sterile technique, cut the healed skin to reveal hernia of muscle layer. Replace organs in the peritoneum, repair muscle layer with absorbable sutures and close the site. | This indicates poor suturing of muscle layer. Improve suturing technique. Use independent knots. | ||
| Kidney failure | 48+ h post-injection | One or more mice. | Weight loss of >20%, possibly becoming uremic, hunched posture | Provide saline and increase or prolong analgesia. If no improvement is observed, sacrifice the affected mice. | Alter the disease state of the animal to make it less severe. Alter the transgene to be less strong or inducible. Inject mice at an earlier timepoint in disease progression. | ||
| Abcess or infection | Days to weeks following surgery | One or more mice. | Palpable abcess or signs of sepsis | Euthanize the affected mice. Request necropsy to confirm suspected infection. | This may occur when the surgical conditions and injections are not sufficiently sterile. The procedure shown is for mice with a normal immune system but further precautions must be taken in the setting of immunocompromised animals such as those treated with cyclophosphamide. | ||
Table 1. Table of potential health problems encountered during the renal pelvis injection protocol. Although the listed health problems are not common, there are a number of investigator-related errors that can occur during the course of the procedure. This table may be of aid in prevention and diagnosis of the health problems, as well as for implementation of potential remedies to prevent such problems from occurring in the future. With practice, investigators should expect infrequent health problems and mortality due to the procedure.
3. Assess injection efficiency and transgene effects
- Use a well-developed assay for the desired transgene to assess the transfection efficiency. Use positive and negative controls carefully to make sure the assay is specific.
NOTE: Depending on the promoter choice, mice will likely have the strongest transgene expression on Day 1; always within the first week following injection. - Perform live animal imaging of luciferase by introducing 10 µg of luciferase-expressing plasmid such as enhanced firefly luciferase to the diluted DNA for injection.
- Clean all surfaces exposed to mice. Place the first cage of mice into the chamber and seal it shut. Ensure there is enough isoflurane for the experiment by checking the gage; if not, add isoflurane until the level reaches the "max" line. Initiate the flow of isoflurane by turning the isoflurane dial to 3.5 and the oxygen to 2.
- Inject each mouse by intraperitoneal injection with 100 µL of thawed 30 mg/mL luciferin. Record the time.
- Open the software to control the imaging machine by clicking on the orange and yellow mouse icon. Under "Select/Add User ID," choose the correct initials from the drop-down menu next to "User ID." Click "OK." On the top menu bar, click "Acquisition" and select "Autosave To…" from the drop-down menu. Select the folder or create a new folder to save the data to.
- Go to the image acquisition box in the lower right-hand corner. Check that the automatic settings are as follows: Binning, medium; F/Stop, 1 for luminescent, 8 for photograph; Emission Filter, open; Imaging Mode, checks next to Luminescent, Photograph, Overlay, and Alignment only. Click "initialize" to initialize the system and wait for initialization to finish, then change the "Field of View" to "E" to image five mice.
- Turn the valve for the anesthesia tubing such that the nosecones inside the machine are administering isoflurane. Place the animals inside the imaging machine in the desired order on their stomachs with their backs facing the camera above to visualize the kidney. If more than one cage is to be imaged, place the next cage into the isoflurane chamber.
- 5-10 min following luciferin injection, press "Acquire" to take the image.
- On the "Tool Palette" on the right-hand side, click "Image Adjust." Under "Color Scale," decrease the "Min" Bar until expression is evident in all expected mice, visualized as purple over each mouse kidney. If any mice do not show expression, reinject the mice with 100 µL of luciferin and wait at least 3 min before reacquiring.
- If the image has saturated pixels, decrease the exposure time and reacquire to obtain a quantitative image as the presence of saturated pixels will result in underestimating the transfection efficiency. The lowest possible exposure time is 0.5 s.
- If the image shows only faint gene expression at the first exposure time, increase the exposure time up to 2 min and reacquire. Each time the image is retaken, the data are autosaved in the chosen folder so all images are available for further analysis later.
- Repeat imaging steps for subsequent cages of mice.
- Remove the mice from the imaging machine. Turn off the gas. Clean the chamber, nosecones, and imaging stage. Close the computer program.
NOTE: The software will ask to "Save Dataset." This refers to the manipulations made to the dataset, not the data itself.- Click "Yes" to save changes to the scale and regions of interest (ROIs). Click "No" to revert back to the original settings for the images.
- For data analysis, open the software. On the "Tool Palette" click "ROI Tools." Click the circle icon and click "1" in the drop-down menu to place a new ROI in the image window as indicated by a red oval with four squares around it. Adjust the size of the ROI by placing the mouse cursor over a red square and dragging to enclose the purple area overlaying the injected kidney.
- With the cursor over a red square on the ROI, right-click then select "Duplicate ROI" to create a new ROI in the image window and move it to the next mouse. Repeat the procedure until all mice in the image have an ROI over the injected kidney.
- In the menu directly above the image, change "Units" from "Counts" to "Radiance (Photons)." To export the ROI data, in the "Tool Palette", click "ROI Tools" then click on the pencil/ruler icon to create a spreadsheet of the measurements. Use the "Avg Radiance" measurement in further analysis in the data analysis and statistics program of choice.
- Perform staining of sections as described11.
NOTE: The injection does not transfect the entire kidney equally so different sections will capture different transfection efficiencies11. Co-injection of beads such as fluorescent latex microspheres may help identify the regions expected to be transgene-positive (Figure 3D)11. Optimization of the staining protocol is extremely important. - Perform Western blot to identify the gene of interest in the transfected kidney.
NOTE: Harvest of organs for protein extracts must be done on ice. Again, do not use a small slice of the organ as this area may not have been transfected well. Transfection is unevenly distributed throughout the organ so combine different areas and pool them. Renal pelvis hydrodynamic injection of a plasmid producing the hormone erythropoietin resulted in a modest rise in the hematocrit, so it is expected that secreted transgene products should be found in the circulation11. - For long-term high-level expression of transgenes, combine an integrating vector system with immunosuppressive agents such as cyclophosphamide. The immune response to the transgene occurs over the first several weeks following injection and is robust11.
Results
The surgery and injection technique are simple to perform once mastered, requiring no major equipment or expensive materials. If new to flank-incision kidney surgery, one training day on several mice scheduled for euthanasia should be allowed in which the mice are not recovered following surgery because the first attempt at this surgery may take much longer than normal. Alternatively, investigators familiar with similar techniques may find it quite simple. Carefully follow the illustratio...
Discussion
In this protocol a robust method for achieving reproducible gene expression specifically in the kidney is described. In the hands of a moderately experienced surgeon we have found the percentage of mice transfected by this technique to be in the range of 50-100%, depending on mouse age and the sensitivity of the readout of the transgene. The level of luciferase gene expression was above background for several months in mice receiving piggyBac transposons and completely maintained for several weeks in immunocompr...
Disclosures
The authors have nothing to disclose and declare no conflict of interest.
Acknowledgements
A Career Development Award from the Department of Veterans Affairs [BX002797] supported L.E.W. and the National Institutes of Health [R01-DK095867] and American Heart Association [15GRNT25700209] supported J.C. The National Institutes of Health [DK093660], Department of Veterans Affairs [BX002190], and the Vanderbilt Center for Kidney Disease supported M.H.W. This material is the result of work supported with resources and use of facilities at the VA Tennessee Valley Healthcare System.
Materials
| Name | Company | Catalog Number | Comments |
| AnaSed Xylazine | Patterson Veterinary | 07-808-1947 | Anesthetic - Not controlled substance |
| BD Insulin Syringe 0.5 mL 29G 1/2 Inch | Cardinal Health | 309306 | Required syringes |
| Buprenex | Pharmacist/Veterinarian | Analgesia - Controlled Substance | |
| Dynarex Disposable Towel Drape | Thermo Fisher Scientific | 19-310-671 | Place over heat pad |
| EndoFree Plasmid Maxi Kit | Qiagen | 12362 | Use only endotoxin-free plasmid DNA |
| Endosafe Gel-Clot LAL Rapid Positive Control | Charles River | PC200 | Positive control for endotoxin test |
| Endosafe Gel-Clot LAL Rapid Single Test Vial | Charles River | R13500 | Endotoxin test |
| Extra Fine Micro Dissecting Scissors | Roboz Surgical Instrument | RS-5882 | Surgical tool |
| Fisherbrand Instant Sealing Sterilization Pouch - 9" | Thermo Fisher Scientific | 01-812-51 | For autoclaving surgical tools |
| Gaymar Heat Pump | Paragon Medical | TP-700 | Water-circulating heat pump |
| Germinator 500 | Roboz Surgical Instrument | DS-401 | To reuse surgical tools during surgery |
| Graefe Forceps | Roboz Surgical Instrument | RS-5136 | Surgical tool |
| Graefe Tissue Forceps | Roboz Surgical Instrument | RS-5153 | Surgical tool |
| Halsey Needle Holder, 5" Length | Roboz Surgical Instrument | RS-7841 | Surgical tool |
| Heat pads - 15" x 21" - need at least 3 | Paragon Medical | TP22G | For use with Gaymar Heat Pump |
| IsoFlo (Isoflurane, USP) | Abbott Animal Health | 5260-04-05 | For imaging and euthanasia |
| Isotec Isoflurane Delivery System Vaporizor | Smiths Medical | VCT3K2 | For imaging and euthanasia |
| Ketamine | Pharmacist/Veterinarian | Anesthetic - Controlled Substance | |
| Kimwipes | Kimberly-Clark Professional | 34120 | Laboratory tissues |
| Living Image software | Caliper Life Sciences | For live animal imaging | |
| Luciferin | Perkin Elmer | 122796 | For live animal imaging |
| Nanodrop 2000 | Thermo Scientific | ND-2000-US-CAN | Spectrophotometer for DNA measurement |
| Prevantics Swabs | Thermo Fisher Scientific | 23-100-110 | For skin surgery prep |
| Prolene 5-0 sutures Taper 30" | Thermo Fisher Scientific | NC0256891 | Non-absorbable sutures for skin |
| Puralube Brand Opthalmic Ointment | Patterson Veterinary | 07-888-2572 | To keep eyes moist during surgery |
| Trans IT - QR Hydrodynamic Delivery Solution | Mirus Bio | MIR-5240 | Hydrodynamic delivery buffer for diluting DNA |
| Vicryl 5-0 Sutures J303H | Thermo Fisher Scientific | NC9816710 | Absorbable sutures for muscle layer |
| Wahl Mini Arco Clipper | Med-Vet International | 8787-1550 | Shaver for skin prep |
| Xenogen IVIS 200 | Caliper Life Sciences | For live animal imaging |
References
- Liu, F., Song, Y., Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 6 (7), 1258-1266 (1999).
- Zhang, G., Budker, V., Wolff, J. A. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Human Gene Therapy. 10, 1735-1737 (1999).
- Fumoto, S., Nishimura, K., Nishida, K., Kawakami, S. Three-Dimensional Imaging of the Intracellular Fate of Plasmid DNA and Transgene Expression: ZsGreen1 and Tissue Clearing Method CUBIC Are an Optimal Combination for Multicolor Deep Imaging in Murine Tissues. PLoS One. 11 (1), e0148233 (2016).
- Yoshino, H., Hashizume, K., Kobayashi, E. Naked plasmid DNA transfer to the porcine liver using rapid injection with large volume. Gene Ther. 13 (24), 1696-1702 (2006).
- Kamimura, K., Suda, T., Xu, W., Zhang, G., Liu, D. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol Ther. 17 (3), 491-499 (2009).
- Kamimura, K., Zhang, G., Liu, D. Image-guided,intravascular hydrodynamic gene delivery to skeletal muscle in pigs. Mol Ther. 18 (1), 93-100 (2010).
- Suda, T., Liu, D. Hydrodynamic gene delivery: its principles and applications. Mol.Ther. 15 (12), 2063-2069 (2007).
- Alino, S. F., et al. Naked DNA delivery to whole pig cardiac tissue by coronary sinus retrograde injection employing non-invasive catheterization. J Gene Med. 12 (11), 920-926 (2010).
- Suda, T., Gao, X., Stolz, D. B., Liu, D. Structural impact of hydrodynamic injection on mouse liver. Gene Ther. 14 (2), 129-137 (2007).
- Skrypnyk, N. I., Harris, R. C., de Caestecker, M. P. Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. J Vis Exp. (78), (2013).
- Woodard, L. E., et al. Kidney-specific transposon-mediated gene transfer in vivo. Sci Rep. 7, 44904 (2017).
- Liang, A., et al. Loss of glutathione S-transferase A4 accelerates obstruction-induced tubule damage and renal fibrosis. Journal of Pathology. 228 (4), 448-458 (2012).
- Liang, M., et al. Protective role of insulin-like growth factor-1 receptor in endothelial cells against unilateral ureteral obstruction-induced renal fibrosis. Am J Pathol. 185 (5), 1234-1250 (2015).
- Corridon, P. R., et al. A method to facilitate and monitor expression of exogenous genes in the rat kidney using plasmid and viral vectors. Am J Physiol Renal Physiol. 304 (9), F1217-F1229 (2013).
- Wallace, D. P., et al. Tracking kidney volume in mice with polycystic kidney disease by magnetic resonance imaging. Kidney Int. 73 (6), 778-781 (2008).
- Woodard, L. E., Wilson, M. H. piggyBac-ing models and new therapeutic strategies. Trends Biotechnol. 33 (9), 525-533 (2015).
- Yeikilis, R., et al. Hydrodynamics based transfection in normal and fibrotic rats. World J Gastroenterol. 12 (38), 6149-6155 (2006).
- Thalhofer, C. J., et al. In vivo imaging of transgenic Leishmania parasites in a live host. J Vis Exp. (41), (2010).
- Wooddell, C. I., et al. Muscle damage after delivery of naked plasmid DNA into skeletal muscles is batch dependent. Hum Gene Ther. 22 (2), 225-235 (2011).
- Crespo, A., et al. Hydrodynamic liver gene transfer mechanism involves transient sinusoidal blood stasis and massive hepatocyte endocytic vesicles. Gene Ther. 12 (11), 927-935 (2005).
- Rocca, C. J., Ur, S. N., Harrison, F., Cherqui, S. rAAV9 combined with renal vein injection is optimal for kidney-targeted gene delivery: conclusion of a comparative study. Gene Ther. 21 (6), 618-628 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved