A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Microcontroller Operated Device for the Generation of Liquid Extracts from Conventional Cigarette Smoke and Electronic Cigarette Aerosol
In This Article
Summary
Here, we describe a programmable laboratory device that can be used to create extracts of conventional cigarette smoke and electronic cigarette aerosol. This method provides a useful tool for making direct comparisons between conventional cigarettes and electronic cigarettes, and is an accessible entry point into electronic cigarette research.
Abstract
Electronic cigarettes are the most popular tobacco product among middle and high schoolers and are the most popular alternative tobacco product among adults. High quality, reproducible research on the consequences of electronic cigarette use is essential for understanding emerging public health concerns and crafting evidence based regulatory policy. While a growing number of papers discuss electronic cigarettes, there is little consistency in methods across groups and very little consensus on results. Here, we describe a programmable laboratory device that can be used to create extracts of conventional cigarette smoke and electronic cigarette aerosol. This protocol details instructions for the assembly and operation of said device, and demonstrates the use of the generated extract in two sample applications: an in vitro cell viability assay and gas-chromatography mass-spectrometry. This method provides a tool for making direct comparisons between conventional cigarettes and electronic cigarettes, and is an accessible entry point into electronic cigarette research.
Introduction
Despite a concentrated effort by health organizations, tobacco product use remains the leading cause of preventable death worldwide, with the majority of these deaths attributed to cigarette smoking1. Since entering the market in 2003, electronic cigarettes have been growing in popularity among tobacco product users. Currently, electronic cigarettes are the most popular alternative to conventional cigarettes among American adults (~5%)2 and the most popular nicotine delivery system among middle (~5.3%) and high schoolers (~16%)3. If current trends continue, electronic cigarettes can be expected to replace conventional cigarettes for future generations. However, the health consequences of electronic cigarette use remain unclear.
Research on electronic cigarettes did not start in earnest until electronic cigarette popularity rapidly increased in 20133,4. Since that time, a number of different models have been employed to address the question of their toxicity. However, the results of many studies are conflicting, and while it seems that electronic cigarettes are generally less toxic than conventional cigarettes there is no current consensus on the health consequences of electronic cigarette use5,6,7. Our previous research indicates that electronic cigarettes are significantly less toxic to the vascular endothelium than conventional cigarettes, despite their ability to cause DNA damage and the induction of oxidative stress and cell death8. However, more research is necessary before we can draw firm conclusions about the health consequences of electronic cigarette use.
As conventional cigarettes are a leading cause of preventable vascular disease9, there is a growing interest in the vascular health risk of electronic cigarette use10,11,12. In order to study the effects of electronic cigarettes on the vascular system, our lab developed a microcontroller operated smoking/vaping device (Figure 1)8. This device is capable of generating liquid extracts of either conventional cigarette smoke or electronic cigarette aerosol in either aqueous or organic solvents. As airflow is controlled by the combination of an adjustable air flow regulator and a PBASIC timing program, the device can be used to generate extracts according to any number of user defined protocols. Here we detail the assembly and operation of this device as well as two potential applications: in vitro cell viability assessment and gas-chromatography mass-spectrometry.
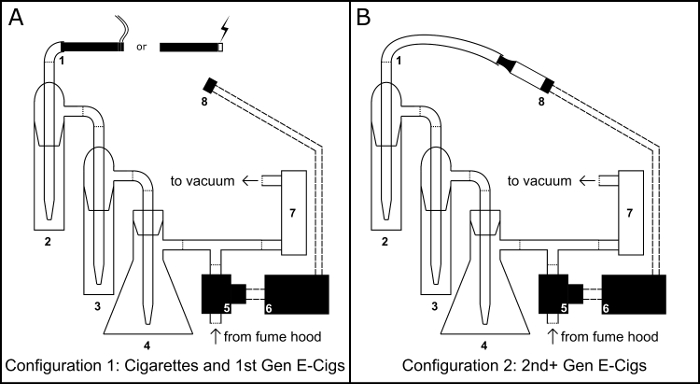
Figure 1: Smoking/Vaping Device. Schematic for the physical assembly of the smoking/vaping device in both the cigarette/cigarette like electronic cigarette (e-cig) configuration (A) and the tank electronic cigarette configuration (B). Component Key: 1) Inhalation port; 2) primary collection impinger; 3) overflow impinger; 4) Buchner flask vacuum trap; 5) normally open solenoid valve; 6) BS1 microcontroller; 7) air flow regulator; 8) 510 threaded electronic cigarette tank base. Please click here to view a larger version of this figure.
Protocol
1. Assembly of the Device
- Secure a 100 mL Buchner flask (Figure 1, #4) to a steel ring stand and create a vacuum trap by filling it with 50 g of calcium chloride to serve as a desiccant. Seal the flask with a rubber through-hole stopper, wrap the stopper junction with paraffin film, and run a pipette through the hole.
- Using vinyl tubing, connect the pipette extending from the stopper to a t-intersection hose connector.
- Using vinyl tubing, connect the two impinger (Figure 1, #2 & #3) to each other and connect the output of the second impinger to the t-intersection hose connector.
- Using vinyl tubing, connect the input port of the first impinger to serve as an inhalation port (Figure 1, #1).
- Using vinyl tubing, connect the side arm of the Buchner flask to the input port of an airflow regulator (Figure 1, #7) and the exit port of the airflow regulator to a vacuum pump.
- Assemble the circuit according to the schematic in Figure 2A.
- Upload the PBASIC program SVL.bs1 (Figure 2B, also available at https://github.com/ChastainAnderson/SVL) to the BS1 microcontroller (Figure 1, #6) by means of a serial adaptor and the manufacturer's software.
- Place the 510 threaded base (Figure 1 #8) in a ring stand clamp.
- Using vinyl tubing, connect the solenoid valve (Figure 1, #5) to the free end of the t-intersection hose connector.
NOTE: The device should be complete and ready for operation, check all joints to make sure that they are air tight and apply hose clamps and vacuum grease as needed.
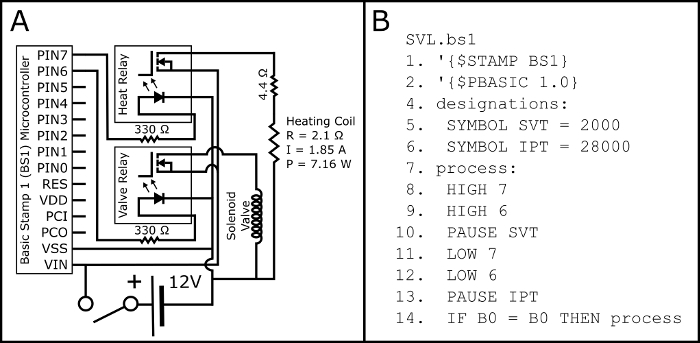
Figure 2: Electrical Schematic and PBASIC Code. Figure 2A displays the electrical schematic for assembling the electrical circuit necessary to activate both the normally open solenoid valve and the heating coil of button activated electronic cigarettes (through the 510 threaded electronic cigarette tank base). The electrical parameters of the heating coil (P: Power; R: Resistance; and I: Current) are projected and should be empirically verified with a multimeter post assembly. Figure 2B displays the PBASIC timing program needed to control the circuit in Figure 2A (also available at https://github.com/ChastainAnderson/SVL). The timing constants SVT & IPT (#5 & #6) are in units of ms and are set to provide an activation time of 2 seconds and a downtime of 28 s. Please click here to view a larger version of this figure.
2. Sample Storage and Preparation
- Store all unopened conventional and electronic cigarette samples in the dark in airtight plastic bags at room temperature.
- Once opened, store samples in airtight plastic bags at 4 ºC, with a paper towel to absorb excess moisture.
- Pre-equilibrate all samples in a room temperature humidor at ~60% humidity for at least 30 minutes prior to use.
3. General Operation of Cigarette Smoke/Electronic Cigarette Aerosol Extraction Device
- Determine the mass of each e-cigarette cartomizer/tank pre-vaping using an analytical balance. The difference in pre/post-post vaping weight will be used to determine appropriate dosing.
NOTE: 3R4F reference cigarettes are assumed to contain 0.7 mg of nicotine, and the nicotine content of commercial cigarette brands can be determined by conventional analytical methods13. - For sample application 1, fill the reservoir of the primary impinger with 4.3 mL of endothelial cell culture medium. For sample application 2, fill the reservoir with 5 mL of acetone.
- Prep the electronic cigarette or conventional cigarette for extraction:
- If using a conventional cigarette, apply a piece of clear tape around the filter and put an easily visible mark where the cigarette paper joins the filter.
- If using a cigarette like e-cigarette, make sure the battery is well charged and the cartomizer tightly screwed to the battery.
- If using an electronic cigarette tank, make sure that an appropriate volume of electronic cigarette liquid is loaded in the tank and screw the tank onto the 510 threaded base.
- Insert the tip of the conventional or electronic cigarette into the inhalation port (Figure 1, #1) and secure with a hose clamp.
- Turn on the vacuum pump.
- Adjust the flow meter to pull 1.65 L/minute to ensure a 55 mL puff over 2 seconds.
- Turn on the microcontroller. If using a conventional cigarette, light the cigarette on the first puff.
- Run until projected desired concentration (in parts per million or % weight/volume) is achieved.
- Determine the mass of each e-cigarette cartomizer/tank after vaporization using an analytical balance. Compare this measurement to the measurement taken in step 3.1 to determine the total mass consumed. Calculate the concentration of the consumed mass/volume of solvent. Use the molar concentration of consumed nicotine to normalize between products.
- If an insufficient mass was consumed, return the electronic cigarette to the device and consume further.
- If a sufficient or excess mass was consumed, proceed.
4. Filtration and Storage
- If extract is to be used for cell culture, filter through a 0.22 µm PES syringe filter.
- Use the extracts immediately or store at -80 ºC. As part of the preparation for Anderson, et al.8, electronic cigarette aerosol was demonstrated to be stable for at least two weeks, and the stability of cigarette smoke for up to two years has been established by Crooks, et al.13.
5. Cleaning the Device
- After each extraction, rinse the tubing and reservoirs of the device with 70% ethanol and deionized water to prevent carry over between samples.
- Following rinsing, briefly run the empty device to allow airflow to assist the drying of the lines.
6. Sample Application 1: Neutral Red Uptake Cell Viability Assay
- Perform extract as above into 4.3 mL of endothelial cell growth medium.
- One day prior, seed human umbilical vein endothelial cells into 96 well plates at a density of 1 x 104 cells/wellin 100 µL of endothelial cell growth medium.
- Treat the cells by replacing the old endothelial cell culture medium with either 100 µL of fresh endothelial cell culture medium to serve as a control or 75 µL of endothelial cell growth medium mixed with 25 µL of a 2 mM consumed nicotine concentration extract (1.4 mg consumed nicotine into 4.3 mL endothelial cell growth medium) for a final concentration of 500 µM to serve as treatment.
- As many of the components of both cigarette smoke and electronic cigarette aerosol are volatile, use a foil seal to keep the wells airtight.
- Incubate the plate 18–24 h at 37 °C and 5% CO2.
- Prepare neutral red staining solution:
- Create a 100x neutral red stock solution by dissolving 33 mg of neutral red dye into 10 mL of buffered salt solution.
- Shortly before use, dilute 100x stock solution 1:100 in cell culture medium to create 1x neutral red staining solution.
- Incubate neutral red staining solution at 37 °C for at least 30 min prior to use and use immediately.
NOTE: It is normal for some crystals to precipitate during incubation. Care should be taken to avoid applying these crystals to the cell culture wells. If needed, a .22 µm filter can be used to filter the neutral red stock and staining solutions.
- Remove extract and add 100 µL of neutral red staining solution per well, use excess to create at least three blank wells for proper quantification.
- Incubate the plate at 2–4 h at 37 °C and 5% CO2.
- Remove neutral red staining solution and wash 3x by submersion in PBS.
- Apply neutral red de-stain solution (50% deionized water, 49% ethanol, 1% acetic acid).
- Incubate at least 10 min at room temperature with shaking.
- Read absorbance at 540 nm.
- Analyze data by subtracting out the average value of the blank wells and normalizing to the average of the blank adjusted control well value.
7. Sample Application 2: Gas Chromatography Mass Spectrometry
- Perform extraction as above into 5 mL of acetone.
- Run the device to achieve a final concentration of ~100 parts per million (weight of e-liquid consumed/volume of acetone) of your sample.
- Using a precision glass syringe, inject 1 µL into the injector of a GC-MS device. Inject at 250 °C with a 1:20 split ratio into a coupled gas chromatograph/quadrupole spectrometer system equipped with a ZB-5 column with the following oven protocol: 1 min at 50 °C; ramp 10 °C/min to 140 °C; ramp 20 °C/min to 300 °C and hold for 10 min.
- Match resulting mass spectra to target library to identify aerosol components.
Results
Within 24 hours of the exposure of human umbilical vein endothelial cells to either conventional cigarette smoke extract (CSE) or electronic cigarette aerosol extract (EAE), there is a significant (control vs. CSE P <0.001; control vs. EAE P <0.01; n = 6) reduction in cell viability (Figure 3A). Extracts were generated with a puffing profile of 2, 2 second, 55 mL puffs per minute and normalized based on molar concentration of nicotin...
Discussion
The most critical elements of this protocol are ensuring the device is clean at the start and finish of each extraction, and ensuring that all seals are maintained so that air flow remains consistent. If the device is not properly cleaned, there is a risk of carry over between samples. Additionally, if the device is left unclean for an extended period of time condensed aerosol and dried solvent can block the system. Note that it is normal for there to be a pressure drop when puffing a conventional cigarette and the airfl...
Disclosures
The Tobacco Product Regulatory Science Research Fellowship program administered by Tulane University is funded by Altria Client Services Regulatory Affairs.
Acknowledgements
The authors acknowledge the assistance of Dr. Robert Dotson of the Tulane University Department of Cell and Molecular Biology for his assistance in editing the manuscript and Dr. James Bollinger of the Tulane University Department of Chemistry for his assistance with mass spectrometry protocol design. The authors further acknowledge the Tulane University Department of Cell and Molecular Biology and the Tulane University Department of Chemistry for their support and the use of space and equipment. This work was supported by a Tobacco Product Regulatory Science Research Fellowship to C. Anderson from the Tulane University School of Science and Engineering.
Materials
| Name | Company | Catalog Number | Comments |
| 12 V AC/DC Wall Mount Adaptor | Digi-Key | T1099-P5P-ND | |
| 2.2 Ohm Resistors | Digi-Key | A105635-ND | Used in tandem to generate the 4.4 Ohm resistance in Figure 2A |
| 330 Ohm Resistors | Digi-Key | 330QBK-ND | |
| 510 Threaded Base | NJoy | N/A | Recovered by dismantalling a second generation NJoy electronic cigarette |
| Acetic Acid, Glacial | Sigma-Aldritch | A6283 | |
| Acetone (Chromatography Grade) | Sigma-Aldritch | 34850 | |
| Basic Stamp Project Board | Digi-Key | 27112-ND | This board contains the BS1 Microcontroller, serial adaptor, power switch, and a barrel pin connector for the AC/DC Wall Mount Adaptor |
| Basic Stamp USB to Serial Adapter | Digi-Key | 28030-ND | An optional component to allow the BS1 serial adaptor to communicate through USB |
| Buchner Flask (Vacuum Flask) 250 mL | VWR | 10545-854 | |
| Clear Tape | 3M | S-9783 | |
| Clear Vinyl Tubing, 3/8" ID | Watts | 443064 | |
| EGM-2 Endothelial Cell Culture Medium | Lonza | CC-3162 | |
| Ethanol | Pharmco-Aaper | 111000200 | |
| Flow Regulator | Dwyer | VFA-23-BV | |
| Gas Chromatograph | Varian | 450-GC | |
| Glass Syringe, 10 mL | Sigma-Aldritch | Z314552 | |
| Glass Syringe, 10 µL | Hamilton | 80300 | |
| High Vacuum Silicon Grease | Dow Corning | 146355D | |
| Hose Clamp | Precision Brand | 35125 | |
| Human Umbilical Vein Endothelial Cells | ATCC | PCS-100-013 | |
| Mass Spectrometer | Varian | 300-MS | |
| Midget Impinger | Chemglass | CG-1820-01 | |
| Neutral Red | Sigma-Aldritch | N4638 | |
| Paraffin Film | 3M | PM-992 | |
| Plate Seal Roller | BioRad | MSR0001 | |
| Plate Seal; Foil | Thermo | 276014 | |
| Ring Stand 20" | American Educational Products | 7-G15-A | |
| Solenoid Valve (normally open) | US Solid | USS2-00081 | |
| Solid State Relay | Digi-Key | CLA279-ND | |
| Stand Clamp | Eisco | CH0688 | |
| Syringe Filter, PES, 0.22 um | Millipore | SLGP033RS | |
| Syringe, 10 mL | BD Syringe | 309604 | |
| Through Hole Stopper, Size 6 | VWR | 59581-287 | |
| Vacuum Pump | KNF Neuberger | N86KTP |
References
- World Health Organization. . WHO Report on the Global Tobacco Epidemic, 2011. , (2011).
- Weaver, S. R., Majeed, B. A., Pechacek, T. F., Nyman, A. L., Gregory, K. R., Eriksen, M. P. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int. J. Public Health. 61 (2), 177-188 (2016).
- Singh, T., et al. Tobacco Use Among Middle and High School Students - United States, 2011–2015. MMWR Morb. Mortal. Wkly. Rep. 65 (14), 361-367 (2016).
- Corey, C. G., Ambrose, B. K., Apelberg, B. J., King, B. A. Flavored Tobacco Product Use Among Middle and High School Students--United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 64 (38), 1066-1070 (2015).
- Pisinger, C., Døssing, M. A systematic review of health effects of electronic cigarettes. Prev. Med. 69, 248-260 (2014).
- Callahan-Lyon, P. Electronic cigarettes: human health effects. Tob. Control. 23 (Suppl 2), ii36-ii40 (2014).
- Dinakar, C., O'Connor, G. T. The Health Effects of Electronic Cigarettes. N. Engl. J. Med. 375 (14), 1372-1381 (2016).
- Anderson, C., Majeste, A., Hanus, J., Wang, S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol. Sci. Off. J. Soc. Toxicol. , (2016).
- U.S. Department of Health and Human Services. . The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. , (2014).
- Farsalinos, K., et al. Comparison of the Cytotoxic Potential of Cigarette Smoke and Electronic Cigarette Vapour Extract on Cultured Myocardial Cells. Int. J. Environ. Res. Public. Health. 10 (10), 5146-5162 (2013).
- Schweitzer, K. S., et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am. J. Physiol. - Lung Cell. Mol. Physiol. 309 (2), L175-L187 (2015).
- Putzhammer, R., et al. Vapours of US and EU Market Leader Electronic Cigarette Brands and Liquids Are Cytotoxic for Human Vascular Endothelial Cells. PLOS ONE. 11 (6), e0157337 (2016).
- Crooks, I., Dillon, D. M., Scott, J. K., Ballantyne, M., Meredith, C. The effect of long term storage on tobacco smoke particulate matter in in vitro genotoxicity and cytotoxicity assays. Regul. Toxicol. Pharmacol. 65 (2), 196-200 (2013).
- Roemer, E., et al. Mainstream Smoke Chemistry and in Vitro and In Vivo Toxicity of the Reference Cigarettes 3R4F and 2R4F. Beitr. Zur Tab. Contrib. Tob. Res. 25 (1), (2014).
- International Organization for Standards. . ISO 3088:2012 Routine analytical cigarette smoking machine – Definitions and standard conditions. , (2012).
- World Health Organization. . Standard Operating Procedure for Intense Smoking of Cigarettes. , (2012).
- Brown, C. J., Cheng, J. M. Electronic cigarettes: product characterisation and design considerations. Tob. Control. 23 (Suppl 2), ii4-ii10 (2014).
- Cooperation Centre for Scientific Research Relative to Tobacco. . CRM No. 81 - Routine Analytical Machine for E-Cigarette Aerosol Generation and Collection - Definitions and Standard Conditions. , (2015).
- Thorne, D., Adamson, J. A review of in vitro cigarette smoke exposure systems. Exp. Toxicol. Pathol. 65 (7-8), 1183-1193 (2013).
- Klus, H., Boenke-Nimphius, B., Müller, L. Cigarette Mainstream Smoke: The Evolution of Methods and Devices for Generation, Exposure and Collection. Beitr. Zur Tab. Contrib. Tob. Res. 27 (4), (2016).
- Baker, R. The Development and Significance of Standards for Smoking-Machine Methodology. Beitr. Zur Tab. Contrib. Tob. Res. 20 (1), (2014).
- Thorne, D., Crooks, I., Hollings, M., Seymour, A., Meredith, C., Gaca, M. The mutagenic assessment of an electronic-cigarette and reference cigarette smoke using the Ames assay in strains TA98 and TA100. Mutat. Res. Toxicol. Environ. Mutagen. 812, 29-38 (2016).
- Thorne, D., Larard, S., Baxter, A., Meredith, C., Gaҫa, M. The comparative in vitro assessment of e-cigarette and cigarette smoke aerosols using the γH2AX assay and applied dose measurements. Toxicol. Lett. 265, 170-178 (2017).
- Herrington, J. S., Myers, C. Electronic cigarette solutions and resultant aerosol profiles. J. Chromatogr. A. 1418, 192-199 (2015).
- Yu, V., et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 52, 58-65 (2016).
- Ji, E. H., et al. Characterization of Electronic Cigarette Aerosol and Its Induction of Oxidative Stress Response in Oral Keratinocytes. PLOS ONE. 11 (5), e0154447 (2016).
- Morgan, D. L., et al. Chemical Reactivity and Respiratory Toxicity of the -Diketone Flavoring Agents: 2,3-Butanedione, 2,3-Pentanedione, and 2,3-Hexanedione. Toxicol. Pathol. 44 (5), 763-783 (2016).
- Cooperation Centre for Scientific Research Relative to Tobacco. . CRM No. 84 - Determination of Glycerin, Propylene Glycol, Water, and Nicotine in the Aerosol of E-Cigarettes by Gas Chromatographic Analysis. , (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved