A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
In Vitro and In Vivo Approaches to Determine Intestinal Epithelial Cell Permeability
* These authors contributed equally
In This Article
Summary
Two methods are presented here to determine intestinal barrier function. An epithelial meter (volt/ohm) is used for measurements of transepithelial electrical resistance of cultured epithelia directly in tissue culture wells. In mice, the FITC-dextran gavage method is used to determine the intestinal permeability in vivo.
Abstract
The intestinal barrier defends against pathogenic microorganism and microbial toxin. Its function is regulated by tight junction permeability and epithelial cell integrity, and disruption of the intestinal barrier function contributes to progression of gastrointestinal and systemic disease. Two simple methods are described here to measure the permeability of intestinal epithelium. In vitro, Caco-2BBe cells are plated in tissue culture wells as a monolayer and transepithelial electrical resistance (TER) can be measured by an epithelial (volt/ohm) meter. This method is convincing because of its user-friendly operation and repeatability. In vivo, mice are gavaged with 4 kDa fluorescein isothiocyanate (FITC)-dextran, and the FITC-dextran concentrations are measured in collected serum samples from mice to determine the epithelial permeability. Oral gavage provides an accurate dose, and therefore is the preferred method to measure the intestinal permeability in vivo. Taken together, these two methods can measure the permeability of the intestinal epithelium in vitro and in vivo, and hence be used to study the connection between diseases and barrier function.
Introduction
Intestinal epithelial cells are not only responsible for the absorption of nutrients, but also form an important barrier to defend against pathogenic microorganisms and microbial toxins. This intestinal barrier function is regulated by tight junction permeability and epithelial cell integrity1,2,3, and dysfunction of the epithelial barrier function is associated with inflammatory bowel disease (IBD). The perijunctional actomyosin ring (PAMR) lies within the cell that is closely contiguous to the tight junctions. The contraction of the PAMR, which is regulated by the myosin light chain (MLC), is crucial for the regulation of tight junction permeability4,5,6,7,8,9,10. Tumor necrosis factor (TNF) is central to intestinal barrier loss by upregulating intestinal epithelial MLC kinase (MLCK) expression and inducing occludin internalization11,12,13.
Ions such as Na+ and Cl- can cross the paracellular space by either the pore or leak pathway14. In a "leaky" epithelium, changes in TER primarily reflect altered tight junction permeability. TER measurement is a commonly used electrophysiological approach to quantify tight junction permeability, primarily to Na+ and Cl-, based on the impedance of cell monolayers. Diverse cell types, including intestinal epithelial cells, pulmonary epithelial cells, and vascular endothelial cells, have been reported for TER measurements. Advantages of this method are that TER measurements are non-invasive and can be used to monitor live cells in real-time. In addition, the TER measurement technique is useful for drug toxicity studies15.
Caco-2BBe cells are human epithelial colorectal adenocarcinoma cells with a structure and function similar to the differentiated small intestinal epithelial cells: for example, these cells have microvilli and enzymes associated with small intestinal brush border. Therefore, cultured Caco-2BBe monolayers are utilized as an in vitro model for testing barrier function.
In mice, one way to study intestinal paracellular permeability is by measuring the ability of FITC-dextran to cross from the lumen into the blood. Thus, the intestinal permeability can be assessed by gavaging FITC-dextran directly into mice and measuring the fluorescence within the blood. The following protocol describes two simple methods to assess intestinal epithelium permeability both in vitro and in vivo.
Protocol
This study was approved by the Animal Care and Use Protocol of Cambridge-Suda Genomic Resource Center (CAM-SU), Soochow University.
1. Plating and Maintenance of Caco-2bbe on Porous Polycarbonate Membranes
- Grow cells in a T75 flask with media (DMEM containing 10% FBS). Flasks should be fed regularly, depending on the cell density.
NOTE: For optimal plating, cells should divide rapidly and have a flat "fried-egg" shape, which indicates that cells are in the growth phase. - Once cells are 80% confluent, take the flask out of the incubator and remove the media. Rinse any residual media with 1-2 mL of sterile PBS (without Ca2+). Pipet 1.5 mL Trypsin-EDTA into the flask and gently rock the flask; then, place the flask in the 37 °C incubator for 20 min without rocking.
- While the cells are trypsinizing, place inserts containing porous polycarbonate membranes (pore size, 0.4 µm; surface area, 0.33 cm2; see Table of Materials) into 24-well plates. Add 1.0 mL of culture media in the basal chamber (the lower space of the membrane).
- Pipet 5 mL of media into the flask and vigorously pipet the cells against the side of the flask 5-10 times to achieve loose, individual cells or 2-3 cell clumps.
- Plate 0.166 mL of cells (achieving a 1:8 dilution ratio) into the apical chamber (the upper space of the membrane). Incubate at 37 °C for up to 3 weeks.
- Feed cells three times weekly by carefully aspirating the media from the basal compartment of each well using a pressure pump. Gently drip 1 mL of media into the apical chamber of each insert.
2. Use of Epithelial Meter (Volt/Ohm) for Measuring TER
NOTE: After about 3 weeks of culture on polycarbonate membranes, Caco-2BBe cells are ready for TER measurement.
- For cytokine studies, one day before measurement, replace the basal media with media containing 10 ng/mL of IFNγ. On the day of the experiment, replace the media with HBSS containing 2.5 or 7.5 ng/mL of TNF.
NOTE: IFNγ treatment increases expression of TNF receptor 2 (TNFR2)16. - To correct the meter, insert the correction electrode into the Input port, and choose "Ohm" mode. Adjust the R Adj screw with a screwdriver until the meter displays a reading of 1,000 Ω.
- Sterilize the electrodes by placing them in 70% ethanol for 15-30 min, and then allow them to air dry for 15 s. Rinse the electrode in the experimental cell culture media.
- Turn on the power, and choose the "Ohm" mode. Carefully place the long ends of the electrode bridges into the basal chamber and the short ends into the apical chamber. Ensure that the longer electrodes touch the bottom of the dish, while keeping the shorter electrodes below the surface of the media but above the tissue culture inserts. Keep the electrodes vertical.
- Measure the resistance of the sample inserts and blank inserts (i.e., the culture inserts without cells but with HBSS) at 0, 1, 2, 3, 4 h after cytokine treatment. Record the resistance.
- To achieve consistency across different plate formats, calculate the product of the resistance and the effective membrane area:
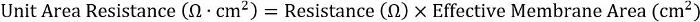
For 24-well inserts, the effective membrane area is 0.33 cm2.
3. Murine Model of Dextran Sulphate Sodium (DSS)-induced Colitis
- Add DSS to autoclaved water to a final concentration of 3.5% (wt/vol).
- Administer 3.5% DSS to 8-week-old male C57BL/6 mice for a total of 7 days. Give regular drinking water without DSS to control mice.
- Switch the DSS-containing water to regular drinking water after day 7.
- Weigh mice and assess clinical scores of each mouse every day. Normalize body weight of each mouse to its initial body weight. Scores are defined according to the disease severity by four parameters: rectal prolapse (0-2), stool consistency (0-2), bleeding (0-2), and activity (0-2)5. Sum the scores from these parameters for a final clinical score.
- To analyze the histopathological condition of colon tissue, euthanize mice by intraperitoneal (i.p.) injection with 1.2% (vol/vol) Avertin (0.6 mL/10 g body weight) 7 days post-DSS treatment. To prepare a stock of 100% avertin, mix 10 g of 2,2,2-tribromoethanol with 10 ml of tert-amyl alcohol. Store in the dark at 4°C. To use, dilute 100% stock to 2% in water.
- Isolate the colon and cecum, and measure the length of the colon5.
- Cut 0.5 cm segments from the distal colon and fix in a 15 mL falcon tube containing 10 mL of 10% formalin overnight. Wash the fixed tissues with graded ethanol (75, 95, and 100%) and xylene. Embed the tissues in paraffin and cut 6 mm sections for the hematoxylin & eosin staining8.
4. Measuring the Epithelial Barrier Permeability in DSS-induced Colitis Mice
- Measure the barrier permeability 7 days after the start of DSS administration.
- On the day of the assay, fast mice for 3 h.
- Autoclave a gavage needle to ensure sterility, then gavage mice with 150 µL 80 mg/mL 4 kDa FITC-dextran in sterile water, and keep the unused FITC-dextran to measure the standard curve after serum collection. Weigh the mice for the permeability calculation.
NOTE: The FITC-dextran solution should be made in water. - Using a pair of scissors, clip a 1 cm piece of the tail, and collect 100 µL of blood from the tail into serum collection tubes. Spin the collected blood at 10,000 x g for 10 min at room temperature.
- Dilute the serum 1:4 in water. To make a standard curve, dilute unused FITC-dextran with water at 1:300, 1:1,000, 1:3,000, 1:10,000, 1:30,000, 1:100,000, 1:300,000, 1:1,000,000, and 1:3,000,000. Add 100 µL/well of the serum and the standard curve samples into 96-well plates.
- Read the fluorescence in a plate reader with 485 excitation/528 emission. Calculate the permeability values based on the standard curve, and multiply by 4 to correct for the dilution.
- Divide the concentration of FITC-dextran by the weight to normalize the values (this helps normalize the difference in FITC-dextran delivery if mice are sick and have lost weight).
Results
In culture, Caco-2BBe cells grow as a monolayer and slowly differentiate into mature absorptive enterocytes that have brush borders. In this protocol, Caco-2BBe cells were plated with a high density on polycarbonate membranes, and cells reached 100% confluency one day after seeding. However, cells are undifferentiated at this stage: To fully differentiate the cells, the media is changed every 2-3 days for 3 weeks. Cells were stained with nuclei and F-actin stains to show the differences b...
Discussion
There are several critical steps in the protocol. Caco-2BBe (brush border-expressing) cells are always used for TER measurement, selected from the Caco-2 cell line for expression of brush-border proteins. Caco-2BBe cells have a villus absorptive phenotype when fully-differentiated (after about 3 weeks of culture post-confluence)17. It is necessary to avoid contamination during the measurement, and to sterilize the electrode. Because the procedure is non-sterile, measurements can only be performed ...
Disclosures
The authors declare no competing financial interests.
Acknowledgements
We thank Dr. Jerrold R. Turner, from Brigham and Women's Hospital, Harvard Medical School, for his generous help in completing this study. This work is supported by the National Natural Science Foundation of China (grant number 81470804, 31401229, and 81200620), the Natural Science Foundation of Jiangsu Province (grant number BK20180838, and BK20140319), The Research Innovation Program for College Graduates of Jiangsu Province (grant number KYLX16-0116), Advanced Research Projects of Soochow University (grant number SDY2015_06), and Crohn's & Colitis Foundation Research Fellowship Award (grant number 310801).
Materials
| Name | Company | Catalog Number | Comments |
| 22 G gavage needle | VWR | 20068-608 | |
| 4 kDa FITC-dextran | Sigma | 46944 | |
| Avertin | Sigma | T48402 | |
| Black 96-well plates for fluorescence | Fisher | 14-245-197A | |
| C57/B6 mice | Nanjing Biomedical Research Institute of Nanjing University | ||
| Caco-2BBe cells | ATCC | CRL-2102 | |
| Dextran sulphate sodium | MP Biomedicals | 2160110 | |
| DMED with high glucose and sodium pyruvate | Hyclone | SH30243.01B | |
| Epithelial (Volt/Ohm) Meter | Millicell-ERS | MERS00002 | |
| Ethanol | Sinopharm ChemicalReagent | 10009218 | |
| Falcon tube (15 mL) | Corning | 430791 | |
| FBS | Gibco | 10437-028 | |
| Fluorescence microscope | Olympus | FV1000 | |
| Fluorometer | Biotek | Synergy 2 | |
| HBSS | 138 mM NaCl, 0.3 mM Na2HPO4, 0.4 mM MgSO4, 0.5 mM MgCl2, 5.0 mM KCl, 0.3 mM KH2PO4, 15.0 mM HEPES, 1.3 mM CaCl2, 25 mM glucose | ||
| IFNg | PeproTech | 315-05-20 | |
| Modular Tissue Embedding Center | Leica | EG1150H | |
| Serum collection tubes | Sarstedt | 41.1378.005 | |
| T75 flask | corning | 430641 | |
| TNF | PeproTech | 315-01A | |
| Parraffin | Sigma | A6330-1CS | |
| Polycarbonate membranes (Transwell) | Costar | 3413 | |
| Pressure pump | AUTOSCIENCE | AP-9925 | |
| Rotary Microtomy | Leica | RM2235 | |
| Trypsin-EDTA | Gibco | 25200-056 | |
| Xylene | Sinopharm ChemicalReagent | 10023418 |
References
- Turner, J. R. Intestinal mucosal barrier function in health and disease. Nature reviews. Immunology. 9, 799-809 (2009).
- Odenwald, M. A., Turner, J. R. The intestinal epithelial barrier: a therapeutic target?. Nature reviews. Gastroenterology & hepatology. 14, 9-21 (2017).
- Clarke, H., Soler, A. P., Mullin, J. M. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J. Cell Sci. 113 (Pt 18), 3187-3196 (2000).
- Clayburgh, D. R., et al. A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability. J.Biol. Chem. 279, 55506-55513 (2004).
- Su, L., et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 145, 407-415 (2013).
- Su, L., et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 136, 551-563 (2009).
- Zha, J. M., et al. Characterization of isoform expression and subcellular distribution of MYPT1 in intestinal epithelial cells. Gene. 588, 1-6 (2016).
- He, W. Q., et al. Altered contractile phenotypes of intestinal smooth muscle in mice deficient in myosin phosphatase target subunit 1. Gastroenterology. 144, e1451-e1455 (2013).
- He, W. Q., et al. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology. 135, 610-620 (2008).
- Li, H. S., et al. Myosin regulatory light chain phosphorylation is associated with leiomyosarcoma development. Biomed. Pharmacother. 92, 810-818 (2017).
- Clayburgh, D. R., et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J. Clin. Invest. 115, 2702-2715 (2005).
- Wang, F., et al. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 166, 409-419 (2005).
- Ye, D., Ma, T. Y. Cellular and molecular mechanisms that mediate basal and tumour necrosis factor-alpha-induced regulation of myosin light chain kinase gene activity. J. Cell Mol. Med. 12, 1331-1346 (2008).
- Turner, J. R., Buschmann, M. M., Romero-Calvo, I., Sailer, A., Shen, L. The role of molecular remodeling in differential regulation of tight junction 300 permeability. Semin. Cell Dev. Biol. 36, 204-212 (2014).
- Srinivasan, B., et al. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 20 (2), 107-126 (2015).
- Wang, F., et al. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 131, 1153-1163 (2006).
- Peterson, M. D., Mooseker, M. S. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line Caco-2. J. Cell Sci. 102 (Pt 3), 581-600 (1992).
- Wang, L., et al. Methods to determine intestinal permeability and bacterial translocation during liver disease. J. Immunol. Methods. 421, 44-53 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved