A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Colorimetric Method for Measuring Iron Content in Plants
In This Article
Summary
We present a simple and reliable protocol for measuring iron content in plant tissues using the colorimetric Prussian Blue method.
Abstract
Iron, one of the most important micronutrients in living organisms, is involved in basic processes, such as respiration and photosynthesis. Iron content is rather low in all organisms, amounting in plants to about 0.009% of dry weight. To date, one of the most accurate methods for measuring iron concentration in plant tissues is flame absorption atomic spectroscopy. However, this approach is time-consuming and expensive and requires specific equipment not commonly found in plant laboratories. Therefore, a simpler, yet accurate method that can be routinely used is needed. The colorimetric Prussian Blue method is regularly used for qualitative iron staining in animal and plant histological sections. In this study, we adapted the Prussian Blue method for quantitative measurements of iron in tobacco leaves. We validated the accuracy of this method using both atomic spectroscopy and Prussian Blue staining to measure iron content in the same samples and found a linear regression (R2 = 0.988) between the two procedures. We conclude that the Prussian Blue method for quantitative iron measurement in plant tissues is precise, simple, and inexpensive. However, the linear regression presented here may not be appropriate for other plant species, due to potential interactions between the sample and the reagent. Establishment of a regression curve is thus needed for different plant species.
Introduction
Iron (Fe) is an important micronutrient in all living organisms. In plants, it is an essential micronutrient1 because of its involvement in basic processes, such as respiration, photosynthesis and chlorophyll biosynthesis. High accumulation of free iron ions is harmful to plant cells due to reactions leading to the release of free radicals causing oxidative stress. To maintain iron homeostasis within the plant cell, ions are stored in vacuoles and sequestered within ferritins, protein cages directly involved in iron homeostasis2 and the principal storage structure of iron in all living organisms. At the same time, iron-deficiency anemia affects a significant proportion of the human population, resulting in an increasing need for plant Fe biofortification. Due to the unique properties of plant ferritin, food enrichment with ferritin-iron offers a promising strategy to fight this problem of malnutrition3.
Iron ions are mainly found in two oxidation states, namely the ferrous (divalent Fe2+ or iron (II)) and ferric (trivalent Fe3+ or iron (III)) forms. Several other forms of iron, such as iron clusters4, are also found in cells. Fe is stored as iron oxide within the cell and naturally forms hematites (Fe2O3) and ferryhidrites ((Fe3+)2O3•0.5H2O) under physiological conditions5. The hydroxides formed in these reactions, especially the ferric form, have very low solubility. Iron retention is consequently affected by the pH of the solution and is largely in a solid state above pH 56.
Considering the poor solubility and high reactivity of Fe, its transfer among plant tissues and organs must be associated with suitable chelating molecules. Moreover, its redox states between the ferrous and ferric forms1 must be controlled. Within leaves, about 80% of the iron is found in photosynthetic cells, due to its essential roles in the electron transport system, in the biosynthesis of cytochromes, chlorophyll and other heme molecules, and in the formation of Fe-S clusters7. In the case of iron excess within the cell, the surplus is translocated into the vacuole where the metal is stored in ferritin molecules8.
Iron can be measured in plant tissues by several methods, including flame atomic absorption spectroscopy9 (FAAS) or colorimetric assays10, the former being far more precise than the latter. FAAS is a highly accurate technique that enables one to determine the elemental composition of a sample on the basis of the electromagnetic emission of the individual elements. FAAS converts metal ions to atomic states by flame-heating of the sample, leading to ion excitation and emission of a specific wavelength when a given ion returns to its ground state. The emissions from the different ions are separated by a monochromator and detected by an absorption sensor11. FAAS thus serves to directly quantify iron concentrations. Other techniques for visualizing iron in biological tissues are, however, available. Inductively-coupled plasma-mass spectroscopy (ICP-MS)12 is a very precise technique for measuring iron and other trace elements but the lack of equipment, both for FAAS and ICP-MS, is a common problem. On the other hand, iron measurement by thiocyanate colorimetry13 lacks precision and fails to detect small variations between samples. Prussian blue staining14,15,16,17 is an indirect method based on the reaction of potassium ferric ferrocyanide (K4Fe(CN)6) with Fe cations, producing a strong blue color, and is used for qualitative iron detection in histological sections of animal and plant tissues.
Metallic (zero-valent) iron is rare in the lithosphere. The dominant non-complexed ionic form of iron in the environment is mostly dictated by the amount of oxygen in the surroundings, with ferrous iron being relatively more abundant in anoxic environments and ferric iron predominating in aerobic sites. This latter form is also dominant in extremely acidic environments, although the causative agents of ferrous iron oxidation often differ in anoxic and acidic surroundings18. When iron is solubilized in 4% HCl (pH 0) in an aerobic environment, the major part of the diluted iron exists as the ferric form (Fe3+)19,20.
The reactions between Fe ions and K4Fe(CN)6 are as follows:
Fe3+: FeCl3 + K4Fe(CN)6 = KFe(III)Fe(II)(CN)6¯ + 3KCl
Fe2+: 4 FeCl2 + 2 K4Fe(CN)6 = Fe4(Fe(CN)6)2 + 8 KCl
In the present study, we asked whether Prussian blue staining can be useful for measuring iron levels in solution.
Initially, we verified the correlation between the concentration of Fe in aqueous solution and Prussian blue staining. The Fe (as FeCl2, FeCl3 or a 1:1 mixture of the two) concentration in aqueous solutions was measured both by atomic spectroscopy and by absorbance (OD) after addition of Prussian blue. Figure 1 shows the linear regression curves for measurements obtained by each method. We concluded that the Prussian blue method can be used for quantitative analysis of iron concentration in solution.
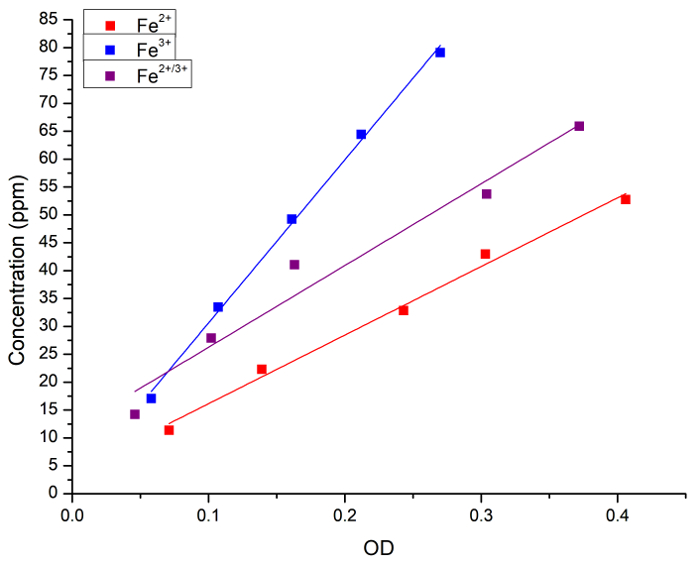
Figure 1: Linear regressions between Fe concentration measured by FAAS and light absorbance (OD, 715 nm) obtained by the Prussian blue method. The blue squares and line represent the Fe2+ solution, the red squares and line represent the Fe3+ solution and the black squares and line represent a 1:1 mixture between Fe2+ and Fe3+. The following regressions were obtained: [Fe2+] = 3 + 123 x OD, r = 0.996, R2 = 0.989; [Fe3+] = 1 + 292 x OD, r = 0.999, R2 = 0.997; and [Fe2+/3+] = 11 + 146 x OD, r = 0.983, R2 = 0.956. The Fe2+ donor was FeCl2 and the Fe3+ donor was FeCl3. Please click here to view a larger version of this figure.
To adapt the colorimetric Prussian blue method for quantitative iron analysis of plant tissues, the iron content of tobacco leaf ashes was measured by flame absorption atomic spectroscopy and Prussian blue staining. There was good correlation between the results from by the two techniques.
Protocol
1. Plant Material and Growth Conditions
- Seed one tobacco (cultivar Samsun) seed per 5 cm x 5 cm pot filled with standard pot medium. Place the pots on trays. Grow the plants in a growth room under long day conditions (16/8 h light/dark) at a constant temperature of 23 °C. Irrigate with tap water until water drains from the pot.
- After 50±5 days, start Fe treatments in the irrigation, according to the concentrations suitable for the experiment. For example, we used a range of iron concentrations from 0 to 6 mM, supplemented by a soluble Fe chelator (Fe EDDHA). Irrigate the plants with the appropriate solution every two days (to avoid dehydration) for 6-8 days.
2. Preparing the Leaves for Iron Measurement
Notes: All materials to be used must be iron-free so as to reduce the risk of iron contamination. Clean the mortar and pestle twice with 4% HCl solution and dry with filter paper each time before use. If any material is reused, clean it twice with 4% HCl solution and dry with filter paper.
- Detach the leaves from the stem by hand, using gloves (do not use any metal equipment). Use about 10 g of leaves (fresh weight) for each sample. Clean each leaf with double distilled water (DDW) using a spray bottle. This step is important to avoid Fe contamination.
- Dry the leaves on a paper towel and put them in a paper bag. Transfer the paper bags to an oven at a constant temperature of 80 °C for 2-3 days.
- When dry, crush the leaves to powder using a mortar and pestle and transfer to sterile 15 mL plastic tubes.
3. Burning the Leaves to Ash
Notes: The use of a low pH (close to 0) solution of HCl is meant to increase iron solubility. The rock wool is used to prevent the gases from escaping the vial during burning.
- Weigh a new, sealed 20 mL scintillation vial without its lid. Note the value or set the value to zero using the tare button. Add the crushed dried leaves (sample) to the vial.
- Weigh the sample and container and note the value. Close the vial with rock wool.
- Weigh 3 additional vials without adding samples and note their values. These vials will be used as controls to evaluate the amount of rock wool that could have led to any increase in sample weight.
- Place the sample and control vials in a furnace and start burning using the following temperature steps: room temperature, fast increase to 425 °C, and, finally, 425°C for 4 hours. By this time, the dry leaves will have turned to ash.
- Let the samples cool down to about 100 °C but not below this temperature for the following two steps to avoid humidity, which could affect the final weight of the sample. Using heavy gloves, remove the samples from the furnace with tweezers, holding the vial exteriorly.
- Place the vials on a flat surface, remove the rock wool and close the vials with their original lids.
- Weigh the 3 control vials (see 3.3) and calculate their average weight gain. If weight gain is equal or above to 1% of the ash weight (see step 4.2), use this value as an estimate of the measurement error.
4. Preparing the Ashes for Iron Measurement
Notes: The final iron concentration in the initial sample is calculated as the weight of the ashes divided by the added volume of HCl.
- Prepare a 1 M HCl solution (4% HCl) by adding 12.5 mL of a 37% HCl stock solution to 87.5 mL of DDW (in a plastic or glass flask).
- Weigh a 15 mL plastic tube and note the value or set the value to zero using the tare button. Transfer the ashes to the tube, weigh, and note the value. This is the ash weight.
- Add 5 mL of 1 M HCl to the ashes. Filter the ashes through a 22 µm filter and add an additional 5 mL of 1 M HCl through the same filter.
- The final volume should be 10 mL. Note that part of the solution will be lost in the filter.
NOTE: The samples are now ready for Fe measurement either by FAAS or by the Prussian Blue method. - Make a calibration curve with the Fe concentration measured by atomic spectrometry and by the Prussian blue method (see Figure 4) for each plant species. Subsequently, Fe concentration can be measured by the Prussian blue method alone.
5. Measuring Fe Concentration by FAAS
- Remove 4 mL from each sample for measurement by FAAS.
- Divide the results obtained from the FAAS measurement by the weight of the ashes. Divide the resulting value by 0.01 (because the ashes were solubilized in 10 mL). The resulting value is the iron concentration per gram ash (ppm).
6. Preparing the Prussian Blue Staining Solution
- Prepare a 4% Prussian blue solution by adding 4 g of K4Fe(CN)6 to 100 mL DDW and vortex (other volumes and/or concentrations can be used for different demands). It should be noted that in this study, a less concentrated Prussian blue solution than previously reported (20%)14 was used.
- Keep the solution in the dark at 4 °C until use. The solution is stable for 6 months when stored at such conditions.
7. Generating a Calibration Curve for the Prussian Blue Method Using FAAS Results
NOTE: Calculate the iron concentration in the ashes using the following formula
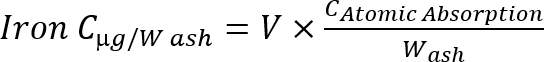 Equation 1
Equation 1
C: concentration, V: sample volume, W: ash weight (g).
- Mix 0.50 mL of Prussian blue solution and 0.50 mL of 1 M HCl. This will serve as the blank solution.
- Mix 0.5 mL of sample (ashes in 4% HCl, as described in section 3) and 0.5 mL of Prussian blue solution (step 6.1) by pipetting. Wait at least 1 minute but not more than 5 minutes. After 5 minutes, sedimentation in the samples will occur.
- Transfer the mix to a cuvette and measure the OD at 715 nm using a spectrophotometer. Note the value.
- Divide the OD value (step 7.3) by the ash weight (step 3.2) of the sample. The result represents OD per gram ash.
- Plot the linear regression between the iron concentrations obtained from the FAAS measurements (Y axis) and the OD values (X axis). Use the results obtained in steps 5.2 and 7.4. Calculate the regression formula, Y = a + bX, where Y represents iron concentration, a represents the absorbance intersect, b represents the absorbance slope and X represents the OD.
8. Using the Prussian Blue Method for Determining Iron Levels in Other Samples from the Same Plant Type
Notes: Since a calibration curve has already been established for this type of plant, iron concentration in any new samples from the same plant type can be directly calculated using the linear regression formula.
- Perform the steps in sections 3 and 4, followed by steps 7.1 to 7.4.
- Calculate the iron concentration in solution using the formula obtained from the linear regression (step 7.5).
Results
When this protocol is carried out correctly, one should get excellent correlation between the results obtained by the Prussian blue and atomic spectroscopy methods. Therefore, the Prussian blue method can be easily used to obtain an accurate measurement of iron concentration in plant samples, as reflected in the following experiment.
Tobacco plants were grown as described in the protocol and irrigated with water containing diffe...
Discussion
Iron measurement in plant tissues is very important for evaluating the effects of irrigation or other environmental conditions. Here, we described an easy and accurate colorimetric method for Fe content measurement in tobacco leaves, which can be readily adapted to other plant species and tissues.
In optimizing conditions for the colorimetric method, we used a low pH medium (pH < 1.0) to allow iron solubility. The burning process was performed to release all forms of iron and to ensure tha...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Israel Ministry of Science, Technology and Spaceand by a grant from the Chief Scientist of the Israeli Ministry of Agriculture (#16-16-0003).
Materials
| Name | Company | Catalog Number | Comments |
| Potassium Hexacyanoferrate(II) | Fisher Chemical | 14459-95-1 | Reagent for the Pussian Blue |
| Millex Syringe Filter Unit, Vial Vent 0.22 μm | Millec | SLGP033RS | Filter used to filter the ashes + 4% HCl Solution |
| Scintillation Vials | Fisherbrand | 03-337-4 | Used to keep the dry powdered plant material during the burning procedure. |
| Disposable Syringe 10 ml | Medi-Plus | 1931 | Syringe used during the filtration |
| Hydrochloric acid | Sigma-Aldrich | 231-595-7 | Used in the 4% HCl solution to dilute the ashes and clean the materials |
| Tobacco, Nicotiana tabacum cv. Samsun NN | Obtained from Prof. Simon Barak and routinely used in the Zaccai Lab | Barak S, Nejidat A, Heimer Y, Volokita M. Transcriptional and posttranscriptional regulation of the glycolate oxidase gene in tobacco seedlings. Plant Molecular Biology. 2001 Mar 1;45(4):399-407. | Tobacco cultivar used in this protocol |
| Glass Wool (Rock Wool) | Sigma-Aldrich | 659997-17-3 | Used in the procedure of burning samples in the furnace. |
References
- Kobayashi, T., Nishizawa, N. K. Iron uptake, translocation, and regulation in higher plants. Annual Review of Plant Biology. 63 (1), 131-152 (2012).
- Bradley, J. M., Le Brun, N. E., Moore, G. R. Ferritins: Furnishing proteins with iron. Journal of Biological Inorganic Chemistry. 21 (1), 13-28 (2012).
- Zielińska-Dawidziak, M. Plant ferritin - a source of iron to prevent its deficiency. Nutrients. 7 (2), 1184-1201 (2015).
- Johnson, D. C., Dean, D. R., Smith, A. D., Johnson, M. K. Structure, function, and formation of biological iron-sulfur clusters. Annual Review of Biochemistry. 74 (1), 247-281 (2015).
- Guo, H., Barnard, A. S. Naturally occurring iron oxide nanoparticles: morphology, surface chemistry and environmental stability. Journal of Materials Chemistry A. 1 (1), 27-42 (2013).
- Hem, J. D., Cropper, W. H. Chemistry of iron in natural water. Report US Geological Survey. , 1-31 (1962).
- Rout, G. R., Sahoo, S. Role of iron in plant growth and metabolism. Reviews in Agricultural Science. 3, 1-24 (2015).
- Speretto, R. A., Ricachenevsky, F. K., Stein, R. J., de Abreu Waldow, V., Fett, J. P. Iron stress in plants Dealing with deprivation and overload. Plant Stress. 4, 57-69 (2010).
- Tautkus, S., Steponeniene, L., Kazlauskas, R. Determination of iron in natural and mineral waters by flame atomic absorption spectrometry. Journal of the Serbian Chemical Society. 69 (5), 393-402 (2006).
- Braunschweig, J., Bosch, J., Heister, K., Kuebeck, C., Meckenstock, R. U. Reevaluation of colorimetric iron determination methods commonly used in geomicrobiology. Journal of Microbiological Methods. 89 (1), 41-48 (2012).
- PerkinElmer. . Atomic Spectroscopy - Guide to Selecting the Appropriate Technique and System. 16, (2011).
- Wachasunder, S. D., Nafade, A. Precision and accuracy control in the determination of heavy metals by atomic absorption spectrometry. Science. 58, 517-528 (2001).
- Woods, J. T., Mellon, M. G. Thiocyanate method for iron. A spectrophotometric study. Industrial & Engineering Chemistry Analytical Edition. 13 (8), 551-554 (1941).
- Perls, M. Nachweis von Eisenoxyd in gewissen Pigmenten. Virchows Archiv Fur Pathologische Anatomie Und Physiologie Und Fur Klinische Medizin. 39 (1), 42-48 (1867).
- Connorton, J. M., Jones, E. R., Rodriguez-Ramiro, I., Fairweather-Tait, S., Uauy, C., Balk, J. Altering expression of a vacuolar iron transporter doubles iron content in white wheat flour. bioRxiv. , 1-25 (2017).
- de la Fuente, V., Rufo, L., Rodríguez, N., Franco, A., Amils, R. Comparison of iron localization in wild plants and hydroponic cultures of Imperata cylindrica (L.) P. Beauv. Plant Soil. 418 (1-2), 25-35 (2017).
- Hsiao, P. Y., Cheng, C. P., Koh, K. W., Chan, M. T. The Arabidopsis defensin gene, AtPDF1.1, mediates defence against Pectobacterium carotovorum subsp. carotovorum via an iron-withholding defence system. Science Reports. 7 (1), 1-14 (2017).
- Johnson, D. B., Kanao, T., Hedrich, S. Redox transformations of iron at extremely low pH: Fundamental and applied aspects. Frontiers in Microbiology. 3, 1-13 (2012).
- Stumm, W., Lee, G. F. Oxygenation of ferrous iron. Industrial & Engineering Chemistry. 53 (2), 143-146 (1961).
- Jones, A. M., Griffin, P. J., Collins, R. N., Waite, T. D. Ferrous iron oxidation under acidic conditions - The effect of ferric oxide surfaces. Geochimica et Cosmochimica Acta. 145, 1-12 (2014).
- Hawkesworth, C. J., Kemp, A. I. S. Evolution of the continental crust. Nature. 443 (7113), (2006).
- Thompson, L. M., Louis, M., Troeh, F. R., Thompson, L. M. . Soils and soil fertility. , (1973).
- Krueger, B. J., Grassian, V. H., Cowin, J. P., Laskin, A. Heterogeneous chemistry of individual mineral dust particles from different dust source regions: The importance of particle mineralogy. Atmospheric Environment. 38 (36), 6253-6261 (2004).
- Bewick, V., Cheek, L., Ball, J. Statistics review 7: Correlation and regression. Journal of Critical Care. 7 (6), 451-459 (2003).
- Asuero, A. G., Sayago, A., González, A. G. The correlation coefficient: An overview. Critical Reviews in Analytical Chemistry. 36 (1), 41-59 (2006).
- . Analytical Chemistry. Calibration Curves Available from: https://www.jove.com/science-education/10188/calibration-curves (2018)
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved