A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Real-time Imaging and Quantification of Fungal Biofilm Development Using a Two-Phase Recirculating Flow System
In This Article
Summary
We describe the assembly, operation, and cleaning of a flow apparatus designed to image fungal biofilm formation in real time while under flow. We also provide and discuss quantitative algorithms to be used on the acquired images.
Abstract
In oropharyngeal candidiasis, members of the genus Candida must adhere to and grow on the oral mucosal surface while under the effects of salivary flow. While models for the growth under flow have been developed, many of these systems are expensive, or do not allow imaging while the cells are under flow. We have developed a novel apparatus that allows us to image the growth and development of Candida albicans cells under flow and in real-time. Here, we detail the protocol for the assembly and use of this flow apparatus, as well as the quantification of data that are generated. We are able to quantify the rates that the cells attach to and detach from the slide, as well as to determine a measure of the biomass on the slide over time. This system is both economical and versatile, working with many types of light microscopes, including inexpensive benchtop microscopes, and is capable of extended imaging times compared to other flow systems. Overall, this is a low-throughput system that can provide highly detailed real-time information on the biofilm growth of fungal species under flow.
Introduction
Candida albicans (C. albicans) is an opportunistic fungal pathogen of humans that can infect many tissue types, including oral mucosal surfaces, causing oropharyngeal candidiasis and resulting in a lower quality of life for affected individuals1. Biofilm formation is an important characteristic for the pathogenesis of C. albicans, and numerous studies have been done on the formation and function of C. albicans biofilms2,3,4,5, many of which have been conducted using static (no flow) in vitro models. However, C. albicans must adhere and grow in the presence of salivary flow in the oral cavity. Numerous flow systems have been developed to allow for live-cell imaging6,7,8,9,10. These different flow systems have been designed for different purposes, and therefore each system has different strengths and weaknesses. We found that many of the flow systems appropriate for C. albicans were costly, required complex fabricated parts, or could not be imaged during flow and had to be stopped prior to imaging. Therefore, we developed a novel flow apparatus to study C. albicans biofilm formation under flow11. During the design of our flow apparatus, we followed these major considerations. First, we wanted to be able to quantify multiple aspects of the biofilm growth and development in real-time without requiring the use of fluorescent cells (allowing us to study mutant strains and unmodified clinical isolates easily). Second, we wanted all parts to be commercially available with little to no modifications (i.e., no custom fabrication), allowing others to more easily recreate our system, and allowing for easy repairs. Third, we also wanted to allow for extended imaging times at reasonably high flow rates. Lastly, we wanted, following a period of cells attaching to the substrate under flow, to be able to monitor the biofilm growth over an extended time without introducing new cells.
These considerations led us to develop the two-flask recirculating flow system illustrated in Figure 1. The two flasks allow us to split the experiment into two phases, an attachment phase that draws from the cell-seeded attachment flask, and a growth phase that uses cell-free media to continue the biofilm growth without the addition of new cells. This system is designed to work with an incubation chamber for the microscope, with the slide and the tubing preceding it (2 to 5, Figure 1) being placed inside the incubator, and all other components placed in a large secondary container outside the microscope. Additionally, a hotplate stirrer with an attached temperature probe is used to maintain fungal cells in the attachment flask at 37 °C. As it is recirculating, this system is capable of continuous imaging during flow (can be over 36 h depending on conditions), and can be used on most standard microscopes, including upright or inverted benchtop microscopes. Here, we discuss the assembly, operation, and cleaning of the flow apparatus, as well as provide some basic ImageJ quantitative algorithms to analyze the videos after an experiment.
Protocol
1. Assemble the Flow Apparatus
- Configure the parts listed in the Table of Materials according to the schematic in Figure 1 with the considerations discussed below.
NOTE: For convenience, the flow apparatus is divided into two sides, the green side (everything upstream of the slide to the media flasks), and the orange side (everything downstream of the slide to the media flasks).- Ensure that all of the flow apparatus is air tight to prevent leaks, with the sole exception of the media flasks (Figure 1, 1). To accomplish this, apply plumbers tape to any male threading before assembling except for the pulsation dampener (PD) and 2 µm filter bottle (FB), which do not require plumbers tape as the rubber gaskets keep them airtight.
- Apply ear clamps at every barbed fitting that is under positive pressure during normal operation (i.e., downstream of the pump).
- Use color coded lab tapes to label the valve locations with an A or G (for attachment and growth, respectively), the pump location, the slide connection locations, and the 0.2 µm filter connection.
- Determine the length of tubing to be used based on the distance between the flow system and microscope, keeping in mind that all the flow apparatus downstream of the pump to the flasks (majority of the orange side) should be in the secondary containment. Add approximately 1 m of extra tubing upstream of the slide (and preferably the bubble trap) to place within the microscope incubation chamber, as this ensures that all media reaching the slide will be at the correct temperature.
- Place the bubble trap as close as reasonably possible to the slide, preferably inside the incubation chamber during an experiment (bubbles often form along the tubing wall); however, keep in mind that it must be connected to a vacuum to operate.
- Ensure that the tubing between the FB and the 0.2 µm disposable filter is about 0.5 m long.
- Add an approximately 2 cm magnetic stir bar to each media flask.
- Obtain some form of tubing clamps to act as shut-off valves (hemostats can be used).
- For ease of use, keep the flow apparatus in an autoclavable basket. It can be helpful to have a second smaller basket in a larger one to allow easy separation of the green side and the orange side.
- For the attachment flask, using a 4 mm drill bit, drill an extra hole in the rubber stopper to accommodate the thermal probe (take care not to go through another hole). To get the tubing through the ports, push the tubing through with tweezers; once through, clamp the tubing to hold it in place, and then pull the tweezers back out.
NOTE: If it is not possible to add the extra hole to the rubber stopper, a wide-mouth screw bottle with a four-port screw cap may work in place of the flask and rubber stopper.
- Once the flow system has been fully assembled, close the valves of both green and orange side growth flasks. Use water with the attachment flask tubing and a graduated cylinder to calibrate the peristaltic pump according to the manufacturer's instructions.
2. Perform an Experiment
- The day before the experiment, begin pre-heating the microscope incubation chamber to 37 °C, and prepare an overnight culture of a fungal strain (fluorescence is not required).
- Gather single use components and pump, and place in a sterile biosafety cabinet.
- Remove the bubble trap and the temperature probe from the flow apparatus and place these in the biosafety cabinet.
- Detangle and organize the tubing, if necessary.
- Autoclave the flow apparatus, including the stir bars, for 30 min to ensure sterility; when finished, transfer to the biosafety cabinet.
- Attach the bubble trap, temperature probe, and all single use components (except the slide) as depicted in Figure 1.
- For the 0.2 µm filter (Figure 1, 11), remove the plunger from the 1 mL syringe to make it as an "adapter". Force the tubing from the FB into this end, and attach the 0.2 µm filter to the tubing leading to the growth flask.
- Apply silicone vacuum grease around the barb of the slide adapter (take care not to get any grease on the inside) prior to connecting it, as this helps prevent air leaks into the system.
- Fill the attachment flask with 100 mL of 1% (w/v) yeast extract, 2% (w/v) peptone, and 2% (w/v) glucose (YPD), and fill the growth flask with 200 mL of YPD. Ensure that the green side tubing reaches the media in each flask.
- Ensure that all valves are open. Attach the bubble trap to a vacuum, and connect the pump to the green side tubing downstream of the bubble trap.
- Pump the fluid at a flow rate of 3.3 mL/min to completely fill the green side, then dispense and discard approximately 1–2 mL of the media because the first couple of milliliters often contain dead cells or random debris. Ensure that the green side of the tubing is filled with media, and has no bubbles downstream of the bubble trap before proceeding.
- Fill the channel slide and the reservoir with YPD, taking care not to introduce bubbles.
- Connect the slide to the flow apparatus, and pump more fluid to create a buffer of about 0.5 m on the orange side. This is to prevent accidentally trapping air in the slide in the event of backflow.
- Prepare the flow apparatus for the transport to the microscope: Clamp closed the inlet and outlet of the bubble trap, and clamp the green and orange side attachment flask valves closed. Ensure that the screw caps for the PD and FB are tight as they can loosen during autoclaving.
- Disconnect the pump from the tubing to make transport easier. Then move all components, including the hotplate stirrer, into a secondary container near the microscope.
- Prepare the flow apparatus for imaging.
- Attach the temperature probe to the hotplate stirrer and begin heating the attachment flask to 37 °C. Stir the media at 300 rpm and maintain this for the whole experiment.
- Mount the slide on the microscope, and use tape if necessary to tightly secure it.
- Attach the bubble trap to a vacuum (do not undo the clamp yet).
- Connect the pump to the flow apparatus at the location indicated on Figure 1.
- Start the pump at a flow rate of 3.3 mL/min, allow it to run for approximately 5–10 s, and then remove the bubble trap inlet/outlet clamp.
- Allow the pump to continue running while the attachment flask heats up. Once media has circulated throughout the flow system, check for normal operation.
- Check fittings for air leaking in upstream of the pump (some bubble formation is normal), or fluid leaking out downstream.
- Check that the growth media flask, PD, and FB are all dripping media from the inlet tube (if not, this could indicate a clogged filter, or an overtightened ear clamp).
- Using the microscope, check for attached or rolling cells on the channel slide. An excessive number of cells may indicate contamination during set-up, or that the polytetrafluoroethylene (PTFE) membrane of the bubble trap needs replacing.
- Once the attachment flask and incubation chamber are both at 37 °C, add enough overnight culture of the fungal cells to the attachment flask to reach 1 x 106 cells/mL.
NOTE: The volume to add in µL can be calculated using this formula:
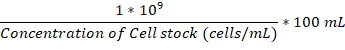
- Wait 15 min to allow the cells to acclimate.
- Open both green and orange side attachment flask valves while closing both growth flask valves to start the flow of cells.
- Wait for approximately 5 min to allow cells to reach the slide, and allow for initial focusing of the microscope (this time may need to be adjusted depending on the length of the green side tubing). During this time, adjust the microscope to the same imaging parameters used in previous experiments. If this is the first run, follow these steps:
- Switch to a low magnification air objective.
- Find and focus on an attached cell or small budding cell.
- Configure condenser for Köhler illumination, then switch to darkfield.
- Set the exposure time to 300 ms.
- Adjust the illuminating intensity until a small cell is dim yet clearly visible against the background (a signal to background ratio of approximately 7–8 for a budding daughter cell is a reasonable value). Note/mark the illuminating intensity for future experiments.
- Configure the software to acquire an image every 2 min over 2 h.
- Begin image acquisition for the attachment phase. Check back after approximately 5 and 10 min to ensure that focus has been maintained. If not, attempt to adjust the focusing immediately after the next image is acquired.
- Immediately after the attachment phase has finished, save the file, and then open both green and orange side growth flask valves while closing both attachment flask valves. Take care not to bump the stage if any valves are inside the incubation chamber.
- Unplug the thermometer probe from the hotplate stirrer.
- Remove the attachment flask from the hotplate stirrer and place the growth flask in its place.
- Configure the software to acquire an image every 15 min over 22 h and begin image acquisition for the growth phase. Re-focusing should not be necessary, but it is highly recommended to check the flow apparatus after a few hours.
- Check fittings for air leaking in upstream of the pump (again some bubble formation is normal), or fluid leaking out downstream
- Check that the growth media flask, PD, and FB are all dripping media (if not, this could indicate a clogged filter, an overtightened ear clamp, or a clog at a barbed fitting if the cells being used flocculate).
- Check the fluid level in the FB. If the media is approaching the top of the bottle (over 1.5 cm above the top of the filter), tighten both screwcaps (do not loosen them, as this flask is under pressure). If they will not tighten further, continue the experiment (though this may result in a leak), and replace the rubber gaskets on the PD and FB after the next cleaning.
- When the growth phase acquisition has finished, save the file, and then open the green and orange side attachment flask valves which may make a noise as the pressure releases on the orange side. Pull up on the green side tubing coming from both media flasks until they are at least several centimeters above the media. Run the pump at a high speed (approximately 100 mL/min or hold the fast forward button on the pump) to remove all the media from the tubing, which makes cleaning much easier. When emptied, disconnect the flow apparatus from the pump, and remove it from the microscope.
3. Clean the Flow Apparatus
- Remove all non-autoclavable components (single use components, bubble trap, and temperature probe), and autoclave the flow apparatus for 30 min. Discard used single-use components, clean probe with 70% ethanol, and set aside bubble trap.
- After autoclaving is finished, discard media, and rinse and set aside media flasks. Then re-connect the bubble trap, connect an ibidi channel slide to be used for cleaning (reusable), and connect the flow system to the pump at the location shown in Figure 1.
- Clamp closed the orange side growth flask valve.
- Place approximately 200 mL of undiluted bleach into a beaker. Place the rubber stoppers into the bleach, and then start the pump at a high speed to circulate bleach throughout the flow apparatus (except all the filters). Once filled with bleach, stop the pump because leaving the pump on at a high speed can wear and break the tubing.
- After bleaching for 15 min, hold the rubber stoppers above the beaker and start the pump again to remove the bleach from the flow apparatus.
- Repeat steps 3.4 and 3.5 twice with excess water instead of bleach to rinse the flow system. During this time, clean the filters only with water because other cleaning agents will corrode or clog the filters.
- Place the tubing that would normally connect to the 0.2 µm media filter (coming from the 2 µm FB) into the beaker water with the rubber stoppers from step 3.6.
- Disconnect the tubing attached to the inlet of the 20 µm inline filter, which can usually be pulled apart with ease despite the ear clamp.
- Use a vacuum filter flask and a long section of tubing through a spare 3-hole stopper to create a vacuum system that can connect to the flow apparatus.
- Connect this vacuum system to the inlet of the 20 µm filter inlet, and start the vacuum; this will pull water through the filters in the reverse direction, removing dead cells.
- Pull at least 200 mL of water through the filters, then remove the tubing from the water to empty the filter lines of water.
- Disconnect the vacuum system from the 20 µm filter, and reconnect the filter to its normal tubing.
4. Quantifying the Videos
NOTE: All files need to be converted to the tag image file (TIF) format to work. Additionally, to compare between experiments, it is critical that all images are taken with the same microscope and imaging parameters, as discussed above.
- Download and install ImageJ if not already installed.
- Download the supplemental macro file, and place it in the ImageJ\macros folder.
- Adjust the provided macro:
- Open an image stack from a previous experiment in ImageJ, and select a time point with cells present.
- Select from the menu via "Image | Type | 8-bit".
- Select from the menu via "Image | Adjust | Threshold". Check the "Dark Background" box. Set the right side dropdown menu to Red.
- Adjust the lower value until all cells are covered in red with minimal excess noise (some non-cell speckling is okay and will be processed out by the macro). Make note of this lower value.
- Close both the Threshold window and the open image.
- Select from the menu via "Plugins | Macros | Edit". When prompted to open a file: "move up one folder level", then select the macros folder and open the flow biofilm quantification macro file.
- Change the 15 value in all instances of "setThreshold(15, 255);" to the value determined in step 4.3.4. Save the file and close this window.
- Select from the menu via "Plugins | Macros | Install" and select the flow biofilm quantification file.
- Now, under the "Plugins | Macros" menu, six new options for various video quantifications appear. Run the Complete analysis and select the attachment and growth video files when prompted to perform all available analyses on the acquired data and automatically generate output files.
Results
Representative images of a normal overnight time-lapse experiment using wild-type C. albicans cells at 37 °C can be seen in Figure 2A and Supplemental Video 1. The images have been contrast enhanced to improve visibility. Quantification of the original data was performed, and representative graphs can be seen in Figure 2B. To generate these graphs, the data were fir...
Discussion
Using the flow system as outlined above allows for the generation of quantitative time-lapse videos of fungal biofilm growth and development. To allow for comparisons between experiments it is of critical importance to ensure that the imaging parameters are kept the same. This includes ensuring that the microscope is set up for Köhler illumination for each experiment (many guides are available online for this process). Aside from imaging parameters, there are some important steps to keep in mind when working with th...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge Dr. Wade Sigurdson for providing valuable input in the design of the flow apparatus.
Materials
| Name | Company | Catalog Number | Comments |
| Pump | Cole Parmer | 07522-20 | 6 |
| Pump head | Cole Parmer | 77200-60 | 6 |
| Tubing | Cole Parmer | 96410-14 | N/A |
| Bubble trap adapter | Cole Parmer | 30704-84 | 3 |
| Bubble trap vacuum adapter for 1/4” ID vacuum line | Cole Parmer | 31500-55 | 3 |
| In-line filter adapter (4 needed) | Cole Parmer | 31209-40 | 8,9 |
| Orange-side Y | Cole Parmer | 31209-55 | 7 |
| Green-side Y | ibidi | 10827 | 2 |
| * Slides | ibidi | 80196 | 4 |
| * Slide luers | ibidi | 10802 | 4 |
| Vacuum assisted Bubble trap | Elveflow/Darwin microfluidics | KBTLarge - Microfluidic Bubble Trap Kit | 3 |
| Media flasks | Corning | 4980-500 | 1 |
| 0.2 µm air filter | Corning | 431229 | 1 |
| Threaded glass bottle for PD and filter flask (2 needed) | Corning | 1395-100 | 5,10 |
| Ported Screw cap for PD and filter flask (2 needed) | Wheaton | 1129750 | 5,10 |
| Screwcap tubing connector | Wheaton | 1129814 | 5,10 |
| Tubing connector beveled washer | Danco | 88579 | 5,10 |
| Tubing connector flat washer | Danco | 88569 | 5,10 |
| Clamps for in-line filters and downstream Y (7 needed) | Oetiker/MSC Industrial Supply Company | 15100002-100 | 7,8,9 |
| Clamp tool | Oetiker/MSC Industrial Supply Company | 14100386 | N/A |
| 20 μm in-line media filter | Analytical Scientific Instruments | 850-1331 | 8 |
| 10 μm in-line media filter | Analytical Scientific Instruments | 850-1333 | 9 |
| 2 μm inlet media filter | Supelco/Sigma-Aldrich | 58267 | 10 |
| * 0.22 µm media filter | Millipore | SVGV010RS | 11 |
| * 0.22 µm media filter “adapter” | BD Biosciences | 329654 | 11 |
| Rubber stopper | Fisher Scientific | 14-131E | 1 |
| Hotplate stirrer with external probe port | ThermoFisher Scientific | 88880006 | N/A |
| Temperature probe | ThermoFisher Scientific | 88880147 | N/A |
References
- Pankhurst, C. L. Candidiasis (oropharyngeal). BMJ clinical evidence. 2012, 1304 (2012).
- Ramage, G., Vandewalle, K., Wickes, B. L., López-Ribot, J. L. Characteristics of biofilm formation by Candida albicans. Revista iberoamericana de micología. 18 (4), 163-170 (2001).
- Nobile, C. J., Mitchell, A. P. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Current biology: CB. 15 (12), 1150-1155 (2005).
- Blankenship, J. R., Mitchell, A. P. How to build a biofilm: a fungal perspective. Current opinion in microbiology. 9 (6), 588-594 (2006).
- Araújo, D., Henriques, M., Silva, S. Portrait of Candida Species Biofilm Regulatory Network Genes. Trends in microbiology. 25 (1), 62-75 (2017).
- Lane, W. O., et al. Parallel-plate flow chamber and continuous flow circuit to evaluate endothelial progenitor cells under laminar flow shear stress. Journal of visualized experiments. (59), e3349 (2012).
- Bakker, D. P., van der Plaats, A., Verkerke, G. J., Busscher, H. J., van der Mei, H. C. Comparison of velocity profiles for different flow chamber designs used in studies of microbial adhesion to surfaces. Applied and environmental microbiology. 69 (10), 6280-6287 (2003).
- Zhang, W., Sileika, T. S., Chen, C., Liu, Y., Lee, J., Packman, A. I. A novel planar flow cell for studies of biofilm heterogeneity and flow-biofilm interactions. Biotechnology and bioengineering. 108 (11), 2571-2582 (2011).
- Uppuluri, P., Lopez-Ribot, J. L. An easy and economical in vitro method for the formation of Candida albicans biofilms under continuous conditions of flow. Virulence. 1 (6), 483-487 (2010).
- Diaz, P. I., et al. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infection and immunity. 80 (2), 620-632 (2012).
- McCall, A., Edgerton, M. Real-Time Approach to Flow Cell Imaging of Candida albicans Biofilm Development. Journal of fungi. 3 (1), 13 (2017).
- Zhang, B., Zerubia, J., Olivo-Marin, J. -. C. Gaussian approximations of fluorescence microscope point-spread function models. Applied optics. 46 (10), 1819-1829 (2007).
- Tati, S., et al. Candida glabrata Binding to Candida albicans Hyphae Enables Its Development in Oropharyngeal Candidiasis. PLoS pathogens. 12 (3), 1005522 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved