Sign In
A subscription to JoVE is required to view this content. Sign in or start your free trial.
Methods Article
Measuring Post-Stroke Cerebral Edema, Infarct Zone and Blood-Brain Barrier Breakdown in a Single Set of Rodent Brain Samples
* These authors contributed equally
In This Article
Summary
This protocol describes a novel technique of measuring the three most important parameters of ischemic brain injury on the same set of rodent brain samples. Using only one brain sample is highly advantageous in terms of ethical and economic costs.
Abstract
One of the most common causes of morbidity and mortality worldwide is ischemic stroke. Historically, an animal model used to stimulate ischemic stroke involves middle cerebral artery occlusion (MCAO). Infarct zone, brain edema and blood-brain barrier (BBB) breakdown are measured as parameters that reflect the extent of brain injury after MCAO. A significant limitation to this method is that these measurements are normally obtained in different rat brain samples, leading to ethical and financial burdens due to the large number of rats that need to be euthanized for an appropriate sample size. Here we present a method to accurately assess brain injury following MCAO by measuring infarct zone, brain edema and BBB permeability in the same set of rat brains. This novel technique provides a more efficient way to evaluate the pathophysiology of stroke.
Introduction
One of the most common causes of morbidity and mortality worldwide is stroke. Globally, ischemic stroke represents 68% of all stroke cases, while in the United States ischemic stroke accounts for 87% of stroke cases1,2. It is estimated that the economic burden of stroke reaches $34 billion in the United States2 and €45 billion in the European Union3. Animal models of stroke are necessary to study its pathophysiology, develop new methods for evaluation, and propose new therapeutic options4.
Ischemic stroke occurs with occlusion of a major cerebral artery, usually the middle cerebral artery or one of its branches5. Thus, models of ischemic stroke have historically involved middle cerebral artery occlusion (MCAO)6,7,8,9,10,11,12. Following MCAO, neurological injury is most commonly assessed by measuring infarct zone (IZ) using a 2,3,5-triphenyltetrazolium chloride (TTC) staining method13, brain edema (BE) using drying or calculating hemispheric volumes14,15,16, and blood brain barrier (BBB) permeability by a spectrometry technique using Evans blue staining17,18,19.
The traditional MCAO method uses separate sets of brains for each of the three brain measurements. For a large sample size, this results in a significant number of euthanized animals, with added ethical and financial considerations. An alternative method to alleviate these costs would involve measurements of all three parameters in a single set of post-MCAO rodent brains.
Previous attempts have been made to measure combinations of parameters in the same brain sample. Simultaneous immunofluorescent staining methods20 as well as other molecular and biochemical analyses21 have been described after TTC staining in the same brain sample. We have previously calculated brain hemisphere volumes to assess brain edema and performed TTC staining to calculate infarct zone in the same brain set15.
In the present protocol, we present a modified MCAO technique that measures ischemic brain injury through determining IZ, BE, and BBB permeability in the same set of rodent brains. IZ is measured by TTC staining, BE is determined by calculating hemispheric volume, and BBB permeability is obtained by spectrometry methods19. In this protocol, we used a modified MCAO model, based on direct insertion and fixation of the monofilament catheter into the internal carotid artery (ICA) and further blocking of blood flow to the middle cerebral artery (MCA)22. This modified method shows a decreased rate of mortality and morbidity compared to the traditional MCAO method16,22.
This new approach provides a financially-sound and ethical model for measuring neurological injury after MCAO. This assessment of the main parameters of ischemic brain injury will help to comprehensively investigate its pathophysiology.
Protocol
The following procedures were conducted according to the recommendations of the Declaration of Helsinki and Tokyo and the Guidelines for the Use of Experimental Animals of the European Community. The experiments were also approved by the Animal Care Committee at the Ben-Gurion University of the Negev.
1. Preparing rats for the experimental procedure
- Select adult male Sprague-Dawley rats without overt pathology, each weighing between 300 and 350 g.
- Maintain all rats at room temperature at 22 °C, with 12 hours of light and dark cycles before experiment.
- Ensure that food and water are available ad libitum.
- Perform all procedures between 6:00 a.m. and 2:00 p.m.
2. Preparing rats for surgery
- Anesthetize the rats for 30 min with isoflurane (4% for induction and 2% for maintenance) and 24% oxygen (1.5 L/min).
- Test the level of anesthesia in the rats by ensuring they do not have a pedal withdrawal reflex.
- Insert the 24-gauge catheter into the tail vein.
NOTE: Tail warming for vasodilation is not performed.- Place the rats on the table in a supine position. Use medical tape to affix all four of the rats’ limbs.
- Place the probe for temperature measurement into the rat rectum before surgery.
- During the procedure, maintain a heating plate to support a 37 °C core body temperature.
- Add ointment in both of the rat’s eyes for protection.
- Shave the surgical area and disinfect with three applications of 10% povidone-iodine followed by 70% isopropyl alcohol.
3. Right side middle cerebral artery occlusion
NOTE: MCAO is performed by a modified technique, as previously described16,22,23, with the use of instruments described by McGarry et al.24 and Uluç et al.25.
- Dissect the skin and superficial fascia at the ventral midline of neck with surgical tweezers and scissors with curved blades.
- Identify the muscle triangle, consisting of the ICA, external carotid artery (ECA) and common carotid artery (CCA).
- Carefully separate the right CCA and ICA from the vagus nerve with microforceps for vascular surgery.
- Expose the right CCA and the ICA. Block the blood flow coming from the CCA to the ICA using either micro-clips or special tourniquets for vascular surgery. Make an incision (approximately 1 mm) on the ICA using microscissors for vascular surgery.
- Insert a monofilament catheter (4-0 nylon) directly through the ICA, about 18.5-19 mm from the bifurcation point of the right CCA into the circle of Willis until reaching a mild resistance, to occlude the MCA26.
- Ligate around the ICA above the bifurcation of CCA.
- For the sham-operated control group, perform an insertion of nylon thread instead of steps 3.5 and 3.616,22.
- Administer 5 mL of 0.9% sodium chloride by intraperitoneal injection.
- Close the wound by suture and take the rat to a recovery area.
NOTE: A few minutes after the end of anesthesia, the rat will wake up and move independently around the cage. - At 23 h after MCAO, inject 2% Evans blue in saline (4 mL/kg)23,26 into the tail vein for both operated groups via a cannula27.
NOTE: This is used as a blood-brain permeability tracer. Allow to circulate for 60 minutes.
4. Determination of infarct zone
- Measure IZ at 24 h after MCAO as described previously9,15,18,19,26.
NOTE: Rats that lost more than 20% of their weight or developed seizures or hemiplegia are excluded from the experiment. - Euthanize the rat by replacing the inspired gas mixture with 20% oxygen and 80% carbon dioxide until the rat ceases to breathe spontaneously.
- Open the chest with a 5-6 cm lateral incision through the abdominal wall under the rib cage using scissors and surgical forceps.
- Perform a diaphragmatic incision along the entire length of the rib cage with scissors and surgical forceps.
- Carefully displacing the lungs, cut through the rib cage up to the collarbone on the right and left sides28.
- Perfuse with 200 mL of normal saline through the left ventricle of the heart.
- Puncture or incise the right atrium of the heart with scissors.
- Perform decapitation using a guillotine and collect brain tissue.
- Using iris scissors, cut from the foramen magnum to the distal edge of the posterior skull surface on both sides.
- Separate the olfactory bulbs, nervous connections along the ventral surface and dorsal surface of the skull from the brain.
- Remove the brain from the head.
- Produce 6 brain slices by creating 2 mm thick horizontal sections with a .009" stainless steel, uncoated, single edge razor blade.
- Incubate for 30 min at 37 °C in 0.05% TTC.
- Place the brain tissue on the microscope slides and perform optical scanning of these 6 brain-slices with a resolution of 1600x1600 dpi (see Supplement 1 for example).
- Add a blue filter with a photo editor (e.g., Adobe Photoshop CS2) using the Channel Mixer function (Image > Adjustments > Channel Mixer) and save the image as a JPEG file format.
NOTE: After applying the blue filter, the image will appear greyscale. - Open the saved image in ImageJ 1.37v29,30.
NOTE: This computer program uses a threshold function to isolate and calculate the pixels that are either black or white (see Figure 1). - For each of the 6 brain slices of the image, select and save each hemisphere (right injured ipsilateral and left uninjured contralateral) as a separate image file using the “polygon selection” tool from the main menu.
- Set the cut-off for determining IZ by using an auto threshold function from the main menu of the ImageJ software by selecting Image > Adjust > Threshold, and measure the number of pixels in each hemisphere of a single brain set.
NOTE: Macros may be used for this step in ImageJ software (see Supplement 2 for the code). The cut off is a critical parameter for determining which pixels to convert to white and which to convert to black depending on the shade of gray (see Supplement 3 and Supplement 4 as examples). ImageJ then compares white and black pixels to determine IZ. Based on the staining protocol and scanner settings, we used a constant cut-off value of 0.220. - Perform measurement of IZ correcting for tissue swelling using the Ratios of Ipsilateral and Contralateral Cerebral Hemispheres (RICH) method13,23 (see example in Supplement 5).
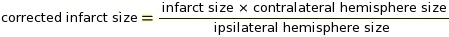
NOTE: Infarct size is assessed as a percentage of the contralateral hemisphere.
5. Determination of brain edema31
NOTE: Use ImageJ 1.37v for measurement of BE32,33.
- Measure BE 24 h after MCAO. For calculation of BE, use the data from left and right hemisphere volume (in units).
- Perform optical scanning with a resolution of 1600x1600 dpi (see Supplement 1 for example).
- Select brain hemispheres and set the cut-off for determining BE with ImageJ 1.37v, as described above in sections 4.17-4.19.
- Express the BE area as a percentage of the standard areas of the unaffected contralateral hemisphere, calculated by the RICH method using following equation (see example in Supplement 5)23,34.

NOTE: Extent of BE is assessed as a percentage of the contralateral hemisphere.
6. Determination of BBB disruption
- Measure BBB disruption 24 h after MCAO.
- Divide right and left hemispheres into six slices and put each one into a microcentrifuge tube.
- Homogenize each slice of the brain tissue in trichloroacetic acid, based on the calculation of 1 g of brain tissue in 4 mL of 50% trichloroacetic acid.
- Centrifuge at 10,000 x g for 20 min.
- Dilute supernatant liquid 1:3 with 96% ethanol.
- Perform luminescence spectrophotometry by utilizing spectrophotometry software, installing the plate, and performing a sample reading using the following parameters: Fluorescence intensity excitation wavelength of 620 nm (bandwidth 10 nm) and an emission wavelength of 680 nm (bandwidth 10 nm)23,35 ; Mod top; Number Flesh 25; Manual 100; Shaking 1 sec, 1 mm.
NOTE: Use an excitation wavelength of 620 nm (bandwidth 10 nm) and an emission wavelength of 680 nm (bandwidth 10 nm).23,35
Results
Infarct zone measurement
An independent-sample t-test indicated that 19 rats that underwent permanent MCAO demonstrated a significant increase in brain infarct volume compared to the 16 sham-operated rats (MCAO=7.49% ± 3.57 vs. Sham = 0.31% ± 1.9, t(28.49) = 7.56, p < 0.01 (see Figure 2A)). The data is expressed as a mean percentage of the contralateral hemisphere ± SD.
Brain...
Discussion
The principal goal of the present protocol was to demonstrate consistent measurements of three main parameters of ischemic injury: IZ, BE and BBB permeability. Previous studies in this field have demonstrated the possibility of performing one or two of these parameters together in the same sample. Besides the cost reduction that this three-part method offers, it also provides a more desirable bioethical model that limits the number of animals that must be operated on and subsequently euthanized. As in all histological te...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Maryna Kuscheriava, Maksym Kryvonosov, Daryna Yakumenko and Evgenia Goncharyk of the Department of Physiology, Faculty of Biology, Ecology, and Medicine, Oles Honchar, Dnipro University, Dnipro, Ukraine for their support and helpful contributions to our discussions. The data obtained are part of Ruslan Kuts’s PhD thesis.
Materials
| Name | Company | Catalog Number | Comments |
| 2 mL Syringe | Braun | 4606027V | |
| 2% chlorhexidine in 70% alcohol solution | Sigma-Aldrich | 500 cc | Provides general antisepsis of the skin in the operatory field |
| 27 G Needle with Syringe | Braun | 305620 | |
| 3-0 Silk sutures | Henry Schein | 1007842 | |
| 4-0 Nylon suture | 4-00 | ||
| Brain & Tissue Matrices | Sigma-Aldrich | 15013 | |
| Cannula Venflon 22 G | KD-FIX | 183603985447 | |
| Centrifuge Sigma 2-16P | Sigma-Aldrich | Sigma 2-16P | |
| Compact Analytical Balances | Sigma-Aldrich | HR-AZ/HR-A | |
| Digital weighing scale | Sigma-Aldrich | Rs 4,000 | |
| Dissecting scissors | Sigma-Aldrich | Z265969 | |
| Eppendorf pipette | Sigma-Aldrich | Z683884 | |
| Eppendorf tube | Sigma-Aldrich | EP0030119460 | |
| Fluorescence detector | Tecan, Männedorf Switzerland | Model: Infinite 200 PRO multimode reader | Optional. |
| Fluorescence detector | Molecular Devices LLC | VWR cat. # 10822 512 SpectraMax Paradigm Multi Mode Microplate Reader Base Instrument | Optional. |
| Gauze sponges | Fisher | 22-362-178 | |
| Heater with thermometer | Heatingpad-1 | Model: HEATINGPAD-1/2 | |
| Hemostatic microclips | Sigma-Aldrich | ||
| Horizon-XL | Mennen Medical Ltd | ||
| Infusion cuff | ABN | IC-500 | |
| Micro forceps | Sigma-Aldrich | ||
| Micro scissors | Sigma-Aldrich | ||
| Multiset | Teva Medical | 998702 | |
| Olympus BX 40 microscope | Olympus | ||
| Operating forceps | Sigma-Aldrich | ||
| Operating scissors | Sigma-Aldrich | ||
| Optical scanner | Canon | Cano Scan 4200F | Resolution 3200 x 6400 dpi |
| Petri dishes | Sigma-Aldrich | P5606 | |
| Purina Chow | Purina | 5001 | Rodent laboratory chow given to rats, mice and hamster is a life-cycle nutrition that has been used in biomedical research for over 5 decades. Provided to rats ad libitum in this experiment. |
| Rat cages | Techniplast | 2000P | Conventional housing for rodents. Cages were used for housing rats throughout the experiment |
| Scalpel blades #11 | Sigma-Aldrich | S2771 | |
| Software | |||
| Adobe Photoshop CS2 for Windows | Adobe | ||
| ImageJ 1.37v | NIH | The source code is freely available. The author, Wayne Rasband (wayne@codon.nih.gov), is at the Research Services Branch, National Institute of Mental Health, Bethesda, Maryland, USA | |
| Office 365 ProPlus | Microsoft | - | Microsoft Office Excel |
| Windows 10 | Microsoft | ||
| Reagents | |||
| 2,3,5-Triphenyltetrazolium chloride | Sigma-Aldrich | 298-96-4 | |
| 50% trichloroacetic acid | Sigma-Aldrich | 76-03-9 | |
| Ethanol 96 % | Romical | Flammable liquid | |
| Evans blue 2% | Sigma-Aldrich | 314-13-6 | |
| Isoflurane, USP 100% | Piramamal Critical Care, Inc | NDC 66794-017 |
References
- Krishnamurthi, R. V., et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Global Health. 1, 259-281 (2013).
- Benjamin, E. J., et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 135, 146 (2017).
- Wilkins, E., et al. . European cardiovascular disease statistics 2017. , (2017).
- Fluri, F., Schuhmann, M. K., Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Design, Development and Therapy. 9, 3445-3454 (2015).
- Lloyd-Jones, D., et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 119, 480-486 (2009).
- Shigeno, T., McCulloch, J., Graham, D. I., Mendelow, A. D., Teasdale, G. M. Pure cortical ischemia versus striatal ischemia. Circulatory, metabolic, and neuropathologic consequences. Surgical Neurology. 24, 47-51 (1985).
- Albanese, V., Tommasino, C., Spadaro, A., Tomasello, F. A transbasisphenoidal approach for selective occlusion of the middle cerebral artery in rats. Experientia. 36, 1302-1304 (1980).
- Hudgins, W. R., Garcia, J. H. Transorbital approach to the middle cerebral artery of the squirrel monkey: a technique for experimental cerebral infarction applicable to ultrastructural studies. Stroke. 1, 107-111 (1970).
- Waltz, A. G., Sundt, T. M., Owen, C. A. Effect of middle cerebral artery occlusion on cortical blood flow in animals. Neurology. 16, 1185-1190 (1966).
- Tamura, A., Graham, D. I., McCulloch, J., Teasdale, G. M. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. Journal of Cerebral Blood Flow & Metabolism. 1, 53-60 (1981).
- Aspey, B. S., Cohen, S., Patel, Y., Terruli, M., Harrison, M. J. Middle cerebral artery occlusion in the rat: consistent protocol for a model of stroke. Neuropathology and Applied Neurobiology. 24, 487-497 (1998).
- Longa, E. Z., Weinstein, P. R., Carlson, S., Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20, 84-91 (1989).
- O'Brien, M. D., Jordan, M. M., Waltz, A. G. Ischemic cerebral edema and the blood-brain barrier. Distributions of pertechnetate, albumin, sodium, and antipyrine in brains of cats after occlusion of the middle cerebral artery. Archives of Neurology. 30, 461-465 (1974).
- Chen, C. H., Toung, T. J., Sapirstein, A., Bhardwaj, A. Effect of duration of osmotherapy on blood-brain barrier disruption and regional cerebral edema after experimental stroke. Journal of Cerebral Blood Flow & Metabolism. 26, 951-958 (2006).
- Boyko, M., et al. Establishment of Novel Technical Methods for Evaluating Brain Edema and Lesion Volume in Stroked Rats: a Standardization of Measurement Procedures. Brain Research. , (2019).
- Boyko, M., et al. An experimental model of focal ischemia using an internal carotid artery approach. Journal of Neuroscience Methods. 193, 246-253 (2010).
- Sifat, A. E., Vaidya, B., Abbruscato, T. J. Blood-Brain Barrier Protection as a Therapeutic Strategy for Acute Ischemic Stroke. AAPS Journal. 19, 957-972 (2017).
- Jiang, X., et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Progress in Neurobiology. 163-164, 144-171 (2018).
- Belayev, L., Busto, R., Zhao, W., Ginsberg, M. D. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Research. 739, 88-96 (1996).
- Li, L., Yu, Q., Liang, W. Use of 2,3,5-triphenyltetrazolium chloride-stained brain tissues for immunofluorescence analyses after focal cerebral ischemia in rats. Pathology - Research and Practice. 214, 174-179 (2018).
- Kramer, M., et al. TTC staining of damaged brain areas after MCA occlusion in the rat does not constrict quantitative gene and protein analyses. Journal of Neuroscience Methods. 187, 84-89 (2010).
- Kuts, R., et al. A middle cerebral artery occlusion technique for inducing post-stroke depression in rats. Journal of Visualized Experiments. , e58875 (2019).
- Kuts, R., et al. A Novel Method for Assessing Cerebral Edema, Infarcted Zone and Blood-Brain Barrier Breakdown in a Single Post-stroke Rodent Brain. Frontiers in Neuroscience. 13, 1105 (2019).
- McGarry, B. L., Jokivarsi, K. T., Knight, M. J., Grohn, O. H. J., Kauppinen, R. A. A Magnetic Resonance Imaging Protocol for Stroke Onset Time Estimation in Permanent Cerebral Ischemia. Journal of Visualized Experiments. , e55277 (2017).
- Uluc, K., Miranpuri, A., Kujoth, G. C., Akture, E., Baskaya, M. K. Focal cerebral ischemia model by endovascular suture occlusion of the middle cerebral artery in the rat. Journal of Visualized Experiments. , e1978 (2011).
- Boyko, M., et al. The effect of blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome in a rat model of subarachnoid hemorrhage. Neurotherapeutics. 9, 649-657 (2012).
- Kuts, R., et al. A Middle Cerebral Artery Occlusion Technique for Inducing Post-stroke Depression in Rats. Journal of Visualized Experiments. , e58875 (2019).
- Gage, G. J., Kipke, D. R., Shain, W. Whole animal perfusion fixation for rodents. Journal of Visualized Experiments. , e3564 (2012).
- Poinsatte, K., et al. Quantification of neurovascular protection following repetitive hypoxic preconditioning and transient middle cerebral artery occlusion in mice. Journal of Visualized Experiments. , e52675 (2015).
- . ImageJ, U. S. National Institutes of Health Available from: https://imagej.nih.gov/ij (2018)
- Boyko, M., et al. Pyruvate's blood glutamate scavenging activity contributes to the spectrum of its neuroprotective mechanisms in a rat model of stroke. European Journal of Neuroscience. 34, 1432-1441 (2011).
- Collins, T. J. ImageJ for microscopy. Biotechniques. 43, 25-30 (2007).
- . ImageJ, U. S. National Institutes of Health Available from: https://imagej.nih.gov/ij (1997)
- Kaplan, B., et al. Temporal thresholds for neocortical infarction in rats subjected to reversible focal cerebral ischemia. Stroke. 22, 1032-1039 (1991).
- Kumai, Y., et al. Postischemic gene transfer of soluble Flt-1 protects against brain ischemia with marked attenuation of blood-brain barrier permeability. Journal of Cerebral Blood Flow & Metabolism. 27, 1152-1160 (2007).
- Schuleri, K. H., et al. Characterization of peri-infarct zone heterogeneity by contrast-enhanced multidetector computed tomography: a comparison with magnetic resonance imaging. Journal of the American College of Cardiology. 53, 1699-1707 (2009).
- Singh, A., Kukreti, R., Saso, L., Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules. 24, (2019).
- Di Napoli, M. Caplan's Stroke: A Clinical Approach. Journal of the American Medical Association. 302, 2600-2601 (2009).
- Deb, P., Sharma, S., Hassan, K. M. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 17, 197-218 (2010).
- Simard, J. M., Kent, T. A., Chen, M., Tarasov, K. V., Gerzanich, V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurology. 6, 258-268 (2007).
- Klatzo, I. Pathophysiological aspects of brain edema. Acta Neuropathology. 72, 236-239 (1987).
- Yang, Y., Rosenberg, G. A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 42, 3323-3328 (2011).
- Lin, T. N., He, Y. Y., Wu, G., Khan, M., Hsu, C. Y. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 24, 117-121 (1993).
- Liu, C., et al. Increased blood-brain barrier permeability in contralateral hemisphere predicts worse outcome in acute ischemic stroke after reperfusion therapy. Journal of NeuroInterventional Surgery. 10, 937-941 (2018).
- Boyko, M., et al. Establishment of novel technical methods for evaluating brain edema and lesion volume in stroked rats: A standardization of measurement procedures. Brain Research. 1718, 12-21 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
ISSN 1940-087X
Copyright © 2025 MyJoVE Corporation. All rights reserved
We use cookies to enhance your experience on our website.
By continuing to use our website or clicking “Continue”, you are agreeing to accept our cookies.