A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Ultrafast Lignin Extraction from Unusual Mediterranean Lignocellulosic Residues
In This Article
Summary
Deep eutectic solvent-based, microwave-assisted pretreatment is a green, fast, and efficient process for lignocellulosic fractionation and high-purity lignin recovery.
Abstract
Pretreatment is still the most expensive step in lignocellulosic biorefinery processes. It must be made cost-effective by minimizing chemical requirements as well as power and heat consumption and by using environment-friendly solvents. Deep eutectic solvents (DESs) are key, green, and low-cost solvents in sustainable biorefineries. They are transparent mixtures characterized by low freezing points resulting from at least one hydrogen bond donor and one hydrogen bond acceptor. Although DESs are promising solvents, it is necessary to combine them with an economic heating technology, such as microwave irradiation, for competitive profitability. Microwave irradiation is a promising strategy to shorten the heating time and boost fractionation because it can rapidly attain the appropriate temperature. The aim of this study was to develop a one-step, rapid method for biomass fractionation and lignin extraction using a low-cost and biodegradable solvent.
In this study, a microwave-assisted DES pretreatment was conducted for 60 s at 800 W, using three kinds of DESs. The DES mixtures were facilely prepared from choline chloride (ChCl) and three hydrogen-bond donors (HBDs): a monocarboxylic acid (lactic acid), a dicarboxylic acid (oxalic acid), and urea. This pretreatment was used for biomass fractionation and lignin recovery from marine residues (Posidonia leaves and aegagropile), agri-food byproducts (almond shells and olive pomace), forest residues (pinecones), and perennial lignocellulosic grasses (Stipa tenacissima). Further analyses were conducted to determine the yield, purity, and molecular weight distribution of the recovered lignin. In addition, the effect of DESs on the chemical functional groups in the extracted lignin was determined by Fourier-transform infrared (FTIR) spectroscopy. The results indicate that the ChCl-oxalic acid mixture affords the highest lignin purity and the lowest yield. The present study demonstrates that the DES-microwave process is an ultrafast, efficient, and cost-competitive technology for lignocellulosic biomass fractionation.
Introduction
Sustainable biorefinery processes integrate biomass processing, its fractionation into molecules of interest, and their conversion to value-added products1. In second-generation biorefining, pretreatment is considered essential for fractionating biomass into its main components2. Traditional pretreatment methods utilizing chemical, physical, or biological strategies have been widely applied3. However, such pretreatment is considered the most expensive step in biorefining and has other disadvantages such as long processing time, high heat and power consumption, and solvent impurities4. Recently, DESs, whose properties are similar to those of ionic liquids3, have emerged as green solvents owing to advantages such as biodegradability, environmental-friendliness, ease of synthesis, and recovery after treatment5.
DESs are mixtures of at least one HBD, such as lactic acid, malic acid, or oxalic acid, and a hydrogen-bond acceptor (HBA) such as betaine or choline chloride (ChCl)6. HBA-HBD interactions enable a catalytic mechanism that permits cleavage of chemical bonds, causing biomass fractionation and lignin separation. Many researchers have reported the DES-based pretreatment of lignocellulosic feedstocks such as ChCl-glycerol on corn's cob and stover7,8, ChCl-urea, and ChCl-oxalic acid on wheat straw9, ChCl-lactic acid on Eucalyptus sawdust10, and ChCl-acetic acid11 and ChCl-ethylene glycol on wood11. To improve DES efficiency, the pretreatment should be combined with microwave treatment to accelerate biomass fractionation5. Many researchers have reported such a combined pretreatment (DES and microwave) of wood8 and of corn stover, switchgrass, and Miscanthus5, which provides new insight into the capacity of DESs for lignocellulosic fractionation and lignin extraction in one easy step over a short period.
Lignin is a phenolic macromolecule valorized as a raw material for the production of biopolymers and presents an alternative for the production of chemicals such as aromatic monomers and oligomers12. In addition, lignin has antioxidant and ultraviolet absorption activities13. Several studies have reported lignin applications in cosmetic products14,15. Its integration in commercial sunscreen products has improved the sun protection factor (SPF) of the product from SPF 15 to SPF 30 with the addition of only 2 wt % lignin and up to SPF 50 with the addition of 10 wt % lignin16. This paper describes an ultrafast approach for lignin-carbohydrate cleavage, assisted by combined DES-microwave pretreatment of Mediterranean biomasses. These biomasses consist of agri-food byproducts, particularly olive pomace and almond shells. Other biomasses that were investigated were those of a marine origin (Posidonia leaves and aegagropile) and those originating from a forest (pinecones and wild grasses). The focus of this study was to test low-cost green solvents to evaluate the effects of this combined pretreatment on feedstock fractionation, to investigate its influence on lignin purity and yield, and to study its effects on the molecular weights and chemical functional groups in the extracted lignin.
Protocol
1. Preparation of biomasses
- Biomass drying
- Place the Posidonia leaves and aegagropile balls (Posidonia oceanica), harvested from Mediterranean beaches, in an oven at 40 °C for 72 h.
- Place the almond shells (Prunus dulcis), generated from food industries, and olive pomace (Olea europaea L.), obtained from olive oil mills, in an oven at 40 °C for 72 h.
- Place the pinecones (Pinus halepensis), collected from a forest, and alfa leaves (Stipa tenacissima), collected from the southern Mediterranean basin, in an oven at 40 °C for 72 h.
NOTE: If the biomass contains sand, it must be rinsed with distilled water before it is placed in the oven. The biomasses are shown in Figure 1A-F.
- Biomass grinding
- Place 20 g of each biomass in a hammer cutter equipped with a 1 mm sieve. Collect the resultant powder in a 0.25 L beaker and feed it to a hammer cutter equipped with a 0.5 mm sieve. Collect the powder in a 0.25 L beaker.
2. Microwave-assisted, ultrafast lignin extraction
- Deep eutectic solvent (DES) preparation
- Prepare DES1 (ChCl-oxalic acid) in a molar ratio of 1:1 by mixing 174 g of ChCl with 126 g of oxalic acid dihydrate in a 500 mL round-bottom flask and melting them in a bath at 70 °C for 4 h until a homogenous and transparent liquid is formed.
- Prepare DES2 (ChCl-lactic acid) in a molar ratio of 1:1 by mixing 174 g of ChCl with 90 g of lactic acid in a 500 mL round-bottom flask and melting them in a bath at 70 °C for 4 h until a homogenous and transparent liquid is formed.
- Prepare DES3 (ChCl-urea) in a molar ratio of 1:12 by mixing 174 g of ChCl with 120 g of urea in a 500 mL round-bottom flask and melting them in a bath at 70 °C for 4 h until a homogenous and transparent liquid is formed.
NOTE: Stir these mixtures continuously with a stir bar at 500 rpm.
- Combined microwave-DES treatment
- Place 5 g of the feedstock in a microwave in a closed polytetrafluoroethylene reactor. Add 50 mL of DES, and place a stirring bar in the sample. Close the microwave container with an appropriate cap, and attach the temperature cap.
- Place the microwave container on the edge of the turntable, ensuring that it is constantly agitated. Set the microwave power at 800 W for 1 min. Using suitable gloves, take the container out of the microwave, and let the mixture cool. Repeat this treatment using the three DESs for each biomass sample.
NOTE: Check and ensure that the temperature probe is correctly placed, and the microwave container has a homogenous temperature.
- Lignin isolation
- Prepare a homogeneous antisolvent solution by mixing ethanol:water in a 50:50 (v:v) ratio. Add 50 mL of the antisolvent solution to the treated feedstock, place the mixture in a centrifugation container (250 mL), and centrifuge for 5 min at 3,000 × g.
- After centrifugation, filter the supernatant (lignin-rich fraction) using a glass filter crucible (porosity 4, 10-16 μm, diameter 10 mm). Wash the remaining cellulose residue collected after centrifugation with 25 mL of the antisolvent solution.
- Centrifuge at 3,000 × g for 5 min after each wash. Repeat washes 4x, and collect and filter the washes through the glass filter crucible (porosity N 4, 10-16 μm, diameter 10 mm).
- Add the filtered lignin-rich fraction from step 2.3.2 to the filtered washes from step 2.3.3 in a 500 mL round bottom flask. Evaporate ethanol using a rotary evaporator at 50 °C and 110 mbar.
- Add 150 mL of deionized water to the concentrated liquor (lignin-rich fraction), and precipitate the lignin by centrifugation. Collect lignin as a pellet, and wash it with 25 mL of distilled water; repeat the washes 4x. Lyophilize lignin, or dry it in an oven at 40 °C.
NOTE: If necessary, wash lignin >4x to remove the salts from the solvents. - Use the following formula to determine the yield:
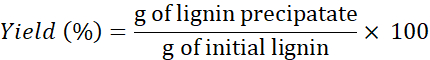
NOTE: Lignin extraction was also performed with two other DESs: choline chloride + resorcinol and choline chloride + butyric acid at 1 min. However, the amounts of lignin recovered using these DESs were extremely small (and unrecoverable) compared with the amounts obtained using the other three DESs.
3. Purity determination of extracted lignin by Klason
- Sample preparation for Klason hydrolysis
- Place the filter crucible (porosity 4, diameter 4.5 mm) in a muffle furnace at 550 °C for 4 h (2 h ramp, from 25°C). Remove the crucible when the oven cools to 150 °C, place it in a desiccator to cool, and weigh.
- Add approximately 30 mg of lignin into a borosilicate glass tube (see the Table of Materials), and note the weight of the sample. Add 1 mL of 72% sulfuric acid (H2SO4) to the sample, place the sample in a 30 °C bath for 60 min, and mix every 10 min by vortexing.
- Remove the sample, transfer it to a 100 mL glass bottle, and add 28 mL of distilled water to dilute the acid to a concentration of 4%. Place the glass bottle in an autoclave at 121 °C for 60 min. Remove the glass bottle, and allow it to cool.
- Analysis of acid-insoluble lignin
- Filter the hydrolysate using a crucible under vacuum. Collect all the solids in a glass bottle containing deionized water. Rinse the crucible with 50 mL of deionized water.
- Dry the crucible containing the solids by placing it in an oven at 105 °C for 16 h. Remove the crucible from the oven, place it in a desiccator, and allow it to cool. Weigh the sample.
- Place the crucible in a muffle furnace at 550 °C for 4 h (2 h ramp). Remove it and place in a desiccator. Weigh the sample.
- Use the following formula to calculate the percentage of the acid-insoluble residue (AIR):
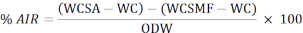
WCSA: weight of crucible + sample after removing them from the oven
WC: weight of crucible
WCSMF: weight of crucible after removing it from the muffle furnace
ODW: oven dry weight of the sample
- Analysis of acid-soluble lignin
- Measure the absorbance of the filtrate obtained in step 3.2.1 with a spectrophotometer at 205 nm using quartz cuvettes. Use distilled water as blank.
- Use the following formula to calculate the percentage of the acid-soluble residue (ASL):
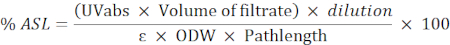
NOTE: Absorbance should be between 0.2 and 0.7. Dilute the sample if necessary.
UVabs: absorbance at 205 nm
Pathlength: light path of the measuring cell (in cm)
ε: absorbency of biomass at a specific wavelength
4. Nitrogen content in extracted lignin
- Preparation of alkali solution
- In a 2.5 L volumetric flask, weigh 1 kg of sodium hydroxide (NaOH) and add deionized water up to the mark. Place a magnetic bar in the flask, and stir until the NaOH is completely dissolved.
- Sulfuric acid solution preparation
- Take 0.1 N H2SO4 (see the Table of Materials) in a 5 L volumetric flask, add deionized water up to the 5 L mark, place a magnetic bar, and stir until the contents dissolve.
- Preparation of receiving solution
- In a 5 L volumetric flask, dissolve 100 g of H3BO3 (boric acid) in deionized water, and bring up the volume to the mark.
- Weigh 100 mg of bromocresol green in a 100 mL volumetric flask, and add technical methanol up to the mark.
- Weigh 100 mg of methyl red in a 100 mL volumetric flask, and add technical methanol up to the mark.
- Pour the 5 L of H3BO3 solution from step 4.3.1, 100 mL of bromocresol green solution from step 4.3.2, 70 mL of the methyl red solution from step 4.3.3, and 5 L of deionized water into a container. Shake the receiving solution well for 30 min.
NOTE: The final color of the solution must be green. If the color is not green, add 50 mL of 1 N NaOH solution.
- Sample preparation
- In a Kjeldahl tube, place 100 mg of lignin weighed on a nitrogen-free paper, add a tablet of Kjeldhal (1.5 g potassium sulfate (K2SO4) + 0.045 g copper sulfate pentahydrate (CuSO4.5H2O) + 0.045 g titanium dioxide (TiO2)), and add 7.2 mL of concentrated H2SO4.
NOTE: Use four tubes with only nitrogen-free paper (without the samples) as blanks.
- In a Kjeldahl tube, place 100 mg of lignin weighed on a nitrogen-free paper, add a tablet of Kjeldhal (1.5 g potassium sulfate (K2SO4) + 0.045 g copper sulfate pentahydrate (CuSO4.5H2O) + 0.045 g titanium dioxide (TiO2)), and add 7.2 mL of concentrated H2SO4.
- Sample digestion
- Switch on the thermostat on the digester 1 h in advance at 360 °C.
- Place the sample tubes on a rack, place the four blank tubes at the four corners of the rack, and fill in the holes (if any) of the rack with empty tubes.
- Place the rack in the preheated digester, cover the suction system, and open the water pump.
NOTE: Take care to avoid fumes; increase the flow of water if fumes appear. - After 2 h, turn off the heating, remove the samples, and place them on a metal support. Allow the rack to cool for approximately 40 min with the suction system on.
- Kjeldhal distillation procedure
- Switch on the Kjeldahl distiller. Allow Self-tests to run until Selection appears on the screen. Switch to Manual mode, insert an empty tube, and close the sliding door.
- Purge the titrant burette (0.02 N H2SO4) (lift the cover) by pressing it at the bottom and top several times, and eliminate air bubbles from the pipes by squeezing the tube of the H2SO4 bottle. Close the hood.
- Purge the H3BO3 receiving solution 3x.
- Add water 3x, and switch to Active steam (10 min). Switch to the Kjeldahl 1 analysis program. Enter Blanco using the arrows at the Result line level.
- Insert the tube. Start with the four blanks, and calculate their averages. Enter the value in the Blanco line.
NOTE: After the tube is inserted, the device automatically and successively adds 30 mL of H2O, 30 mL of H3BO3, and 40 mL of 10 N NaOH. - Switch to mL of titrant at the result line. Insert the tube, and note the amount of H2SO4 used.
NOTE: To test the Kjeldahl distiller, consider that 50 mg of glycerin correspond to 18.60% ± 5% of % N. At the end of each titration, the device automatically empties and cleans the tube. - Calculate the percentage of N.

V s.a : Volume of sulfuric acid
T s.a : 0.02 N H2SO4
S: sample mass
5. Ash content in extracted lignin
- Dry the ceramic crucibles for 1 h at 105 °C. Leave them to cool in a desiccator.
- Weigh a crucible, and note its number. Add approximately 1 g of the sample powder. Place the crucible in the muffle furnace with the following program: a 2 h ramp up to 575 °C; a plateau of 4 h at 575 °C.
- Allow the oven to cool to 100 °C. Remove the crucibles, place them in the desiccator, and weigh them.
6. Carbohydrate content
- Preparation of sodium borohydride (NaBH4)/dimethylsulfoxide (DMSO) solution
- Place 2 g of NaBH4 in a 100 mL volumetric flask, and fill to the mark with DMSO. Heat to 100 °C in a Mayor's bath, and stir the solution until completely dissolved.
- Preparation of MIX solution
- Place 20 mg each of xylose, arabinose, rhamnose, glucose, galactose, mannose, and 2-deoxyglucose in a 100 mL volumetric flask, and fill up to the mark of 100 mL with deionized water.
- Hydrolysis of the sample
- Weigh a 50 mg sample of lignin in a borosilicate glass tube, add 3 mL of 1 M H2SO4, and heat the mixture for 3 h at 100 °C.
- Cool the sample, add 1 mL of 15 M ammonium hydroxide (NH4OH), and check the pH to ensure that it is neutral or alkaline. Add exactly 1 mL of internal standard (2-deoxyglucose) to each sample.
NOTE: The 2-deoxyglucose added as an internal standard makes it possible to quantify the quantity of each dose present in the sample.
- Reduction and acetylation of monosaccharides into alditol acetate
- Take 400 µL of the solution from step 6.3.2, and place it in special tubes. Take 400 µL of the control MIX solution, and place it in special tubes.
NOTE: Using the MIX solution facilitates the calculation of response factors (RFs) and monosaccharide percentages. - Add 2 mL of the NaBH4/DMSO solution prepared in section 6.1. Close the tube, and incubate for 90 min at 40 °C in a water bath. Remove the tube from the water bath, and add 0.6 mL of glacial acetic acid.
NOTE: As this is an exothermic reaction, bubbles and smoke will appear. - Add approximately 0.4 mL of 1-methylimidazole and approximately 4 mL of acetic anhydride. After 15 min, add 10 mL of distilled water, cool, and add ~3 mL of dichloromethane (CH2Cl2).
- After at least 2 h, collect ~1 mL of the lower (organic) phase, and inject it into a gas chromatograph equipped with a flame ionization detector capillary column, HP1-methylsiloxane (30 m (length) x 320 µm (internal diameter), 0.25 µm (film thickness)). Analyze the data.
- Use the following formula to calculate the response factor (RF).
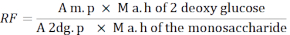
A m. p: Average of the area of the monosaccharide peak in the MIX solution
M a. h of 2 - deoxy glucose: Mass of 2-deoxyglucose after hydrolysis
A 2dg. p: Average of the area of the 2-deoxyglucose peak in the MIX solution
M a. h of the monosaccharide: Mass of the monosaccharide after hydrolysis
NOTE: Anhydro correction is 0.8 for rhamnose, 0.88 for arabinose and xylose, and 0.9 for mannose, glucose, and galactose. Mass after hydrolysis = anhydro correction x mass (g) of the monosaccharide used in the MIX solution. - Use the following formula to calculate the monosaccharide mass.
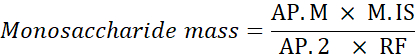
AP.M: Monosaccharide peak area in the analyzed sample
M. IS: Mass of internal standard added; here, C SI=1 mg/mL
AP.2: Peak area of 2-deoxyglucose in the sample
RF: response factor - Calculate the percentage of each monosaccharide using the following formula.

- Take 400 µL of the solution from step 6.3.2, and place it in special tubes. Take 400 µL of the control MIX solution, and place it in special tubes.
7. Chemical functions in extracted lignin (Fourier-transformed infrared)
- To identify the chemical functional groups in extracted lignin, use an FT-IR spectrometer equipped with an attenuated total reflectance (ATR) module. Open the spectroscopy software, and adjust the parameters: resolution 4 cm-1, sample scan time 32, background scan time 16, save data from 4000 to 400 cm-1, result spectrum transmittance.
- Do not add any sample; press background single channel. Now place 1 mg of the sample on the crystal, and press sample single channel. Process the obtained spectra.
8. Molecular weight of extracted lignin (gel permeation chromatography)
- Prepare a solution of dimethylformamide (DMF) with 0.5% lithium chloride (LiCl). Take 5 g of LiCl in a 1 L volumetric flask, add DMF up to the gauge line, and mix the contents until a homogenous liquid is obtained.
- Dissolve 3 mg of the lignin sample in 3 mL of DMF with 0.5% LiCl. Centrifuge in a 10 mL centrifuge tube, and separate the soluble fraction into a vial.
- Dissolve 3 mg of polystyrene standard 1 kDa, 2 kDa, 3 kDa, 10 kDa, 20 kDa, and 30 kDa in the solution of DMF with 0.5% LiCl. Centrifuge in 10 mL borosilicate glass tubes, and transfer the soluble fraction to a vial.
- Prepare a high-performance liquid chromatography-ultraviolet (UV) system.
- Open the data system, and check the UV detector.
- Purge the system with distilled water. Install the plunger in the eluent (DMF with 0.5% LiCl). Open the purging valve, and purge the line with a flow rate of 1 mL/min for 15 min. Stop the flow and close the purging valve.
- Set the flow rate to 1 mL/min for 10 min to clean the eluent pathway to the detector. Stop the flow rate.
- Install the column preceded by a guard column (see the Table of Materials). Switch on the column heater at 45 °C, switch on the UV detector, and set the flow rate gradually until a flow rate of 0.6 mL/min is reached.
- Inject 30 µL of each sample for 40 min at a wavelength of 270 nm. Process the obtained data, and calculate the mass distribution using the calibration line.
- Calculate the number average molecular weight (Mn), weight average molecular weight (Mw), and polydispersity index (PDI).
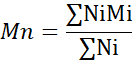
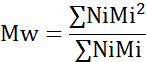
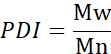
Mi: molecular weight of a chain
Ni: number of chains for that molecular weight
9. Data treatment and statistical analyses
- Perform all analytical experiments in triplicate and express the results as % of dry matter.
- Perform one-way analysis of variance (ANOVA), and compare the means using Tukey's multiple comparison test.
- Perform principal component analysis (PCA) .
Results
Figure 2A-C depict the lignin yield of extraction from the six feedstocks, shown in Figure 1A-F, after the combined microwave-DES pretreatment. The results show that the lignin yield obtained with DES1 (ChCl-oxalic acid) (Figure 2A) was lower than the yields obtained with DES2 (ChCl-lactic acid) and DES3 (ChCl-urea) (Figure 2B
Discussion
This study had many objectives; the first of which was to prepare and use low-cost green solvents with the characteristics of both ionic liquids and organic solvents. The second objective was to fractionate the biomass and extract lignin in a single step, without requiring preliminary steps such as the extraction of extractables using Soxhlet or hemicellulose using alkaline solvents, basic, or thermophysical techniques. The third aim was to recover lignin by simple filtration after the treatment, without adjustment of pH...
Disclosures
The authors report no conflict of interest.
Acknowledgements
MK and TB thanks Haitham Ayeb for statistical analyses and figure preparation, Walloon Region (European Regional Development-VERDIR) and Minister of Higher Education and Scientific Research (Taoufik Bettaieb) for funding.
Materials
| Name | Company | Catalog Number | Comments |
| HPLC Gel Permeation Chromatography | Agilent 1200 series | ||
| 1 methylimadazole | Acros organics | ||
| 2-deoxy-D-glucose (internal standard) | Sigma Aldrich (St. Louis, USA) | ||
| Acetic acid | Sigma Aldrich (St. Louis, USA) | ||
| Acetic anhydride | Sigma Aldrich (St. Louis, USA) | ||
| Adjustables pipettors | |||
| Alkali | alkali-extracted lignin | ||
| Arabinose (99%) | Sigma Aldrich (St. Louis, USA) | ||
| Autoclave | CERTO CLAV (Model CV-22-VAC-Pro) | ||
| Water Bath at 70 °C | |||
| Boric acid | Sigma Aldrich (St. Louis, USA) | ||
| Bromocresol | Sigma Aldrich (St. Louis, USA) | ||
| Catalyst | CTQ (coded A22) (1.5 g K2SO4 + 0.045 g CuSO4.5 H2O + 0.045 g TiO2) | Merck | |
| Centrifugation container | |||
| Centrifuge | BECKMAN COULTER | Avanti J-E centrifuge | |
| Ceramic crucibles | |||
| Choline chloride 99% | Acros organics | ||
| Column | Agilent PLGel Mixed C (alpha 3,000 (4.6 × 250 mm, 5 µm) preceded by a guard column (TSK gel alpha guard column 4.6 mm × 50 mm, 5 µm) | ||
| Column | HP1-methylsisoxane (30 m, 0.32 mm, 0.25 mm) | ||
| Crucible porosity N°4 ( Filtering crucible) | Shott Duran Germany | boro 3.3 | |
| Deonized water | |||
| Dessicator | |||
| Dimethylformamide | VWR BDH Chemicals | ||
| Dimethylsulfoxide | Acros organics | ||
| Erlenmeyer flask | |||
| Ethanol | Merck (Darmstadtt, Germany) | ||
| Filtering crucibles, procelain | |||
| Filtration flasks | |||
| Fourrier Transformed Inra- Red | Vertex 70 Bruker apparatus equipped with an attenuated total reflectance (ATR) module. Spectra were recorded in the 4,000–400 cm−1 range with 32 scans at a resolution of 4.0 cm−1 | ||
| Galactose (98% | Sigma Aldrich (St. Louis, USA) | ||
| Gaz Chromatography | Agilent (7890 series) | ||
| Glass bottle 100 mL | |||
| Glass tubes ( borosilicate) with teflon caps 10 mL | |||
| Glucose (98% | Sigma Aldrich (St. Louis, USA) | ||
| Golves | |||
| Graduated cylinder 50 mL /100 mL | |||
| H2SO4 Titrisol (0.1 N) | Merck (Darmstadtt, Germany) | ||
| H2SO4 (95-98%) | Sigma Aldrich (St. Louis, USA) | BUCHI R-114) | |
| Hummer cutter equiped with 1 mm and 0.5 mm sieve | Mill Ttecator (Sweden) | Cyclotec 1093 | |
| Indulin | Raw lignin control | ||
| Kjeldahl distiller | Kjeltec 2300 (Foss) | ||
| Kjeldahl tube | FOSS | ||
| Kjeldhal rack | |||
| Kjeldhal digester | Kjeltec 2300 (Foss) | ||
| Kjeldhal suction system | |||
| Lab Chem station Software | GC data analysis | ||
| Lactic acid | Merck (Darmstadtt, Germany) | ||
| Lithium chloride LiCl | Sigma Aldrich (St. Louis, USA) | ||
| Mannose (98%) | Sigma Aldrich (St. Louis, USA) | ||
| Methyl red | |||
| Microwave | START SYNTH MILESTONE Microwave laboratory system | ||
| Microwave temperature probe | |||
| Microwave container | |||
| Muffle Furnace | |||
| NaOH | Merck (Darmstadtt, Germany) | ||
| Nitrogen free- paper | |||
| Opus | spectroscopy software | ||
| Oven | GmbH Memmert SNB100 | Memmert SNB100 | |
| Oxalic acid | VWR BDH Chemicals | ||
| P 1000 | Soda-processed lignin | ||
| pH paper | |||
| precision balance | |||
| Infrared spectroscopy | |||
| Quatz cuvette | |||
| Rhamnose (98%) | Sigma Aldrich (St. Louis, USA) | ||
| Rotary vacuum evaporator | Bucher | ||
| Round-bottom flask 500 mL | |||
| sodium borohydride NaBH4 | |||
| Schott bottle | glass bottle | ||
| Sovirel tubes | sovirel | Borosilicate glass tubes | |
| Spatule | |||
| Special tube | |||
| Spectophotometer | UV-1800 Shimadzu | ||
| Sterilization indicator tape | |||
| Stir bar in teflon | |||
| Stirring plate | |||
| Syringes | |||
| Sodium borohydride | Sigma Aldrich (St. Louis, USA) | ||
| Titrisol | Merck | Merck 109984 | 0.1 N H2SO4 |
| Urea | VWR BDH Chemicals | ||
| Vials | |||
| VolumetriC flask 2.5 L /5 L | Bucher | ||
| Vortex | |||
| Xylose (98%) | Sigma Aldrich (St. Louis, USA) |
References
- Kammoun, M., et al. Hydrothermal dehydration of monosaccharides promoted by seawater fundamentals on the catalytic role of inorganic salts. Frontiers in Chemistry. 7, 132 (2019).
- Kammoun, M., Ayeb, H., Bettaieb, T., Richel, A. Chemical characterisation and technical assessment of agri-food residues, marine matrices, and wild grasses in the South Mediterranean area: A considerable inflow for biorefineries. Waste Management. 118, 247-257 (2020).
- Zhang, C. W., Xia, S. Q., Ma, P. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresource Technology. 219, 1-5 (2016).
- Mora-Pale, M., Meli, L., Doherty, T. V., Linhardt, R. J., Dordick, J. S. Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Biotechnology and Bioengineering. 108 (6), 1229-1245 (2011).
- Chen, Z., Wan, C. Ultrafast fractionation of lignocellulosic biomass by microwave-assisted deep eutectic solvent pretreatment. Bioresource Technologie. 250, 532-537 (2018).
- Francisco, M., Van Den Bruinhorst, A., Kroon, M. C. New natural and renewable low transition temperature mixtures ( LTTMs ): screening as solvents for lignocellulosic biomass processing. Green Chemistry. 14 (8), 2153-2157 (2012).
- Liu, Y. C., et al. Efficient cleavage of lignin - carbohydrate complexes and ultrafast extraction of lignin oligomers from wood biomass by microwave-assisted treatment with deep eutectic solvent. Chem sus chem. 10, 1692-1700 (2017).
- Xu, G. C., Ding, J. C., Han, R. Z., Dong, J. J., Ni, Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresource Technologie. 203, 364-369 (2016).
- Jablonský, M., Andrea, &. #. 3. 5. 2. ;., Kamenská, L., Vrška, M., Šima, J. Deep eutectic solvents fractionation of wheat straw deep eutectic solvents fractionation of wheat straw. Bioresources. 10 (4), 8039-8047 (2015).
- Shen, X. J., et al. Facile fractionation of lignocelluloses by biomass-derived deep eutectic solvent (DES) pretreatment for cellulose enzymatic hydrolysis and lignin valorization. Green Chemistry. 21, 275-283 (2019).
- Alvarez-Vasco, C., et al. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): a source of lignin for valorization. Green Chemistry. 18, 5133-5141 (2016).
- Banu, J. R., et al. A review on biopolymer production via lignin valorization. Bioresource Technologie. 290, 121790 (2019).
- Gordobil, O., Olaizola, P., Banales, J. M., Labidi, J. Lignins from agroindustrial by-products as natural ingredients for cosmetics chemical structure and in vitro sunscreen and cytotoxic activities. Molecules. 25 (5), 1131 (2020).
- Lee, C. S., Thu Tran, T. M., Weon Choi, J., Won, K. Lignin for white natural sunscreens. International Journal of Biological Macromolecules. 122, 549-554 (2019).
- Widsten, P. Lignin-based sunscreens-state-of-the-art, prospects and challenges. Cosmetics. 7, 85 (2020).
- Qian, Y., Qiu, X., Zhu, S. Lignin: a nature-inspired sun blocker for broad-spectrum sunscreens. Royal Society of Chemistry. 17, 320-324 (2015).
- Zijlstra, D. S., et al. Extraction of lignin with high β-O-4 content by mild ethanol extraction and its effect on the depolymerization yield. Journal of Visualized Experiments. (143), e58575 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved