A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Generalized Method for Determining Free Soluble Phenolic Acid Composition and Antioxidant Capacity of Cereals and Legumes
In This Article
Summary
Phenolic acids are important phytochemicals that are present in whole grains. They possess bioactive properties such as antioxidant protective functions. This work aimed at reporting on a generalized method for the HPLC identification, total phenolic content estimation, and determination of the antioxidant capacity of phenolic acids in cereals and legumes.
Abstract
Phenolic acids are a class of organic compounds that bear both a phenolic group, and a carboxylic group. They are found in grains and concentrate in the bran of cereals or seed coat of legumes. They possess antioxidant properties that have generated much research interest in recent years, about their potential antioxidant protective health functions. This work presents a generalized method for the extraction of free soluble phenolic acids from whole grains and analysis of their antioxidant capacity. Five whole grain samples comprising two cereals (wheat and yellow corn) and three legumes (cowpea bean, kidney bean, and soybean), were used. The grains were milled into flour and their free soluble phenolic acids extracted using aqueous methanol. The compounds were then identified using a high-pressure liquid chromatograph (HPLC). The Folin-Ciocalteu method was used to determine their total phenolic content while their antioxidant capacities were determined using the DPPH radical scavenging capacity, Trolox equivalent antioxidant capacity (TEAC) and oxygen radical absorbance capacity (ORAC) assays. The phenolic acids identified included vanillic, caffeic, p-coumaric and ferulic acids. Vanillic acid was identified only in cowpea while caffeic acid was identified only in kidney bean. p-Coumaric acid was identified in yellow corn, cowpea, and soybean, while ferulic acid was identified in all the samples. Ferulic acid was the predominant phenolic acid identified. The total concentration of phenolic acids in the samples decreased in the following order: soybean > cowpea bean > yellow corn = kidney bean > wheat. The total antioxidant capacity (sum of values of DPPH, TEAC and ORAC assays) decreased as follows: soybean > kidney bean > yellow corn = cowpea bean > wheat. This study concluded that HPLC analysis as well as DPPH, TEAC, and ORAC assays provide useful information about the phenolic acid composition and antioxidant properties of whole grains.
Introduction
Phenolic acids are among the most important phytochemicals studied in plants due to the vital role they play in plant defense against herbivory and fungal infection, as well as maintaining structural support and integrity in plant tissues1,2. They are abundant in the bran of cereals and seed coat of legumes3. Structurally, they are divided into two groups: the hydroxybenzoic acids (Figure 1) and hydroxycinnamic acids (Figure 2). The common hydroxybenzoic acids in cereals and legumes include gallic, p-hydroxybenzoic, 2,4-dihydroxybenzoic, protocatechuic, vanillic, and syringic acids, while the common hydroxycinnamic acids include caffeic, p-coumaric, ferulic, and sinapic acids3. Phenolic acids also possess antioxidant properties since they are able to scavenge free radicals, which cause oxidative rancidity in fats, and initiate and propagate radical-induced oxidative stress in physiological systems4,5. Due to this vital physiological role as antioxidants, they are the subject of recent research. This is because when consumed as components of plant foods, they can exert antioxidant protection.
Cereals and cereal products are major carbohydrate food sources for humans and animals worldwide6. Cereals include wheat, rice, corn (maize), barley, triticale, millets, and sorghum. Among them, corn is the most utilized, with an estimated global utilization of 1,135.7 million tonnes in 2019/2020, followed by wheat with an estimated global utilization of 757.5 million tonnes during the same period7. Cereal foods are great sources of energy to consumers since they are rich sources of carbohydrates. They also provide some protein, fat, fiber, vitamins and minerals6. In addition to their nutritional value, cereals are good sources of phytochemical antioxidants, particularly phenolic acids, which have the potential to protect the physiological system from radical-induced oxidative damage3. Legumes are also good sources of nutrients and are generally higher in protein than cereals. They also contain vitamins and minerals and are used in the preparation of various foods8. Additionally, legumes are good sources of a variety of phytochemical antioxidants, including phenolic acids, flavonoids, anthocyanins, and proanthocyanidins9,10. Different varieties of cereals and legumes may have a different phenolic acid composition. There is therefore the need to study the phenolic acid composition of cereals and legumes and their varieties, in order to know their potential health benefits with respect to phenolic antioxidants.
A number of assays have been reported for measuring the quantity of phenolic acids in cereal and legume grains, and determining their antioxidant activities. The most common methods of analysis for whole grain phenolic acids are spectrophotometry and liquid chromatography11. The aim of this work was to demonstrate a generalized high pressure liquid chromatographic method for determining free soluble phenolic acid composition, and spectrophotometric methods for determining total phenolic content and antioxidant capacity of some whole grain cereals and legumes.
Protocol
1. Type of samples
- Use five whole grain samples, comprising two cereals (e.g., durum wheat and yellow corn) and three legumes (e.g., Blackeye cowpea bean, soybean, and red kidney bean) for this study.
- Mill 50 g of each grain in triplicates into flour, using a coffee grinder, and pass them through a 500 µm sieve.
- Store them at -20 °C.
2. Sample preparation
- Determination of dry matter content and expression of dry weight basis
NOTE: Determine the dry matter content of each powdered sample according to the method of AOAC (2000)12.- Turn on a forced-convection oven and set the temperature to 130 °C.
- Dry desiccant (silica gel) in the oven for 30 min to 1 h and transfer the dried silica gel to a desiccator.
- Accurately weigh 2 g of each sample into a clean, pre-dried, and weighed aluminum can.
- Dry the weighed samples at 130 °C for 1 h in a forced convection oven.
- Transfer the dried sample to the desiccator and allow to cool to ambient temperature.
- Weigh the dried, cooled sample and record its weight.
- Calculate the dry matter content of each sample as follows:
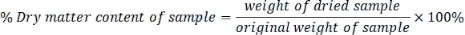
- Express each parameter measured in dry weight basis using the following formula:

- Phenolic acid extraction
NOTE: Extract the soluble free phenolic compounds in the grain samples using a modification of the method of Y. Qiu et al.5 that enables phenolic extraction from milligram quantities of whole grains.- Accurately weigh 100 mg of the whole grain flour sample directly into an amber-colored 2 mL capacity microcentrifuge tube. The dark color of the tube helps to prevent exposure of the mixture to light.
- Add 1 mL of 80% aqueous HPLC-grade methanol to each of the tubes containing the samples.
- Vortex briefly to mix the methanol solution and the samples.
- Sonicate the samples for 60 min to extract the free soluble phenolic compounds. Put a cover over the samples for the duration of the sonication for added protection from light.
- After sonication, centrifuge the mixture at 20,000 × g for 5 min to sediment the solid residues leaving the supernatant on top. Free phenolic compounds will be present in the supernatant after centrifugation.
- Transfer the supernatant into a clean microcentrifuge tube.
NOTE: The supernatant needs to be filtered prior to injecting into the HPLC instrument. To filter the supernatant, remove the plunger of a 3 mL syringe and attach a syringe filter. The filter should have a pore size of no larger than 0.22 µm. - Pipette approximately 0.4 mL of the supernatant into the top of the syringe. Re-insert the plunger and push the liquid through the filter into an HPLC vial containing a vial insert.
- Once the instrument has been set up to run the method outlined in the manuscript for HPLC analysis, load the vials into the carousel to correspond with the sample list.
- Obtain HPLC chromatograms at 320 nm and 280 nm showing distinct peaks representing different phenolic compounds.
- Using appropriate standard curves, quantify hydroxycinnamic acids at 320 nm since they have maximum absorbance at this wavelength. By the same principle, quantify hydroxybenzoic acids at 280 nm.
- Store the remaining extracts at -20 °C for other analyses.
3. Phenolic composition
- Use a high-pressure liquid chromatograph (Table of Materials) to identify and quantify the extracted phenolic compounds in the samples, based on the method of J. Xiang, F. B. Apea-Bah, V. U. Ndolo, M. C. Katundu, and T. Beta 4.
- Prepare phenolic acid standards (vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, and sinapic acid) to identify and quantify constituent phenolic compounds in the extracts.
- To do this, weigh 1 mg of each standard and dissolve in 1 mL of 50% aqueous methanol to produce 1,000 µg/mL of each standard.
- Mix equal volumes of all five standards in a 2 mL amber centrifuge tube to produce a cocktail of standards, each having a concentration of 200 µg/mL.
- Prepare serial dilutions of the standard cocktail by taking a volume into a new tube and diluting with equal volume of the aqueous methanol solvent.
- Repeat the serial dilutions down to a concentration of 3.125 µg/mL.
- Also, separately dilute each standard 40 times with the solvent to obtain 25 µg/mL concentration of each standard.
- Set the column temperature to 35 °C and the sample oven temperature to 15 °C.
- To prepare mobile phase A (0.1% aqueous formic acid), transfer 1 mL of formic acid into a 1 L capacity volumetric flask and add HPLC-grade water to the 1 L mark. Shake well to mix.
- To prepare mobile phase B (0.1% formic acid in methanol), transfer 1 mL of formic acid into a 1 L capacity volumetric flask and add HPLC-grade methanol to the 1 L mark. Shake well to mix.
- Adjust the volumes to the 1 L mark if necessary.
- Filter both mobile phases through a 0.45 µm hydrophilic filter paper.
- For the analysis, inject 10 µL of each extract or standard (25 µg/mL standards and the standard cocktails) on to the reverse phase column.
- Elute with the mobile phases according to the linear gradient program for a 25 min run as follows: 0-3.81 min, 9%-14% B; 3.81-4.85 min, 14%-15% B; 4.85-5.89 min, 15%-15% B, 5.89-8.32 min, 15%-17% B; 8.32-9.71 min, 17%-19% B; 9.71-10.40 min, 19%-19% B; 10.40-12.48 min, 19%-26% B; 12.48-13.17 min, 16%-28% B; 13.17-14.21 min, 28%-35% B; 14.21-15.95 min, 35%-40% B; 15.95-16.64 min, 40%-48% B; 16.64-18.37 min, 48%-53% B; 18.37-22.53 min, 53%-70% B; 22.53-22.88 min, 70%-90% B; 22.88-25.00 min, 90% B.
- Compare the retention times of chromatographic peaks obtained for the authentic standards of concentrations 25 µg/mL, at 280 nm and 320 nm, with those of the extracts, in order to identify the constituent phenolic compounds in the samples.
- Plot calibration curves for the phenolic acid standard cocktails, with concentration of the standards on the horizontal axis, and the peak area on the vertical axis
- Use the calibration curves to estimate the concentrations of the identified phenolic compounds, by comparing the peak areas on the plot with that of the identified compounds at 280 nm and 320 nm as mentioned in the earlier section.
4. Total phenolic content
NOTE: Determine the total phenolic content of the extracts using the Folin-Ciocalteu method described by F. B. Apea-Bah et al.13.
- Prepare ferulic acid and gallic acid standards within the concentration range of 0.025 to 0.150 mg/mL to plot calibration curves in comparison, for the estimation of total phenolic content.
- To do this, accurately weigh 1 mg each of ferulic acid and gallic acid standards into 2 mL capacity centrifuge tubes.
- Add 1 mL of 50% aqueous methanol to each standard and vortex to dissolve, producing 1 mg/mL stock of each standard.
- Prepare a series of dilutions from each stock solution in a total of 500 µL.
- Pipette 18.2 µL of each extract or standard into a separate well on a 96-well microplate.
- Add 36.4 µL of 10% (v/v) aqueous Folin-Ciocalteu reagent to each extract or standard.
- Then, add 145.4 µL of 700 mM sodium carbonate to each reaction mixture.
- Incubate the reaction mixtures in the dark at room temperature (20-25 °C) for 2 h.
- Read the absorbance on a microplate reader at 750 nm.
- Plot calibration curves of change in absorbance against concentration of the phenolic acid standards and use them to estimate the total phenolic contents.
- Express the results as milligram ferulic acid equivalents per gram of milled sample (mg FAE/g) and milligram gallic acid equivalents per gram of milled sample (mg GAE/g) on dry weight basis.
5. Antioxidant assays
NOTE: Determine the antioxidant capacity of the grain extracts using the following three assays: 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity; 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging capacity, which is also called the Trolox equivalent antioxidant capacity (TEAC); and oxygen radical absorbance capacity (ORAC).
- Preparation of Trolox standards for standard curves
- Use Trolox, a water-soluble analogue of vitamin E, as a standard to estimate the in vitro antioxidant capacity of the whole grain extracts.
- Accurately weigh 1 mg of Trolox into a 15 mL tube. Dissolve in 4 mL of 50% aqueous methanol. Vortex to dissolve to prepare a stock solution of 1 mM (1000 µM).
- Prepare six concentrations of Trolox, that is, 50, 100, 200, 400, 600, and 800 µM to plot standard curves for the estimation of DPPH radical scavenging capacity and Trolox equivalent antioxidant capacity (TEAC). Similarly, prepare 6.25, 12.5, 25, and 50 µM concentrations of Trolox for estimating oxygen radical absorbance capacity (ORAC). Make up the total volume of each concentration to 500 µL as shown in Table 1.
- Dilution of sample extracts
- Dilute the sample extracts with methanol prior to analysis. Here, yellow corn and cowpea extracts were diluted two times, the wheat and kidney bean were diluted five times while the soybean extract was diluted 10 times with methanol.
- Trolox equivalent antioxidant capacity (TEAC) assay
- Measure the Trolox equivalent antioxidant capacity (TEAC) of the samples using the method described previously by F. B. Apea-Bah et al.14.
- Weigh 8.23 mg of ABTS into a clean 2 mL capacity amber centrifuge tube.
- Next, weigh 1.62 mg of potassium persulfate into another clean 2 mL capacity amber centrifuge tube.
- Add 1 mL distilled water to each of them and vortex to dissolve them.
NOTE: This results in 16 mM ABTS stock solution with 6 mM aqueous potassium persulfate solution. - Prepare the ABTS stock solution by mixing the ABTS and potassium persulfate solutions in equal volumes. The solution will immediately change to a dark color.
- Incubate the reagent mixture in the dark for 12-16 h.
- Dilute the ABTS stock solution 30 times with 200 mM phosphate buffered saline (PBS), to form the ABTS working solution. To do this, add 58 mL of 200 mM PBS to 2 mL of the ABTS stock solution. The working solution will contain 0.27 mM ABTS and 0.1 mM potassium persulfate.
- For the analysis, place 10 µL of each diluted extract or Trolox into a 96-well microplate.
- Add 190 µL of ABTS working solution to each well and incubate the reaction mixtures for 60 min.
- Measure the absorbance of the reaction mixtures at 750 nm in a microplate reader.
- Use the Trolox standards in a concentration ranging from 100 to 800 µmol/L to plot a calibration curve.
- Estimate the ABTS radical scavenging capacity from the calibration curve.
- Express the results as micromole Trolox equivalents per gram (µmol TE/g) sample on dry weight basis.
- DPPH assay
NOTE: Determine the DPPH radical scavenging capacity of the samples using the method described previously by F. B. Apea-Bah et al.13. The DPPH antioxidant assay requires a radical-generating compound, DPPH (2,2-diphenyl-1-picrylhydrazyl).- Accurately weigh 1.2 mg of DPPH into an empty 50 mL capacity centrifuge tube. Dissolve the DPPH in 30 mL of methanol to prepare a 60 µmol/L methanolic solution.
NOTE: The DPPH assay tests the ability of the sample extracts to scavenge free radicals produced by DPPH. - For the analysis, add 5 µL of the sample extracts or Trolox solution into the microplate wells.
- Next, add 195 µL of the 60 µmol/L DPPH methanolic solution and incubate for 60 min.
- Measure the absorbance of the reaction mixture at 515 nm.
- Use the Trolox standards (50-800 µmol/L) to plot a calibration curve, with the change in absorbance on the vertical axis and the Trolox concentrations on the horizontal axis.
- Estimate the DPPH scavenging capacity from the calibration curve.
- Express the results as micromole Trolox equivalents per gram (µmol TE/g) sample on dry weight basis.
- Accurately weigh 1.2 mg of DPPH into an empty 50 mL capacity centrifuge tube. Dissolve the DPPH in 30 mL of methanol to prepare a 60 µmol/L methanolic solution.
- Oxygen radical absorbance capacity
- Determine the oxygen radical absorbance capacity (ORAC) of the samples based on the method of F. B. Apea-Bah et al.13.
- To begin, prepare Trolox standards of concentrations 6.25, 12.5, 25, and 50 µM from a 1,000 µM Trolox standard stock solution in 75 mM potassium phosphate (K2HPO4/KH2PO4) buffer.
- To do this, weigh 1 mg Trolox powder and dissolve in 4 mL of the buffer solution under sonication to prepare a 1,000 µM stock solution.
- Then, take 50 µL of the stock solution into a 2 mL capacity centrifuge tube and dilute with 950 µL of buffer solution to obtain 1,000 µL of 50 µM Trolox solution.
- Pipette 500 µL of the 50 µM solution into a new tube and dilute with equal volume of buffer to obtain a 25 µM Trolox solution.
- Repeat the serial dilution of the 25 µM to obtain 12.5 µM, and then similarly repeat the dilution of 12.5 µM to obtain 6.25 µM.
- Prepare appropriate dilutions of the sample extracts.
- Dilute the yellow corn and cowpea extracts 20 times, the wheat and kidney bean extracts 50 times, and the soybean extract 100 times with the buffer solution.
- Transfer 200 µL of each sample or Trolox standard into the wells of a black 96-well microplate for automatic pipetting.
- Then, fill the three reagent tanks provided with the ORAC equipment, with the following: (1) the buffer solution; (2) 0.816 nM fluorescein in the buffer; and (3) 153 mM of 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) in buffer.
- Place them in their receptacles for automatic pipetting.
- Set up the ORAC machine and automatic pipetting system for analysis, based on the standard operating procedure.
- For the analysis, set up the automated pipetting system to transfer 25 µL of each diluted extract or standard to the wells of a clear bottom black 96-well microplate.
- Then, automatically add 150 µL of 0.816 nM fluorescein in buffer.
- Incubate the reaction mixture at 37 °C for 15 min in the ORAC machine.
- Afterwards, automatically add 25 µL of the AAPH to each reaction mixture.
- Incubate them at 37 °C for 50 min in the ORAC machine.
- Set up the ORAC machine to measure the fluorescence decay, over the incubation period, at excitation and emission wavelengths of 485 nm and 520 nm, respectively.
- After the measurements, plot a Trolox standard curve, with Trolox (6.25-50 µM) on the horizontal axis and fluorescence decay on the vertical axis.
- Estimate the oxygen radical absorbance capacity of the extracts from the Trolox standard curve.
- Express the results as µmol TE/g sample on dry weight basis.
- Statistical analysis
- Present all the results as means ± standard deviation of at least triplicates.
- Perform analysis of variance (ANOVA) to determine the effect of grain type on the response variables.
- Where significant differences exist at p < 0.05, use least significant difference (LSD) to compare the means.
- Perform Pearson correlation analysis to estimate the relationship between the phenolic content and antioxidant capacities.
Results
Table 2 shows the phenolic acids that were identified in the cereal and legume grains. Based on available authentic standards, four phenolic acids were identified in the samples and they are: vanillic, caffeic, p-coumaric, and ferulic acids. Vanillic acid is a hydroxybenzoic acid while the other three are hydroxycinnamic acids. Vanillic acid was identified only in Blackeye cowpea bean while caffeic acid was identified only in kidney bean. p-Coumaric acid was identified in yellow corn, c...
Discussion
The whole grains were selected as representative cereal grains and legumes that find wide food applications worldwide. While variations may exist among cultivars of each grain, the focus of this study was to demonstrate a generalized method for free phenolic acid extraction and analysis for whole grains. The extraction method was modified by substantially reducing the amounts of samples and solvents, in order to reduce the amount of chemicals that would be released into the environment when such experiments are conducted...
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge the technical support of Ms. Alison Ser and Ms. Hannah Oduro-Obeng, as well as the video editing support by Ms. Janice Fajardo and Mr. Miguel del Rosario.
Materials
| Name | Company | Catalog Number | Comments |
| 15 mL Falcon conical centrifuge tubes | Fisher Scientific | 05-527-90 | |
| 2 mL Amber glass ID Surestop vial | Thermo Scientific | C5000-2W | |
| 2 mL Amber microcentrifuge tubes | VWR | 20170-084 | |
| 2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH) | Sigma-Aldrich | 440914-100G | |
| 2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) (C18H18N4O6S4) ≥98%, | Sigma Aldrich | A1888-2G | |
| 2,2-Diphenyl-1pikrylhydrazyl (DPPH) (C18H12N5O6) | Sigma Aldrich | D913-2 | |
| 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) (C14H18O4), ≥98% | Fluka Chemika | 56510 | |
| 9 mm Autosampler Vial Screw Thread Caps | Thermo Scientific | 60180-670 | |
| 96 well flat bottom plates | Fisher Scientific | 12565501 | |
| Agilent BioTek ELx800 microplate reader | Fisher Scientific | BT-ELX800NB | |
| Agilent BioTek Precision 2000 96/384 Automated Microplate Pipetting System | Fisher Scientific | N/A | |
| Agilent BioTek FLx800 Microplate Fluorescence Reader | Fisher Scientific | N/A | |
| Analytical balance SI-114 | Denver Instrument | SI-114.1 | |
| Autosampler, Waters 717 Plus | Waters | WAT078900 | |
| BD 3 mL syringe Luer-Lok Tip | BD | 309657 | |
| Bransonic ultrasonic cleaner, Branson 5510 | Millipore Sigma | Z245143 | |
| Corning LSE Vortex Mixer | Corning | 6775 | |
| Durapore Filter (0.45 µm PVDF Membrane) | Merck Millipore Ltd | HVLP04700 | |
| Durapore Membrane Filters (0.45 µm HV) | Merck Millipore Ltd | HVHP04700 | |
| Eppendorf Research plus, 0.5-10 µL | Eppendorf | 3123000020 | |
| Eppendorf Research plus, 0.5-5 mL | Eppendorf | 3123000071 | |
| Eppendorf Research plus, 100-1000 µL | Eppendorf | 3123000063 | |
| Eppendorf Research plus, 10-100 µL | Eppendorf | 3123000047 | |
| Ethyl acetate, HPLC grade | Fisher Chemical | E195-4 | |
| Ferulic acid standard | Sigma Aldrich | 128708-5G | |
| Fluorescein | Fisher Scientific | AC119245000 | |
| Folin & Ciocalteu phenol reagent | Sigma Aldrich | F9252 | |
| Formic acid, 99% | Acros Organics, Janssen Pharmaceuticalaan 3a | 27048-0010 | |
| Gallic acid standard | Sigma | G7384 | |
| High performance liquid chromatograph (HPLC), Waters 2695 | Waters | 960402 | |
| Methanol, HPLC grade | Fisher Chemical | A452-4 | |
| Micro pipet tips, 0.5-10 µL | Fisherbrand | 21-197-2F | |
| Microcentrifuge Sorvall Legend Micro 21 centrifuge | Thermo Scientific | 75002435 | |
| Multichannel micropipette, Proline Plus, 30-300 µL | Sartorius | 728240 | |
| Photodiode array detector, Waters 2996 | Waters | 720000350EN | |
| Pipet tips, 1000 µL | VWR | 83007-382 | |
| Pipet tips, 1-5 mL | VWR | 82018-840 | |
| Potassium persulfate (K2S2O8), ≥99.0% | Sigma Aldrich | 216224-100G | |
| Potassium phosphate dibasic anhydrous (K2HPO4) | Fisher Scientific | P288-500 | |
| Potassium phosphate monobasic (KH2PO4) | Fisher Scientific | P285-500 | |
| PYREX 250 mL Short Neck Boiling Flask, Round Bottom | Corning | 4321-250 | |
| Reversed phase C18 Analytical Column (100 x 3 mm) Accucore aQ | Thermo Scientific | 17326-103030 | |
| Roto evaporator, IKA RV 10 | IKA | 0010005185 | |
| Sodium carbonate (NaCO3) anhydrous | Fisher Chemical | S263-1 | |
| Sodium chloride (NaCl) | Mallinckrodt AR® | 7581 | |
| Sodium phosphate dibasic anhydrous (Na2HPO4) | Fisher Scientific | BP332-500 | |
| Sodium phosphate monobasic anhydrous (NaH2PO4) | Fisher bioreagents | BP329-500 | |
| Standardization pipet tips 0-200µL | Fisherbrand | 02-681-134 | |
| Syringe Driven Filter unit (0.22 µm) | Millex®-GV | SLGVR04NL | |
| Target micro-serts vial insert (400 µL) | Thermo Scientific | C4011-631 | |
| Ultrapure water (Direct Q-3 UV system with pump) | Millipore | ZRQSVP030 |
References
- Huitu, O., et al. Silicon, endophytes and secondary metabolites as grass defenses against mammalian herbivores. Frontiers in Plant Science. 5, 478 (2014).
- Joshi, J. R., Burdman, S., Lipsky, A., Yariv, S., Yedidia, I. Plant phenolic acids affect the virulence of Pectobacterium aroidearum and P. carotovorum ssp. brasiliense via quorum sensing regulation. Molecular Plant Pathology. 17 (4), 487-500 (2016).
- Dykes, L., Rooney, L. W. Phenolic compounds in cereal grains and their health benefits. Cereal Foods World. 52 (3), 105-111 (2007).
- Xiang, J., Apea-Bah, F. B., Ndolo, V. U., Katundu, M. C., Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chemistry. 275, 361-368 (2019).
- Qiu, Y., Liu, Q., Beta, T. Antioxidant properties of commercial wild rice and analysis of soluble and insoluble phenolic acids. Food Chemistry. 121 (1), 140-147 (2010).
- Beverly, R. L., Motarjemi, Y. . Encyclopedia of Food Safety. 3, 309-314 (2014).
- FAO. Food Outlook - Biannual report on global food markets. Food and Agriculture Organization. , (2020).
- Erbersdobler, H. F., Barth, C. A., Jahreis, G. Legumes in human nutrition. Nutrient content and protein quality of pulses. Ernahrungs Umschau. 64 (9), 134-139 (2017).
- Dueñas, M., Hernández, T., Estrella, I. Assessment of in vitro antioxidant capacity of the seed coat and the cotyledon of legumes in relation to their phenolic contents. Food Chemistry. 98 (1), 95-103 (2006).
- Khang, D. T., Dung, T. N., Elzaawely, A. A., Xuan, T. D. Phenolic profiles and antioxidant activity of germinated legumes. Foods. 5 (2), 27 (2016).
- Hefni, M. E., Amann, L. S., Witthöft, C. M. A HPLC-UV method for the quantification of phenolic acids in cereals. Food Analytical Methods. 12 (12), 2802-2812 (2019).
- AOAC. . Official Methods of Analysis. 17th edn. , (2000).
- Apea-Bah, F. B., Head, D., Scales, R., Bazylo, R., Beta, T. Hydrothermal extraction, a promising method for concentrating phenolic antioxidants from red osier dogwood (Cornus stolonifer) leaves and stems. Heliyon. 6 (10), 05158 (2020).
- Apea-Bah, F. B., Minnaar, A., Bester, M. J., Duodu, K. G. Sorghum-cowpea composite porridge as a functional food, Part II: Antioxidant properties as affected by simulated in vitro gastrointestinal digestion. Food Chemistry. 197, 307-315 (2016).
- Robbins, R. J., Bean, S. R. Development of a quantitative high-performance liquid chromatography-photodiode array detection measurement system for phenolic acids. Journal of Chromatography A. 1038 (1-2), 97-105 (2004).
- Singleton, V. L., Rossi, J. A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 16, 144-158 (1965).
- Prior, R. L., Wu, X., Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agricultural and Food Chemistry. 53 (10), 4290-4302 (2005).
- Ainsworth, E. A., Gillespie, K. M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nature Protocols. 2 (4), 875-877 (2007).
- Waterhouse, A. L. Determination of total phenolics. Current Protocols in Food Analytical Chemistry. 1, 1-8 (2002).
- Huang, D., Ou, B., Prior, R. L. The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry. 53 (6), 1841-1856 (2005).
- Esterbauer, H., Wäg, G., Puhl, H. Lipid peroxidation and its role in atherosclerosis. British Medical Bulletin. 49 (3), 566-576 (1993).
- Esterbauer, H., Gebicki, J., Puhl, H., Jürgens, G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radical Biology and Medicine. 13 (4), 341-390 (1992).
- Apea-Bah, F. B., Serem, J. C., Bester, M. J., Duodu, K. G. Phenolic composition and antioxidant properties of Koose, a deep-fat fried cowpea cake. Food Chemistry. 237, 247-256 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved