A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Automatic Identification of Dendritic Branches and their Orientation
In This Article
Summary
Presented is a computational tool that allows simple and direct automatic measurement of orientations of neuronal dendritic branches from 2D fluorescence images.
Abstract
The structure of neuronal dendritic trees plays a key role in the integration of synaptic inputs in neurons. Therefore, characterization of the morphology of dendrites is essential for a better understanding of neuronal function. However, the complexity of dendritic trees, both when isolated and especially when located within neuronal networks, has not been completely understood. We developed a new computational tool, SOA (Segmentation and Orientation Analysis), which allows automatic measurement of the orientation of dendritic branches from fluorescence images of 2D neuronal cultures. SOA, written in Python, uses segmentation to distinguish dendritic branches from the image background and accumulates a database on the spatial direction of each branch. The database is then used to calculate morphological parameters such as the directional distribution of dendritic branches in a network and the prevalence of parallel dendritic branch growth. The data obtained can be used to detect structural changes in dendrites in response to neuronal activity and to biological and pharmacological stimuli.
Introduction
Dendritic morphogenesis is a central subject in neuroscience, as the structure of the dendritic tree affects the computational properties of synaptic integration in neurons1,2,3. Moreover, morphological abnormalities and modifications in dendritic branches are implicated in degenerative and neuro-developmental disorders4,5,6. In neuronal cultures where dendritic ramification can be more readily visualized, the interactions between non-sister dendritic branches regulate the sites and extent of synaptic clustering along the branches, a behavior that may affect synaptic coactivity and plasticity7,8,9. Therefore, characterization of the morphological parameters of the dendritic tree using two-dimensional (2D) neuronal cultures is advantageous for understanding dendritic morphogenesis and functionality of single and networks of neurons. Yet, this is a challenging task because dendritic branches form a complex mesh even in "simplified" 2D neuronal cultures.
Several tools have been developed to automatically trace and analyze dendritic structures10,11,12,13. However, most of these tools are designed for 3D neuronal networks and are naturally too complex to use with 2D networks. In contrast, less advanced morphological analysis tools typically involve a significant component of computer-assisted manual labor, which is very time-consuming and susceptible to operator bias14. Existing semi-automatic tools, such as 'ImageJ'15 (an NIH open-source image processing package with a vast collection of community-developed biological image analysis tools), largely reduce user manual labor. However, some manual interventions are still needed during image processing, and the quality of the segmentation can be less than desirable.
This paper presents the SOA, a simple automated tool that allows direct segmentation and orientation analysis of dendritic branches within 2D neuronal networks. The SOA can detect various line-like objects in 2D images and characterize their morphological properties. Here, we used the SOA for segmenting dendritic branches in 2D fluorescence images of dendritic networks in culture. The software identifies the dendritic branches and successfully performs measurements of morphological parameters such as parallelism and spatial distribution. The SOA can be easily adapted for the analysis of cellular processes of other cell types and for studying non-biological networks.
Protocol
NOTE: The Israeli Ministry of Health approved the use of mice under protocol IL-218-01-21 for the ethical use of experimental animals. SOA is only compatible with Windows 10 and Python 3.9. It is available as an open-source code: https://github.com/inbar2748/DendriteProject. At this link, there is also a README.DM file that has directions for downloading the software, a link to the software's website, and a requirements file containing information on the required versions of all the packages. Additional examples of analysis performed using the software have been provided there as well.
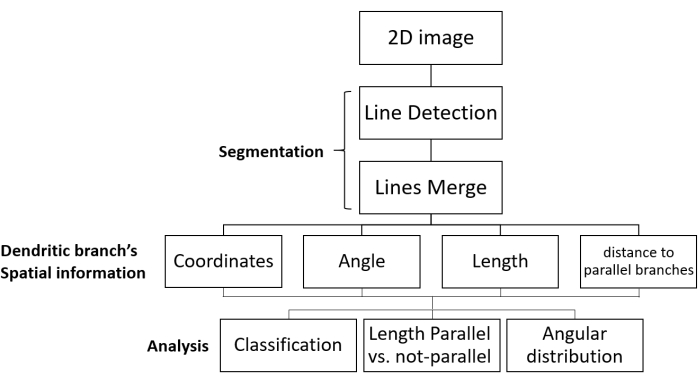
Figure 1: SOA workflow for segmentation and growth direction analysis. Shown are the processing steps of fluorescent images of dendritic networks and data analysis. The 2D image is uploaded, segmented (in two steps: dendritic branches are detected as lines, and then the relevant lines are merged), and the spatial information of each dendritic branch is obtained. The data are collected for all the dendritic branches in the image. Finally, the data are analyzed to give the desired morphological parameters. Abbreviation: SOA = segmentation and orientation analysis. Please click here to view a larger version of this figure.
1. Open the SOA application.
- Open the URL address: https://mega.nz/folder/bKZhmY4I#4WAaec4biiGt4_1lJlL4WA, find the SOA.zip zipped folder, and download the ZIP file by double-clicking.
- Unzip the folder by right-clicking on SOA.zip and choose Extract Files. Observe the Extraction Path and Options window that opens and the Destination Address text box that displays the path for the extracted files. To extract to a different location, click on one of the folders in the window's right panel to make it the destination folder. Click OK to extract the files to that folder.
- Open the extracted SOA file and double-click on SOA.exe. Wait for a black window to open, after which the application will appear.
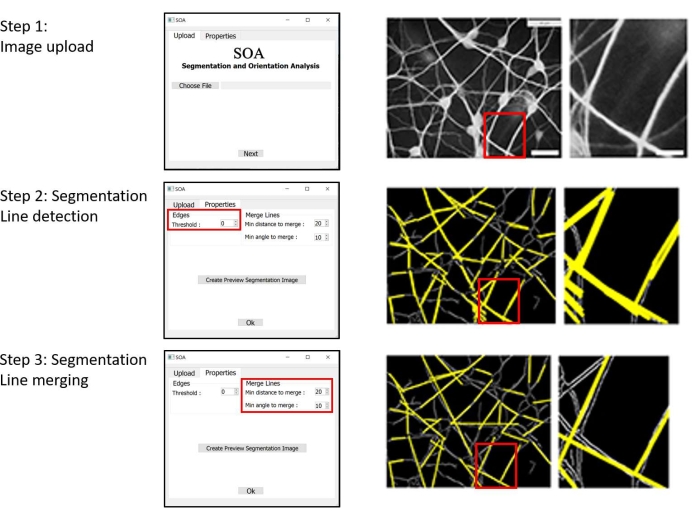
Figure 2: Example of a workflow using the SOA's GUI. Left column: GUI sections of the workflow. Middle column: image of a dendritic network, processed during the workflow (Scale bar: 20 µm). Right column: magnification of the area marked by a red rectangle in the images of the middle column (Scale bar: 4 µm). Step 1: Selection and uploading of an image. Step 2: The first stage of segmentation is the detection of lines that represent the identified dendritic branches. Step 3: The second stage of segmentation is the proximity-based merger of segment lining in individual dendritic branches. The settings of all steps can be modified. Abbreviations: SOA = segmentation and orientation analysis; GUI = graphical user interface. Please click here to view a larger version of this figure.
2. Open an image to analyze.
- In the SOA Viewer Upload menu bar | select Choose File | choose an image from the computer files | click on it (.png .jpg .tif .bmp files only) | Open | observe the path of the file | Next.
3. Segmentation optimization
NOTE: In the SOA Viewer Properties menu bar, change the values of the selected parameters to adjust the segmentation process settings. A detailed description of the parameters, such as the Threshold, is given in the Supplemental Material.
- In Edges, adjust the threshold for the display by selecting the Threshold and entering a number.
NOTE: The lower the number for the threshold, the more lines are detected. Threshold is a number that ranges from 0 to 255. The default value has been set to 0. - In Merge Lines:
- Adjust the minimum distance to merge for the display by selecting the Min distance to merge and entering a number.
NOTE: The Min distance to merge ranges from 0 to 30 pixels. The default value is set to 20. - Adjust the minimum angle to merge for the display by selecting the Min angle to merge and entering a number.
NOTE: The Min angle to merge ranges from 0 to 30°. The default value is set to 10.
- Adjust the minimum distance to merge for the display by selecting the Min distance to merge and entering a number.
- Click on Create Preview Segmentation Image.
NOTE: A preview image of the segmentation results will be displayed according to the updated values. In addition, the number of lines before merging and the number of lines after merging will be displayed. - Change the parameters to achieve maximum identification of segments. If there is a need to change the Properties, click on the Close window button and follow steps 3.1-3.4.
4. Create the output files.
- Press OK to visualize the segmentation images and the analyzing graphs. Observe the window that appears for selecting a location where the .xlsx file will be saved.
- insert a file name | Choose Save | wait for the .xlsx file with data to be created and saved.
NOTE: In addition to the .xlsx file, the following files will be automatically displayed: a file that presents the original image, the line recognition image, the final image of the segmentation, and three analysis graphs.
5. Navigation toolbar
NOTE: A navigation toolbar is included in all figure windows and can be used to navigate through the data set. Each of the buttons at the bottom of the toolbar is described below.
- To navigate back and forth between previously defined views, use the Forward and Back buttons.
NOTE: The Home, Forward, and Back buttons are similar to the Home, Forward, and Back controls on a web browser. Home returns to the default screen, the original image. - Use the Zoom button to pan and zoom. To activate panning and zooming, press the Zoom button, then move the mouse to a desired location in the image.
- To pan the figure, press and hold the left mouse button while dragging it to a new position. Release the mouse button, and the selected point in the image will appear in the new position. While panning, hold down the x or y keys to restrict the motion to the x or y axes, respectively.
- To zoom, hold down the right mouse button and drag it to a new location. Move right to zoom in on the x-axis and move left to zoom out on the x-axis. Do the same for the y-axis and up/down motions. When zooming, note that the point under the mouse remains stationary, allowing to zoom in or out around that point. Use the modifier keys x, y, or CONTROL to limit the zoom to the x, y, or aspect ratio preserve, respectively.
- To activate the Zoom-to-rectangle mode, click the Zoom-to-rectangle button. Place the cursor over the image and press the left mouse button. Drag the mouse to a new location while holding the button to define a rectangular region.
NOTE: The axes view limits will be zoomed to the defined region when the left mouse button is pressed. The axes view limits will be zoomed out when the right mouse button is pressed, placing the original axes in the defined region. - Use the Subplot-configuration tool to configure the appearance of the subplot.
NOTE: The left, right, top, and bottom sides of the subplot, as well as the space between rows and columns, can be stretched or compressed. - To open a file save dialog, click the Save button and save the file in the following formats: .png, .ps, .eps, .svg, or .pdf.
Results
A representative analysis was performed on images of dendritic networks in culture. Cells were extracted as described by Baranes et al.16,17. Briefly, hippocampal cells were extracted from the brains of postnatal rats and cultivated on 2D glass coverslips for 1-2 weeks. The cultures were then fixed and stained through indirect immunofluorescence using an antibody against the dendritic protein marker, microtubule-associated protein 2 (MAP2). Images of den...
Discussion
Effective strategies for extracting morphological information from 2D images are urgently required to keep up with biological imaging data. Although imaging data can be generated in hours, in-depth analysis of the images takes a long time. As a result, image processing has clearly become a major obstacle in many fields. This is due in part to the high complexity of the data, especially when dealing with biological samples. Furthermore, as many users lack specialized programming and image processing skills, automated tool...
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
The authors would like to thank Dr. Orly Weiss for the preparation of the culture images.
Materials
| Name | Company | Catalog Number | Comments |
| Matplotlib | 2002 - 2012 John Hunter, Darren Dale, Eric Firing, Michael Droettboom and the Matplotlib development team; 2012 - 2021 The Matplotlib development team. | 3.4.2 | a Python 2D plotting library |
| matplotlib-scalebar | Philippe Pinard | 0.7.2 | artist for matplotlib to display a scale bar |
| NumPy | The NumPy community. | 1.20.3 | fundamental package for scientific computing library |
| OpenCV | OpenCV team | 4.5.2.54 | Open Source Computer Vision Library |
| PyCharm | JetBrains | 2020.3.1 (Community Edition) version | Build #PC-203.6682.86, built on December 18, 2020. Runtime version: 11.0.9.1+11-b1145.37 amd64. VM: OpenJDK 64-Bit Server VM by JetBrains s.r.o. Windows 10 10.0. Memory: 978M, Cores: 4 |
| PyQt5 | Riverbank Computing | 5.15.4 | manage the GUI |
| python | Python Software Foundation License | 3.9 version | |
| Qt Designer | The QT Company Ltd. | 5.11.1 version | |
| scipy | Community library project | 1.6.3 | Python-based ecosystem of open-source software for mathematics, science, and engineering |
| Seaborn | Michael Waskom. | 0.11.1 | Python's Statistical Data Visualization Library. |
| Windows 10 | Microsoft | ||
| Xlsxwriter | John McNamara | 1.4.3 | Python module for creating Excel XLSX files |
References
- Ferrante, M., Migliore, M., Ascoli, G. Functional impact of dendritic branch-point morphology. Journal of Neuroscience. 33 (5), 2156-2165 (2013).
- Spruston, N. Pyramidal neurons: dendritic structure and synaptic integration. Nature Reviews Neuroscience. 9 (3), 206-221 (2008).
- Chklovskii, D. Synaptic Connectivity and Neuronal Morphology: Two Sides of the Same Coin. Neuron. 43 (5), 609-617 (2004).
- Chapleau, C., Larimore, J., Theibert, A., Pozzo-Miller, L. Modulation of dendritic spine development and plasticity by BDNF and vesicular trafficking: fundamental roles in neurodevelopmental disorders associated with mental retardation and autism. Journal of Neurodevelopmental Disorders. 1 (3), 185-196 (2009).
- Irwin, S. Dendritic Spine Structural Anomalies in Fragile-X Mental Retardation Syndrome. Cerebral Cortex. 10 (10), 1038-1044 (2000).
- Kaufmann, W. Dendritic anomalies in disorders associated with mental retardation. Cerebral Cortex. 10 (10), 981-991 (2000).
- Pinchas, M., Baranes, D. Dendritic branch intersections are structurally regulated targets for efficient axonal wiring and synaptic clustering. PLoS ONE. 8 (12), 82083 (2013).
- Cove, J., Blinder, P., Baranes, D. Contacts among non-sister dendritic branches at bifurcations shape neighboring dendritic branches and pattern their synaptic inputs. Brain Research. 1251, 30-41 (2009).
- Blinder, P., Cove, J., Foox, M., Baranes, D. Convergence among non-sister dendritic branches: An activity-controlled mean to strengthen network connectivity. PLoS ONE. 3 (11), 3782 (2008).
- Glaser, J., Glaser, E. Neuron imaging with neurolucida - PC-based system for image combining microscopy. Computerized Medical Imaging and Graphics. 14 (5), 307-317 (1990).
- Scorcioni, R., Polavaram, S., Ascoli, G. L-Measure: a web-accessible tool for the analysis, comparison and search of digital reconstructions of neuronal morphologies. Nature Protocols. 3 (5), 866-876 (2008).
- Torben-Nielsen, B. An efficient and extendable python library to analyze neuronal morphologies. Neuroinformatics. 12 (4), 619-622 (2014).
- Parekh, R., Ascoli, G. Neuronal morphology goes digital: A research hub for cellular and system neuroscience. Neuron. 78 (1), 206 (2013).
- heng, J., Zhou, X., Sabatini, B. L., Wong, S. T. C. NeuronIQ: A novel computational approach for automatic dendrite SPINES detection and analysis. 2007 IEEE/NIH Life Science Systems and Applications Workshop. , 168-171 (2007).
- Image processing and analysis in Java. NIH. ImageJ Available from: https://imagej.nih.gov/ij (2021)
- Peretz, H., Talpalar, A. E., Vago, R., Baranes, D. Superior survival and durability of neurons and astrocytes on 3-dimensional aragonite biomatrices. Tissue Engineering. 13, 461-472 (2007).
- Morad, T. I., Hendler, R. M., Weiss, O. E., Canji, E. A., Merfeld, I., Dubinsky, Z., Minnes, R., Francis, Y. I., Baranes, D. Gliosis of astrocytes cultivated on coral skeleton is regulated by the matrix surface topography. Biomedical Materials. 14 (4), 045005 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved