A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Light-Controlled Fermentations for Microbial Chemical and Protein Production
* These authors contributed equally
In This Article
Summary
Optogenetic control of microbial metabolism offers flexible dynamic control over fermentation processes. The protocol here shows how to set up blue light-regulated fermentations for chemical and protein production at different volumetric scales.
Abstract
Microbial cell factories offer a sustainable alternative for producing chemicals and recombinant proteins from renewable feedstocks. However, overburdening a microorganism with genetic modifications can reduce host fitness and productivity. This problem can be overcome by using dynamic control: inducible expression of enzymes and pathways, typically using chemical- or nutrient-based additives, to balance cellular growth and production. Optogenetics offers a non-invasive, highly tunable, and reversible method of dynamically regulating gene expression. Here, we describe how to set up light-controlled fermentations of engineered Escherichia coli and Saccharomyces cerevisiae for the production of chemicals or recombinant proteins. We discuss how to apply light at selected times and dosages to decouple microbial growth and production for improved fermentation control and productivity, as well as the key optimization considerations for best results. Additionally, we describe how to implement light controls for lab-scale bioreactor experiments. These protocols facilitate the adoption of optogenetic controls in engineered microorganisms for improved fermentation performance.
Introduction
Optogenetics, the control of biological processes with light-responsive proteins, offers a new strategy to dynamically control microbial fermentations for chemical and protein production1,2. The burden of engineered metabolic pathways and the toxicity of some intermediates and products often impairs cell growth3. Such stresses can lead to poor biomass accumulation and reduced productivity3. This challenge can be addressed by temporally dividing fermentations into a growth and production phase, which devote metabolic resources to biomass accumulation or product synthesis respectively4. We recently showed that the transition from growth to production in this two-phase fermentation can be induced with changes in illumination conditions5,6,7. The high tunability, reversibility, and orthogonality of light inputs8 offer unique advantages to light-controlled fermentations that are difficult or impossible to replicate with chemical inducers used in dynamical control of conventional two-phase fermentations4,9,10,11.
The blue-light responsive EL222 protein derived from Erythrobacter litoralis has been used to develop several optogenetic circuits for metabolic engineering in Saccharomyces cerevisiae5,7,12,13. EL222 contains a light-oxygen-voltage sensor (LOV) domain that undergoes a conformational shift upon blue light activation (465 nm), which allows it to bind to its cognate DNA sequence (C120)13. Fusing EL222 to the viral VP16 activation domain (VP16-EL222) results in a blue-light responsive transcription factor that can reversibly activate gene expression in S. cerevisiae7 and other organisms14 from the synthetic promoter PC120. Several circuits based on EL222 have been developed and used for chemical production in S. cerevisiae, such as the basic light-activated OptoEXP system7, in which the gene of interest is directly expressed from PC120 (Figure 1A). However, concerns of light penetration at the high cell densities typically encountered in the production phase of fermentations motivated us to develop inverted circuits that are induced in the dark, such as the OptoINVRT and OptoQ-INVRT circuits (Figure 1B)5,7,13. These systems harness the galactose (GAL) or quinic acid (Q) regulons from S. cerevisiae and N. crassa, respectively, controlling their corresponding repressors (GAL80 and QS) with VP16-EL222, to repress gene expression in the light and strongly induce it in the dark. Combining OptoEXP and OptoINVRT circuits results in bidirectional control of gene expression, enabling two-phase fermentations in which the growth phase is induced with blue light, and the production phase with darkness (Figure 2A)5,7.
Using light instead of darkness to induce gene expression during the production phase would greatly expand the capabilities of optogenetic controls but would also require overcoming the light penetration limitations of the high cell densities typically encountered in this phase of fermentation. To this end, we have developed circuits, known as OptoAMP and OptoQ-AMP, that amplify the transcriptional response to blue light stimulation. These circuits use wild-type or hypersensitive mutants of VP16-EL222 to control production of the transcriptional activators Gal4p or QF2 of the GAL or Q regulons, respectively, achieving enhanced sensitivity and stronger gene expression with light12,13 (Figure 1C). OptoAMP circuits can achieve complete and homogeneous light induction in 5 L bioreactors at an optical density (measured at 600 nm; OD600) values of at least 40 with only ~0.35% of illumination (5% light dose on only ~7% of the bulk surface). This demonstrates a higher degree of sensitivity compared to OptoEXP, which requires close to 100% illumination12. The ability to effectively induce gene expression with light at high cell densities opens new opportunities for dynamical control of fermentations. This includes operating fermentations in more than two temporal phases, such as three-phase fermentations, in which growth, induction, and production phases are established with unique light schedules to optimize chemical production (Figure 2B)12.

Figure 1: Optogenetic circuits for dynamic control of S. cerevisiae. The OptoEXP, OptoINVRT, and OptoAMP circuits are based on the light-sensitive VP16-EL222 system. (A) In the OptoEXP circuit, exposure to blue light causes a conformational change and dimerization of VP16-EL222, which exposes a DNA-binding domain and allows for transcription from PC120. The figure has been modified from Zhao et al.7. (B) OptoINVRT circuits harnesses the GAL (shown) or Q regulons to induce expression in the dark. In GAL-based circuits, VP16-EL222 and GAL4 are constitutively expressed, while PC120 drives expression of the GAL80 repressor (in Q-based circuits, GAL4 and GAL80 are replaced by QF2 and QS, respectively, and a synthetic QUAS-containing promoter is used instead of a GAL promoter). In light, Gal80p prevents activation of the gene of interest from PGAL1. In the dark, GAL80 is not expressed and rapidly degraded by fusing it to a constitutive degron domain (small brown domain), which allows for activation of PGAL1 by Gal4p. The figure has been modified from Zhao et al.5. (C) OptoAMP circuits also use VP16-EL222 to control the GAL (shown) or Q regulons. In these circuits, the GAL80 repressor (or QS) is constitutively expressed and fused to a photo-sensitive degron (small blue domain) ensuring tight repression in the dark. PC120 and a hypersensitive VP16-EL222 mutant control expression of GAL4 (or QF2) with light, which strongly activates PGAL1 (or a QUAS-containing promoter) in the light. GAL-derived circuits can use engineered forms of PGAL1, such as PGAL1-M or PGAL1-S, which have increased activity, as well as wild-type promoters controlled by the GAL regulon (PGAL1, PGAL10, PGAL2, PGAL7). The figure has been modified from Zhao et al.12. Please click here to view a larger version of this figure.

Figure 2: Two- and three-phase fermentations through time. (A) Two-phase fermentations operated with inverted circuits consist of a light-driven growth phase and a dark production phase. In the growth phase, biomass accumulates as the production pathway stays repressed. Upon reaching the desired OD600, cells are shifted to the dark to metabolically adjust before being resuspended in fresh media for the production phase. (B) In a three-phase process, the growth, incubation, and production phases are defined by unique light schedules, which may consist of a dark growth period, pulsed incubation, and fully illuminated production phase. Figure created with Biorender. Please click here to view a larger version of this figure.
Optogenetic circuits have also been developed for dynamical control of chemical and protein production in E. coli. OptoLAC circuits control the bacterial LacI repressor using the light-responsive pDawn circuit, which is based on the YF1/FixJ two-component system6 (Figure 3). Similar to OptoINVRT5, OptoLAC circuits are designed to repress gene expression in the light and induce it in the dark. Expression levels using OptoLAC circuits can match or exceed those achieved with standard isopropyl β-d-1-thiogalactopyranoside (IPTG) induction, thus maintaining the strength of chemical induction while offering enhanced tunability and reversibility6. Therefore, OptoLAC circuits enable effective optogenetic control for metabolic engineering in E. coli.
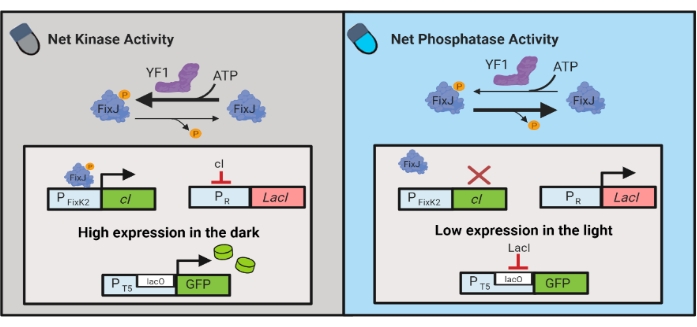
Figure 3: OptoLAC circuits for dynamic control of E. coli. The OptoLAC circuits adapt the pDawn system and lac operon to achieve activation in the dark and repression in the light. In the dark, YF1 phosphorylates FixJ, which then activates the PFixK2 promoter to express the cI repressor. The cI repressor prevents expression of the lacI repressor from the PR promoter, which permits transcription of the gene of interest from a lacO-containing promoter. Conversely, blue light reduces YF1 net kinase activity, reversing FixJ phosphorylation and thus cI expression, which derepresses expression of lacI and prevents expression from the lacO-containing promoter. The figure has been modified from Lalwani et al.6. Please click here to view a larger version of this figure.
We describe here the basic protocols for light-controlled fermentations of S. cerevisiae and E. coli for chemical or protein production. For both yeast and bacteria, we first focus on fermentations with a light-driven growth phase and a darkness-induced production phase enabled by OptoINVRT and OptoLAC circuits. Subsequently, we describe a protocol for a three-phase (growth, induction, production) light-controlled fermentation enabled by OptoAMP circuits. Furthermore, we describe how to scale up optogenetically controlled fermentations from microplates to lab-scale bioreactors. With this protocol, we aim to provide a complete and easily reproducible guide for performing light-controlled fermentations for chemical or protein production.
Protocol
1. Light-controlled chemical production using the S. cerevisiae OptoINVRT7 circuit
- Strain construction
- Obtain a strain with his3 auxotrophy, as this marker is necessary for most existing OptoINVRT plasmids5. If seeking optogenetic regulation of a gene that is native to S. cerevisiae, construct a strain in which any endogenous copy of the gene is deleted.
- Linearize the plasmid containing the OptoINVRT7 circuit, such as EZ-L4395, and integrate it into the his3-locus of the auxotrophic strain using standard lithium-acetate transformation methods15. If using the EZ-L439 plasmid, which contains the components to repress PGAL1 in the light and activate it in the dark, linearize at the PmeI restriction site.
- Following the transformation, centrifuge the cells at 150 x g for 1 min and gently resuspend in 200 µL of fresh histidine-dropout synthetic complete medium (SC-His) medium.
- Plate the entire cell volume onto SC-His agar plates and incubate at 30 °C for 2-3 days until colonies appear.
- Prepare competent cells from this strain using standard lithium acetate transformation protocols, and transform them with a plasmid containing the gene(s) to be controlled optogenetically downstream of either the PGAL1-M or PGAL1-S promoter5.
NOTE: Using a plasmid that integrates at δ-sites (YARCdelta5) and selects with Zeocin allows for stable multicopy integration7,16,17,18. - After transformation, centrifuge the culture at 150 x g for 1 min and gently resuspend in 200 µL of fresh SC-dropout medium.
NOTE: The PGAL1-M promoter is a synthetic version of the PGAL1 promoter with the Mig1p repression sites deleted, while PGAL1-S is an engineered version of PGAL1-M, which has extra Gal4p activator binding sites. The regular PGAL1 promoter can be used to control the expression; however, the expression strength will be lower than from these engineered promoters. - Plate the entire cell volume on a yeast extract peptone dextrose (YPD) agar plate if integrating into δ-sites, or an SC-dropout plate if transforming with a plasmid containing a selection marker. Incubate at 30 °C for 16 h under constant blue light to keep the optogenetically controlled gene repressed.
NOTE: For some strains, colonies might grow faster when incubated in blue light pulses (e.g., 1 s on/79 s off, 5 s on/75 s off, 10 s on/70 s off, etc.) rather than in constant illumination, which must be determined experimentally for each strain, if necessary. - Use any 465 nm light source and place an LED panel ~40 cm above the plate such that the light intensity is ~80-110 µmol/m2/s. Measure the intensity using a quantum meter (see Table of Materials).
- If integrating into δ-sites, create a replica of the plate onto YPD plates containing a range of Zeocin concentrations between 400 µg/mL and 1,200 µg/mL to select for a variety of integration copy numbers5,7,12,16,17,18. Incubate the replica plates at 30 °C under constant or pulsed blue light for 2-3 days until the colonies appear.
- Preliminary screening for the best colonies
- Select eight colonies from each plate and use them to inoculate 1 mL of SC-His medium supplemented with 2% glucose in individual wells of a 24-well plate. Grow in 24-well plates under the cells overnight (16-20 h) at 30 °C with 200 rpm (19 mm orbital diameter) shaking under constant blue light illumination.
- The next morning, dilute each culture in 1 mL of a fresh SC-His medium with 2% glucose to OD600 values ranging from 0.01-0.3 and grow in 24-well plates under constant or pulsed light at 30 °C with 200 rpm shaking until they reach cell densities between 2 and 9 OD600 values (Figure 4A). The amount of time needed for this growth phase will depend on the strain.
- Incubate the plates in dark for 4 h at 30 °C with 200 rpm shaking by turning off the light panel and wrapping the plates in aluminum foil.
NOTE: This step allows the cells to metabolically transition to the production phase before resuspension in the production media. - To start the production phase, centrifuge the cultures in the 24-well plate at 234 x g for 5 min and resuspend cells in 1 mL of fresh SC-dropout medium with 2% glucose. Seal the plates to prevent evaporation of the desired product by using sterile microplate sealing tape.
- Ferment the sealed plates in the dark for 48 h at 30 °C with shaking at 200 rpm. Ensure that the plates are wrapped in aluminum foil to prevent any exposure to light.
NOTE: Wrapping the plates in foil does not limit oxygen or gas availability in fermentations; however, the sterile sealing tape does limit gas transfer. Small holes can be poked in the tape to introduce oxygen, if necessary.
- Harvesting and analysis
- To harvest the fermentations, centrifuge the plates for 5 min at 234 x g and transfer 800 µL of the supernatant into 1.5 mL microcentrifuge tubes.
- Depending on the chemical of interest, analyze using high-performance liquid chromatography (HPLC), gas chromatography-mass spectrometry (GC-MS), or another analytical method using the sample preparation technique best suited for the instrument used.
2. Light-controlled protein production using the E. coli OptoLAC system
- Strain construction
- Co-transform electrocompetent BL21 DE3 ΔlacI ΔlacI-DE3 with a plasmid containing the OptoLAC1B or OptoLAC2B circuit6 and a plasmid that expresses the recombinant protein of interest from the PT7 promoter19.
- After transforming, recover the cells for 1 h in 1 mL of super optimal broth with catabolite repression (SOC; 2% tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4, 20 mM glucose) at 37 °C with rotation or shaking.
NOTE: The plasmid containing the protein of interest must be compatible with the OptoLAC plasmid (i.e., different resistance marker and origin of replication) and must not contain a copy of lacI. - Centrifuge the cells at 4845 x g for 3 min and concentrate the pellet in 200 µL of lysogeny broth (LB) media. Plate the entire concentrated cells onto an LB agar plate with appropriate antibiotics and grow at 37 °C overnight under constant blue light to keep protein expression repressed.
- Initial screening to verify expression
- Take three single colonies and use them to inoculate 1 mL of LB media with appropriate antibiotics in individual wells of a 24-well plate. Grow overnight (16-20 h) at 37 °C with 200 rpm shaking under constant blue light illumination (Figure 4A).
- The next day, use 1.5 µL of culture to measure OD600 in a spectrophotometer with a microvolume measurement. Dilute cultures into 1 mL of fresh LB in 24-well plates to OD600 values ranging from 0.01-0.1.
- Grow the cultures at 37 °C with 200 rpm shaking under blue light for 2-3 h. Starting from the second hour, take OD600 measurements every 15 min to ensure that the cultures do not overgrow the OD600 range of 0.1-1.5.
- Once the cultures are at the desired OD600, turn off the light panel and wrap the plate in aluminum foil to initiate the production phase. Keep the plate in the dark for 8 h (37 °C), 20 h (30 °C), or 48 h (18 °C), with 200 rpm shaking.
- Measure and record the final OD600 value of each culture.
- Harvesting and analysis
- Transfer 800 µL of each culture into a 1.5 mL microcentrifuge tube and centrifuge for 5 min at 17,000 x g.
- Resuspend the cell pellet in 200 µL of resuspension buffer (Tris 50 mM, pH 8.0, NaCl 300 mM).
NOTE: The concentration of NaCl can be adjusted based on the recombinant protein being analyzed. - Add 50 µL of 6x sodium dodecyl sulfate (SDS) sample buffer (Tris 375 mM, pH 6.8, SDS 9%, glycerol 50%, bromophenol blue 0.03%, DTT 9%). Incubate at 100 °C for 10 min with shaking at 700 rpm in a thermomixer.
- Load ~3-20 µL of the culture in a 12% SDS-PAGE gel. Using the final OD600 measurement as a guide, load approximately the same amount of protein for each sample (equivalent to 10 µL of the sample corresponding to a final OD600 value of 1). Use a power supply to run electrophoresis at 100 V until the gel is fully resolved.
- Stain the gel with Coomassie brilliant blue G-250 solution by heating for 30-40 s in a microwave oven, and then incubating on a platform rotator for at least 15 min.
- Rinse with deionized water twice and destain on a platform rotator for at least 30 min (or overnight), adding two cleaning wipes tied into a knot to help absorb the stain. Boil the gel in sufficient amount of water in the microwave oven for 15 min to speed up the destaining process.
3. Three-phase fermentation using the S. cerevisiae OptoAMP system
- Strain construction
- Obtain a strain with a his3 auxotrophic marker, as this marker is necessary in order to use the existing OptoAMP plasmids5. If seeking optogenetic regulation of a gene that is native to S. cerevisiae, construct a strain in which the endogenous copy of this gene is deleted.
- Linearize a plasmid containing the OptoAMP4 circuit, such as EZ-L58012, and integrate it in the his3 locus of the auxotrophic strain using standard lithium-acetate transformation methods15. If using EZ-L580, linearize the plasmid at the PmeI restriction site.
- Following the transformation, centrifuge the cells at 150 x g for 1 min and gently resuspend in 200 µL of fresh SC-His medium.
- Plate the entire cell volume on selective media (SC-His-agar) and incubate at 30 °C for 2-3 days until colonies appear.
- Prepare competent cells from this strain and transform them with a plasmid containing the gene(s) to be controlled optogenetically downstream of the PGAL1-S promoter12.
NOTE: Using a plasmid that integrates at δ-sites and selects with Zeocin allows for stable multicopy integration and selection. - After transforming, centrifuge the culture at 150 x g for 1 min and gently resuspend in 200 µL of fresh SC-dropout medium.
NOTE: The PGAL1-S promoter is a synthetic version of the PGAL1 promoter in which the Mig1p repression sites are deleted and extra Gal4p activator binding sites are added. The regular PGAL1 promoter can be used; however, the expression strength will be lower than this engineered promoter. - Plate the entire cell volume on a YPD or SC-dropout agar plate and incubate at 30 °C for 16 h in the dark (wrapped in aluminum foil). Incubating in the dark keeps the optogenetically-controlled gene repressed, which allows the cells to direct their metabolic resources toward cell growth rather than chemical production.
- If integrating into δ-sites, replica plate onto YPD plates containing a range of Zeocin concentrations to select for a variety of integration copy numbers. Incubate the plates at 30 °C in the dark (wrapped in aluminum foil) for 2-3 days until colonies appear.
- Preliminary screening for the best colonies
- Select eight colonies from each plate and use them to inoculate 1 mL of SC-His medium with 2% glucose in individual wells of a 24-well plate. Grow the cells overnight (16-20 h) in the dark at 30 °C with 200 rpm shaking.
- The next morning, dilute each culture in 1 mL of fresh SC-His medium with 2% glucose to 0.1 OD600 and grow in dark at 30 °C with 200 rpm shaking until they reach an OD600 of 3. Wrap the plates in aluminum foil to prevent exposure to light. The amount of time needed for this growth phase will depend on the strain.
- To start the induction phase, incubate the plates under pulsed light (for example, 5 s on/95 s off) for 12 h at 30 °C with 200 rpm shaking. Use any 465 nm light source and place an LED panel above the plate such that the light intensity is ~80-110 µmol/m2/s for optimum results (Figure 4A).
NOTE: The optimal light pulse duration for this incubation will vary based on the chemical being produced. It is recommended to screen a range of light schedules from 0.1% (e.g., 1 s on 999 s off) to 100% (full light). - To start the production phase, centrifuge the cultures at 234 x g for 5 min and resuspend in fresh SC-His medium with 2% glucose. Seal the plates to prevent evaporation of the desired product using sterile microplate sealing tape.
- Ferment the sealed plates in light for 48 h at 30 °C with shaking at 200 rpm. Optimize the light schedule during this step as some chemicals benefit from a pulsed production phase rather than full light.
- Harvesting and analysis
- Harvest the fermentations by centrifuging the plates for 5 min at 234 x g and transferring 800 µL of the supernatant into 1.5 mL microcentrifuge tubes.
- Depending on the chemical of interest, analyze using HPLC, GC-MS, or another analytical method using the sample preparation technique best suited for the instrument used.
4. Chemical (mevalonate) production from E. coli in a light-controlled bioreactor
- Initial inoculation and bioreactor setup
- Inoculate a colony of an E. coli strain engineered with light-controlled chemical production into 5 mL of M9 minimal salts (3.37 mM Na2HPO4, 2.2 mM KH2PO4, 0.855 mM NaCl, 0.935 mM NH4Cl) supplemented with 0.2% w/v casamino acids, 5% w/v glucose, and trace metal mixture20 (0.0084 g/L EDTA, 0.0025 g/L CoCl2, 0.015 g/L MnCl2, 0.0015 g/L CuCl2, 0.003 g/L H3BO3, 0.0025 g/L Na2MoO4, 0.008 g/L ZnCl2, 0.06 g/L Fe (III) citrate, 0.0045 g/L thiamine, 1.3 g/L MgSO4) in a 50 mL conical tube.
- Grow the culture overnight at 30 °C with 200 rpm shaking under blue light illumination.
- Set up the bioreactor vessel head plate, making sure that the following ports are installed: insert for thermal probe; dissolved oxygen (DO) probe; gas sparger - connect to the air source through a 0.2 µm filter; impeller; gas condenser - connect to a 0.2 µm filter; cooling line (x2); feed lines (x2) - one for media addition, one for pH control; sampling line - ensure it reaches the bottom of the vessel; empty port; pH probe - calibrate the probe with standards of pH = 4 and pH = 7 prior to installation, either autoclave with the rest of the vessel or sterilize with 95% ethanol and aseptically insert before setup.
- Fill the vessel with 1 L of filtered water, and attach the head plate and tighten, making sure that the O-ring snugly fits and seals.
- Cover any openings into the reactor with aluminum foil.
- Prepare three strips of tubing: one for removing the water, one for inserting the feed, and one for pH control. Cover the ends with aluminum foil and wrap all the tubing in aluminum foil.
NOTE: NH4OH, which is used for pH adjustment, does not flow smoothly in silicone tubing, which may lead to inaccurate flow rates and over-basification of the culture. To avoid this issue, use biocompatible pump tubing (BPT) for the NH4OH feed. - Autoclave the bioreactor and tubing, using a 30 min liquid cycle.
- Remove the materials from the autoclave. Once cool enough to handle, connect the impeller, pH and DO probes, air source, condenser inlet and outlet, and cooling inlet and outlet to the control station.
- Insert the thermal probe and cover the vessel with a heating jacket. Secure the jacket toward the top of the vessel to avoid blocking the culture from light exposure.
- Connect one of the sterile tubes to the sampling line and secure through the sampling pump. Place the other end such that it flows into an empty container that can hold at least 1 L. Drain the water inside the vessel.
- Connect another sterile tube to one of the feed lines and secure through one of the feed pumps. Connect the other end of the tube to a bottle of M9 media. Feed the media into the reactor.
- Connect another sterile tube to one of the feed lines and secure through one of the feed pumps. Connect the other end of the tube to the bottle containing 28%-30% NH4OH.
CAUTION: NH4OH is corrosive. Work in a fume hood while transferring to a feed bottle and make sure the feed bottle is placed in secondary containment. - Place three light panels in a triangular formation ~20 cm away from the reactor, checking that the light intensities on the surface of the vessel reach ~80-110 µmol/m2/s from each side (Figure 4B).
- The next day, turn on the bioreactor system and chiller. Set the reactor temperature setpoint to 37 °C, the pH setpoint to 7.0, and the agitation to 200 rpm. The heating jacket should turn on.
- Calibrate the DO probe by first waiting until the temperature and DO measurements become constant (this will be the 100% setpoint). Then, disconnect the probe from the system (this will be the 0% setpoint). Repeat until the DO measurement stabilizes at 100% when the probe is connected, and then set the DO setpoint to 20%.
- Light-controlled chemical production
- Inoculate the bioreactor to an initial OD600 of 0.001-0.1. Turn on the light panels to initiate growth.
- After 3 h, begin taking samples from the sampling line to take OD600 measurements to avoid overgrowing the optimal cell density of induction (ρs). Once the optimal ρs is reached (the optimal value for mevalonate production is 0.17), turn off the light panels, cover the reactor in aluminum foil, and wrap the setup in black cloth to initiate the dark production phase.
- Add 50 µL of antifoam, 8 h after switching to darkness. Unscrew the empty port and pipette the antifoam directly into the reactor.
- Use the sampling port to periodically take samples for HPLC or GC analysis.
- Disassembly and analysis
- After the experiment is concluded, turn off the system. Carefully unscrew the DO and pH probes and wash them with soap and water. Unscrew the head plate and wash it with soap and water using a brush.
- Transfer the culture into an empty container and add bleach to a final concentration of 10% v/v. Place in a fume hood and dispose of after 30 min.
- Wash the reactor vessel with soap and water using a brush.
- Prepare the samples for analysis based on the product of interest. For mevalonate production, mix 560 µL of culture with 140 µL of 0.5 M HCl and vortex at high speed for 1 min. This converts mevalonate into (±)-mevalonolactone.
CAUTION: HCl is a health hazard. Handle with proper PPE and ensure sample tubes are properly capped prior to vortexing. - Centrifuge at 17,000 x g for 45 min at 4 °C. Transfer 250 µL of the supernatant to an HPLC vial.
- For mevalonate, analyze samples using an organic acids ion exchange column. Quantify production using a refractive index detector (RID), comparing peak areas to a standard of (±)-mevalonolactone.
Results
Optogenetic regulation of microbial metabolism has been successfully implemented to produce a variety of products, including biofuels, bulk chemicals, proteins, and natural products5,6,7,12,13. Most of these processes are designed for cell growth to occur in the light (when low cell density poses minimal challenges with light penetration), and for production t...
Discussion
Dynamic control has long been applied to improve yields for metabolic engineering and recombinant protein production4. Shifts in enzymatic expression are most typically implemented using chemical inducers such as IPTG21, galactose22, and tetracycline23, but have also been mediated using process conditions such as temperature and pH. Optogenetic control of gene expression eliminates the need for changes to fermentation paramete...
Disclosures
The authors have applied for several patents for the optogenetic circuits and methods described in this article.
Acknowledgements
This research was supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research Award Number DE-SC0019363, the NSF CAREER Award CBET-1751840, The Pew Charitable Trusts, and the Camille Dreyfus Teacher-Scholar Award.
Materials
| Name | Company | Catalog Number | Comments |
| Light-controlled chemical production using S. cerevisiae | |||
| 24-well culture plate | USA Scientific | CC7672-7524 | |
| Agar powder | Thermo Fisher Scientific | 303991049 | |
| Aluminum foil | Reynolds | B004NG90YO | |
| BioSpectrometer with μcuvette | Eppendorf | 6135000923 | |
| Blue LED panel | HQRP | 884667106091218 | |
| EZ-L439 OptoINVRT7 Plasmid | N/A | N/A | See Reference 1 |
| Glucose | Thermo Fisher Scientific | 501879892 (G8270-5KG) | |
| Microcentrifuge | Thermo Fisher Scientific | 75002403 | |
| Microcentrifuge tubes | USA Scientific | 1615-5510 | |
| Orbital Shaker | Yamato Scientific America | SOU-300 | |
| Petri dish | Celltreat | 229656 | |
| PmeI | New England Biolabs | R0560L | |
| Quantum meter | Apogee Instruments | MQ-510 | |
| Replica-plating device | Thomas Scientific | F37848-0000 | |
| Replica-plating pads | Sunrise Science Products | 3005-012 | |
| SC-His powder | Sunrise Science Products | 1303-030 | |
| SC Complete powder | Sunrise Science Products | 1459-100 | |
| Sterile sealing film | Excel Scientific | STR-SEAL-PLT | |
| YPD agar plates | VWR | 100217-054 | |
| Zeocin | Thermo Fisher Scientific | R25005 | |
| Light-controlled protein production using E. coli | |||
| 6X SDS Sample Buffer | Cepham Life Sciences | 10502 | |
| 12% Acrylamide protein gels | Thermo Fisher Scientific | NP0341BOX | |
| 24-well culture plate | USA Scientific | CC7672-7524 | |
| Aluminum foil | Reynolds | B004NG90YO | |
| BioSpectrometer with μcuvette | Eppendorf | 6135000923 | |
| Blue LED panel | HQRP | 884667106091218 | |
| Coomassie Brilliant Blue G-250 | Thermo Fisher Scientific | 20279 | |
| Electrophoresis cell | Bio-Rad | 1658004 | |
| Electrophoresis power supply | Bio-Rad | 1645050 | |
| LB broth (Miller) | Fisher Scientific | BP97235 | |
| Microcentrifuge | Thermo Fisher Scientific | 75002403 | |
| Microcentrifuge tubes | USA Scientific | 1615-5510 | |
| NaCl | Thomas Scientific | SX0425-1 | |
| OptoLAC plasmids | N/A | N/A | See Reference 2 |
| Orbital Shaker | Yamato Scientific America | SOU-300 | |
| Petri dish | Celltreat | 229656 | |
| Quantum meter | Apogee Instruments | MQ-510 | |
| SOC medium | Thermo Fisher Scientific | 15544034 | |
| Thermomixer | Eppendorf | 5382000015 | |
| Tris base | Fisher Scientific | BP1521 | |
| Three-phase fermentation using S. cerevisiae | |||
| Same materials as "Light-controlled chemical production using S. cerevisiae" protocol plus the following: | |||
| EZ-L580 OptoAMP4 Plasmid | N/A | N/A | See Reference 10 |
| Chemical production in a light-controlled bioreactor | |||
| Aluminum foil | Reynolds | B004NG90YO | |
| Antifoam | Sigma-Aldrich | A8311 | |
| Bioreactor with control station | Eppendorf | B120110001 | |
| BioSpectrometer with μcuvette | Eppendorf | 6135000923 | |
| Bleach | VWR Scientific | 89501-620 (CS) | |
| Blue LED panel | HQRP | 884667106091218 | |
| BPT tubing | Fisher Scientific | 14-170-15 | |
| Glucose | Thermo Fisher Scientific | 501879892 (G8270-5KG) | |
| Hydrochloric acid (HCl) | Fisher Scientific | 7647-01-0 | |
| M9 Minimal Salts | Thermo Fisher Scientific | A1374401 | |
| Microcentrifuge | Thermo Fisher Scientific | 75002403 | |
| Microcentrifuge tubes | USA Scientific | 1615-5510 | |
| NH4OH Solution | Sigma-Aldrich | I0503-1VL | |
| Orbital Shaker | Yamato Scientific America | SOU-300 | |
| Quantum meter | Apogee Instruments | MQ-510 | |
| SC Complete powder | Sunrise Science Products | 1459-100 |
References
- Figueroa, D., Rojas, V., Romero, A., Larrondo, L. F., Salinas, F. The rise and shine of yeast optogenetics. Yeast. 38 (2), 131-146 (2021).
- Pouzet, S., et al. The promise of optogenetics for bioproduction: Dynamic control strategies and scale-up instruments. Bioengineering. 7 (4), 151 (2020).
- Venayak, N., Anesiadis, N., Cluett, W. R., Mahadevan, R. Engineering metabolism through dynamic control. Current Opinion in Biotechnology. 34, 142-152 (2015).
- Lalwani, M. A., Zhao, E. M., Avalos, J. L. Current and future modalities of dynamic control in metabolic engineering. Current Opinion in Biotechnology. 52, 56-65 (2018).
- Zhao, E. M., et al. Design and characterization of rapid optogenetic circuits for dynamic control in yeast metabolic engineering. ACS Synthetic Biology. 9 (12), 3254-3266 (2020).
- Lalwani, M. A., et al. Optogenetic control of the lac operon for bacterial chemical and protein production. Nature Chemical Biology. 17 (1), 71-79 (2021).
- Zhao, E. M., et al. Optogenetic regulation of engineered cellular metabolism for microbial chemical production. Nature. 555 (7698), 683-687 (2018).
- Baumschlager, A., Khammash, M. Synthetic biological approaches for optogenetics and tools for transcriptional light-control in bacteria. Advanced Biology. 5 (5), 2000256 (2021).
- Dvorak, P., et al. Exacerbation of substrate toxicity by IPTG in Escherichia coli BL21(DE3) carrying a synthetic metabolic pathway. Microbial Cell Factories. 14, 201 (2015).
- Hartline, C. J., Schmitz, A. C., Han, Y., Zhang, F. Dynamic control in metabolic engineering: Theories, tools, and applications. Metabolic Engineering. 63, 126-140 (2021).
- Ni, C., Dinh, C. V., Prather, K. L. J. Dynamic control of metabolism. Annual Review of Chemical and Biomolecular Engineering. 12, 519-560 (2021).
- Zhao, E. M., et al. Optogenetic amplification circuits for light-induced metabolic control. ACS Synthetic Biology. 10 (5), 1143-1154 (2021).
- Lalwani, M. A., Zhao, E. M., Wegner, S. A., Avalos, J. L. The Neurospora crassa Inducible Q System Enables Simultaneous Optogenetic Amplification and Inversion in Saccharomyces cerevisiae for Bidirectional Control of Gene Expression. ACS Synthetic Biology. 10 (8), 2060-2075 (2021).
- Motta-Mena, L. B., et al. An optogenetic gene expression system with rapid activation and deactivation kinetics. Nature Chemical Biology. 10 (3), 196-202 (2014).
- Gietz, R. D., Woods, R. A. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods in Enzymology. 350, 87-96 (2002).
- Marx, H., Mecklenbräuker, A., Gasser, B., Sauer, M., Mattanovich, D. Directed gene copy number amplification in Pichia pastoris by vector integration into the ribosomal DNA locus. FEMS Yeast Research. 9 (8), 1260-1270 (2009).
- Nordén, K., et al. Increasing gene dosage greatly enhances recombinant expression of aquaporins in Pichia pastoris. BMC Biotechnology. 11, 47 (2011).
- Zhao, E. M., et al. Light-based control of metabolic flux through assembly of synthetic organelles. Nature Chemical Biology. 15 (6), 589-597 (2019).
- Dowee, W. J., Miller, J. F., Ragsdale, C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Research. 16 (13), 6127-6145 (1988).
- Zhou, K., Edgar, S., Stephanopoulos, G. Engineering microbes to synthesize plant isoprenoids. Methods in Enzymology. 575, 225-245 (2016).
- Arfman, N., Worrell, V., Ingram, L. O. Use of the tac promoter and lacI(q) for the controlled expression of Zymomonas mobilis fermentative genes in Escherichia coli and Zymomonas mobilis. Journal of Bacteriology. 174 (22), 7370-7378 (1992).
- Steen, E. J., et al. Metabolic engineering of Saccharomyces cerevisiae for the production of n-butanol. Microbial Cell Factories. 7 (1), 1-8 (2008).
- Tan, S. Z., Manchester, S., Prather, K. L. J. Controlling central carbon metabolism for improved pathway yields in Saccharomyces cerevisiae. ACS Synthetic Biology. 5 (2), 116-124 (2015).
- Jayaraman, P., et al. Blue light-mediated transcriptional activation and repression of gene expression in bacteria. Nucleic Acids Research. 44 (14), 6994 (2016).
- Fernandez-Rodriguez, J., Moser, F., Song, M., Voigt, C. A. Engineering RGB color vision into Escherichia coli. Nature Chemical Biology. 13 (7), 706-708 (2017).
- Ding, Q., et al. Light-powered Escherichia coli cell division for chemical production. Nature Communications. 11 (1), 1-14 (2020).
- Senoo, S., Tandar, S. T., Kitamura, S., Toya, Y., Shimizu, H. Light-inducible flux control of triosephosphate isomerase on glycolysis in Escherichia coli. Biotechnology and Bioengineering. 116 (12), 3292-3300 (2019).
- Ramakrishnan, P., Tabor, J. J. Repurposing synechocystis PCC6803 UirS-UirR as a UV-violet/green photoreversible transcriptional regulatory tool in E. Coli. ACS Synthetic Biology. 5 (7), 733-740 (2016).
- Tabor, J. J., Levskaya, A., Voigt, C. A. Multichromatic control of gene expression in escherichia coli. Journal of Molecular Biology. 405 (2), 315-324 (2011).
- Stewart, C. J., McClean, M. N. Design and implementation of an automated illuminating, culturing, and sampling system for microbial optogenetic applications. Journal of Visualized Experiments:JoVE. (120), e54894 (2017).
- Grødem, E. O. S., Sweeney, K., McClean, M. N. Automated calibration of optoPlate LEDs to reduce light dose variation in optogenetic experiments. BioTechniques. 69 (4), 313-316 (2020).
- Gerhardt, K. P., et al. An open-hardware platform for optogenetics and photobiology. Scientific Reports. 6, (2016).
- Bugaj, L. J., Lim, W. A. High-throughput multicolor optogenetics in microwell plates. Nature Protocols. 14 (7), 2205-2228 (2019).
- Steel, H., Habgood, R., Kelly, C., Papachristodoulou, A. In situ characterisation and manipulation of biological systems with Chi.Bio. PLoS Biology. 18 (7), (2020).
- Carrasco-López, C., García-Echauri, S. A., Kichuk, T., Avalos, J. L. Optogenetics and biosensors set the stage for metabolic cybergenetics. Current Opinion in Biotechnology. 65, 296-309 (2020).
- Milias-Argeitis, A., Rullan, M., Aoki, S. K., Buchmann, P., Khammash, M. Automated optogenetic feedback control for precise and robust regulation of gene expression and cell growth. Nature Communications. 7 (1), 1-11 (2016).
- Melendez, J., et al. Real-time optogenetic control of intracellular protein concentration in microbial cell cultures. Integrative Biology: Quantitative Biosciences From Nano to Macro. 6 (3), 366-372 (2014).
- Milias-Argeitis, A., et al. In silico feedback for in vivo regulation of a gene expression circuit. Nature Biotechnology. 29 (12), 1114-1116 (2011).
- Castillo-Hair, S. M., Baerman, E. A., Fujita, M., Igoshin, O. A., Tabor, J. J. Optogenetic control of Bacillus subtilis gene expression. Nature Communications. 10 (1), 1-11 (2019).
- Xia, A., et al. Optogenetic modification of pseudomonas aeruginosa enables controllable twitching motility and host infection. ACS Synthetic Biology. 10 (3), 531-541 (2021).
- Pu, L., Yang, S., Xia, A., Jin, F. Optogenetics manipulation enables prevention of biofilm formation of engineered pseudomonas aeruginosa on surfaces. ACS Synthetic Biology. 7 (1), 200-208 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved