A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Reliable Porcine Fascio-Cutaneous Flap Model for Vascularized Composite Allografts Bioengineering Studies
In This Article
Summary
The present protocol describes the porcine fascio-cutaneous flap model and its potential use in vascularized composite tissue research.
Abstract
Vascularized Composite Allografts (VCA) such as hand, face, or penile transplant represents the cutting-edge treatment for devastating skin defects, failed by the first steps of the reconstructive ladder. Despite promising aesthetic and functional outcomes, the main limiting factor remains the need for a drastically applied lifelong immunosuppression and its well-known medical risks, preventing broader indications. Therefore, lifting the immune barrier in VCA is essential to tip the ethical scale and improve patients' quality of life using the most advanced surgical techniques. De novo creation of a patient-specific graft is the upcoming breakthrough in reconstructive transplantation. Using tissue engineering techniques, VCAs can be freed of donor cells and customized for the recipient through perfusion-decellularization-recellularization. To develop these new technologies, a large-scale animal VCA model is necessary. Hence, swine fascio-cutaneous flaps, composed of skin, fat, fascia, and vessels, represent an ideal model for preliminary studies in VCA. Nevertheless, most VCA models described in the literature include muscle and bone. This work reports a reliable and reproducible technique for saphenous fascio-cutaneous flap harvest in swine, a practical tool for various research fields, especially vascularized composite tissue engineering.
Introduction
Vascularized composite allografts (VCA) have revolutionized the treatment of hard-to-repair body part losses, such as hands, face, and penis1,2,3. Unfortunately, the first long-term outcomes4 have shown that lifelong administration of high-dose immunosuppressive agents can lead to severe collateral medical conditions, including diabetes, infections, neoplasia, and reno-vascular dysfunction5. Lately, expert VCA teams have had to manage the risk of chronic rejection leading to graft loss and perform the first face retransplantation cases6,7. Different strategies have been described to overcome the limitations of immunosuppression in VCA. The first relies on establishing long-term graft tolerance by inducing an immune mixed chimerism state in the allograft recipient8,9. The second involves de novo creation of a patient-specific graft via tissue engineering.
Recently, perfusion decellularization of biological tissues has generated native extracellular matrix (ECM) scaffolds, allowing the preservation of the vascular network and tissue architecture of whole organs10. Hence, the recellularization of these ECM with recipient-specific cells would create a customized graft free of immune constraints. In research on VCA bioengineering, multiple teams have decellularized and obtained such ECM preserving the entire architecture11,12,13. However, the recellularization process remains challenging and has not been successful in large animal models14,15. Developing these breakthrough technologies creates a need for reliable and reproducible large animal composite tissue models. Swine models represent the utmost choice in the bioengineering developmental pipeline, as porcine skin presents the closest anatomical and physiological characteristics to human skin16. The use of fascio-cutaneous flaps (FCF) is ideal during the first steps towards the creation of 'tailored' vascularized composite tissue grafts. Indeed, FCF is an elementary VCA model containing skin, fat, fascia, and endothelial cells. A description of swine myocutaneous flaps17 and osteomyocutaneous flaps18 can be found in the literature. Nonetheless, there is a lack of focus on fascio-cutaneous flaps harvest techniques.
Hence, this study aims to provide researchers with a detailed description of a swine saphenous FCF procurement technique and depict all the flap's characteristics for its use in many research fields, especially in vascularized composite tissue engineering.
Protocol
All animals received human care following the National Institute of Health Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee approved the experimental protocol (IACUC- protocol #2020N000015). Seven female Yorkshire pigs (20-25 kg) were used for all experiments.
1. Preoperative care
- Fast the animal for solid food 12 h prior to the surgery.
- Sedate the animal with 4.4 mg/kg of Telazol, 2.2 mg/kg of Xylazine, and 0.04 mg/kg (IM) of Atropine sulfate (see Table of Materials).
- Place an 18 G peripheral intravenous catheter in an ear vein.
- Intubate the swine with an appropriate endotracheal tube (6-15 mm can be used for 10-200 kg pigs) and connect the tube to a ventilator. Administer pre-operative analgesia with buprenorphine (0.05 mg/kg, IM) (see Table of Materials).
2. Intraoperative monitoring
- Maintain anesthesia with an inhalation mixture of 1.5%-3% isoflurane with 1.5 L/min oxygen flow.
- Continuously monitor the heart rate, pulse oximetry, and end-tidal CO2. Assess blood pressure and body temperature every 5 min.
NOTE: The target range for the heart rate is between 90-100 beats/min, the oxygen saturation must be higher than 93%, and the end-tidal CO2 range is between 5%-6% of CO2. - Administer 5-10 mL/kg per hour 0.9% saline throughout the procedure to regulate the mean arterial pressure between 60 mmHg and 90 mmHg.
3. Bilateral saphenous FCF procurement
- Place the animal in a supine position. Shave and scrub both groins and hindlimbs, include the entire hindlimbs in the surgical site, and drape in a sterile fashion.
- Palpate the pulse of the saphenous artery ~3 finger-widths medial from the patella and tag it.
- Identify and draw the limits of the flap.
NOTE: The superior limit is an axis parallel to the inguinal crease 3 cm below it. The lateral limit is an axis from the anterior superior iliac spine to the medial part of the patella. - Draw a 10 cm diameter oval-like flap centered on the saphenous pedicle and contained in the previously described flap limits (step 3.3).
- Make a 1.5 cm skin incision regarding the distal portion of the pedicle on the flap landmark.
- Open the fascia and blunt dissect to expose the saphenous artery and its two venae comitantes. Perform a double ligature and separate in one bundle.
- Incise the remaining skin of the flap with a blade.
- Use cautery to open the subcutaneous tissue and the surrounding fascia. Perform thorough hemostasis using bipolar forceps (see Table of Materials).
- Attach the skin component of the flap to the underlying fascia with 3-0 non-absorbable sutures to avoid inadvertent traction and disruption of perforating vessels.
- Free the flap from the gracilis by dissecting the fascia away from the muscle.
NOTE: The distal part of the saphenous pedicle runs in a plane between the gracilis muscle and the fascia. Appropriate tension and cautious bipolar hemostasis of side branches are crucial elements to ease the pedicle dissection. - Use a scalpel to make a 12 cm incision in the inguinal crease. Perform a perpendicular incision joining the inguinal crease to the proximal part of the flap. Lift away the connecting skin and open the subcutaneous layer using cautery.
- Continue the pedicle dissection by following the saphenous vessels down towards the femoral vessels.
NOTE: The proximal portion of the saphenous pedicle can either run through the intermuscular septum or dive into the gracilis muscle. - Skeletonize the femoral vessels and ligate them distally to the saphenous branch in two separate bundles. Continue the dissection of the femoral vessels from distal to proximal until reaching the level of the inguinal ligament. Use bipolar forceps to cauterize or vascular clips and 2-0 silk ties to ligate the deep femoral vessels, then cut.
NOTE: Vascular clips can also be used before cutting the vessels. - Repeat steps 3.2-3.13 on the contralateral hindlimb to harvest the second saphenous flap.
- Heparinize the animal with an intravenous (IV) heparin injection (100 IU/kg) 5 min before step 3.16.
- Ligate the femoral pedicle (artery and vein) as proximal to the inguinal ligament as possible and separate the flap from the donor pig.
- Dilate the femoral vessel ends and insert a 20 G angiocatheter in both artery and vein. Use 3-0 silk ties to secure the catheter to the vessels.
- Slowly flush the fascio-cutaneous flap artery with 10 mL of heparin saline (100 IU/mL) until a clear venous outflow is observed (Figure 1).
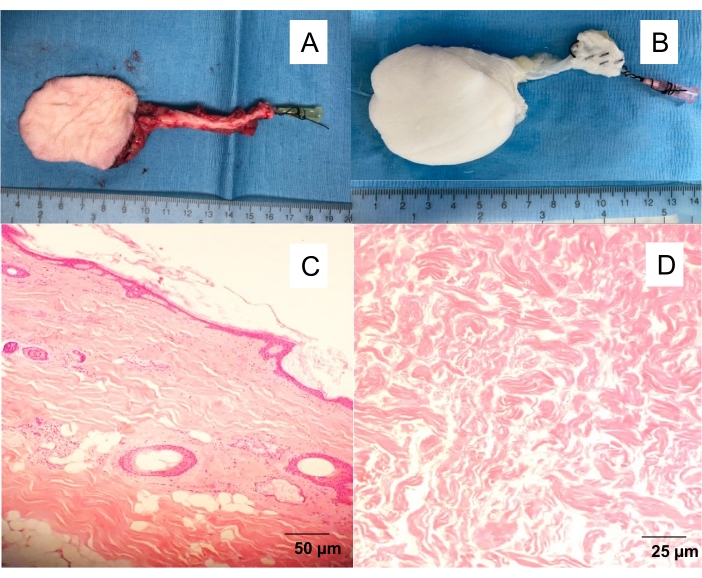
Figure 1: Native and decellularized saphenous fascio-cutaneous flap. (A) Isolated skin flap with a 20 G angiocatheter inserted in the femoral artery, allowing to wash the flap from the blood and proceed with different experiments (angiography, perfusion decellularization). (B) Decellularized skin flap. Perfusion decellularization yielding white, acellular scaffolds after 10 days of detergent perfusion. H&E-stained full-thickness cross-sections of (C) native skin flap and (D) decellularized skin flap. Please click here to view a larger version of this figure.
- Euthanize the animal with an IV injection of sodium phenobarbital (100 mg/kg). Confirm death by the absence of heartbeat and respiratory movements.
Results
This work on living animals was preceded by determining the saphenous perforasome on three cadaveric specimens (Figure 2). A colored filling solution was injected into the saphenous artery to opacify the specific vascular network coming from the artery. The solution is composed of 10 mL blue-colored glycerin agent mixed with 10 mL of the diluent agent (see Table of Materials). This generated a colored map of the skin vascularized by the saphenous artery and allowed drawing t...
Discussion
This article describes a reliable and reproducible fasciocutaneous flap harvested on swine hindlimbs. Following this step-by-step surgical protocol will allow the procurement of two flaps on only one animal in less than 2 h. The most critical step of the surgery is the skeletonization of the vascular pedicle within the gracilis muscle fibers, which requires a thorough dissection by a skilled surgeon. Securing the skin to the fascia using cutaneous sutures is a crucial tip to avoid a shearing effect disrupting the perfora...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by Shriners Hospitals for Children grants #85127 (BEU and CLC) and #84702 (AA). The authors would like to thank the "Gueules Cassées" foundation for the salary support to the fellows involved in that project.
Materials
| Name | Company | Catalog Number | Comments |
| 18 G angiocatheter | BD Insyte Autoguard | 381409 | |
| 20 G angiocatheter | BD Insyte Autoguard | 381411 | |
| Adson Tissue Forceps, 11 cm, 1 x 2 Teeth with Tying Platform | ASSI | ASSI.ATK26426 | |
| Atropine Sulfate | AdvaCare | 212-868 | |
| Bipolar cords | ASSI | 228000C | |
| Buprenorphine HCl | Pharmaceutical, Inc | 42023-179-01 | |
| Dilating Forceps | Fine science tools (FST) | 18131-12 | |
| Endotrachel tube | Jorgensen Labs | JO615X | size from 6 to 15mm depending on the pig weight |
| Ethilon 3-0 16 mm 3/8 | Ethicon | MPVCP683H | |
| Euthasol | Virbac AH | 200-071 | |
| Heparin Lock Flush Solution, USP, 100 units/mL | BD PosiFlush | 306424 | |
| Isoflurane | Patterson Veterinary | 14043-704-06 | |
| Jewelers Bipolar Forceps Non Stick 11 cm, straight pointed tip, 0.25 mm tip diameter | ASSI | ASSI.BPNS11223 | |
| Metzenbaum scissors 180 mm | B Braun | BC606R | |
| Microfil blue | Flow tech | LMV-120 | |
| Microfil dilution | Flow tech | LMV-112 | colored filing solution |
| Monopolar knife | ASSI | 221230C | |
| N°15 scalpel blade | Swann Morton | NS11 | |
| Omnipaque | General Electric | 4080358 | contrast product |
| Perma-Hand Silk 3-0 | Ethicon | A184H | |
| Small Ligaclip | Ethicon | MCM20 | |
| Stevens scissors 115 mm | B Braun | BC008R | |
| Telazol | Zoetis | 106-111 | |
| Xylamed (xylazine) | Bimeda | 200-529 |
References
- Dubernard, J. M., et al. Human hand allograft: Report on first 6 months. The Lancet. 353 (9161), 1315-1320 (1999).
- Meningaud, J. P., et al. Procurement of total human face graft for allotransplantation: A preclinical study and the first clinical case. Plastic and Reconstructive Surgery. 126 (4), 1181-1190 (2010).
- Cetrulo, C. L., et al. Penis transplantation: First US experience. Annals of Surgery. 267 (5), 983-988 (2018).
- Lantieri, L., et al. Face transplant: Long-term follow-up and results of a prospective open study. Lancet. 388 (10052), 1398-1407 (2016).
- Derek, E., Dhanireddy, K. Immunosuppression. Current Opinion in Organ Transplantation. 17 (6), 616-618 (2012).
- Lantieri, L., et al. First human facial retransplantation: 30-month follow-up. Lancet. 396 (10264), 1758-1765 (2020).
- Kauke, M., et al. Full facial retransplantation in a female patient-Technical, immunologic, and clinical considerations. American Journal of Transplantation. 21 (10), 3472-3480 (2021).
- Leonard, D. A., et al. Vascularized composite allograft tolerance across MHC barriers in a large animal model. American Journal of Transplantation. 14 (2), 343-355 (2014).
- Kawai, T., et al. HLA-mismatched renal transplantation without maintenance immunosuppression. The New England Journal of Medicine. 368 (19), 1850-1852 (2013).
- Badylak, S. F., Taylor, D., Uygun, K. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix scaffolds. Annual Review of Biomedical Engineering. 13, 27-53 (2011).
- Jank, B. J., et al. Creation of a bioengineered skin flap scaffold with a perfusable vascular pedicle. Tissue Engineering Part A. 23 (13-14), 696-707 (2017).
- Jank, B. J., et al. Engineered composite tissue as a bioartificial limb graft. Biomaterials. 61, 246-256 (2015).
- Duisit, J., et al. Decellularization of the porcine ear generates a biocompatible, nonimmunogenic extracellular matrix platform for face subunit bioengineering. Annals of Surgery. 267 (6), 1191-1201 (2018).
- Lupon, E., et al. Engineering Vascularized composite allografts using natural scaffolds: A systematic review. Tissue Engineering Part B: Reviews. , (2021).
- Duisit, J., Maistriaux, L., Bertheuil, N., Lellouch, A. G. Engineering vascularized composite tissues by perfusion decellularization/recellularization: Review. Current Transplantation Reports. 8, 44-56 (2021).
- Sullivan, T. P., Eaglstein, W. H., Davis, S. C., Mertz, P. The pig as a model for human wound healing. Wound Repair and Regeneration: Official Publication of the Wound Healing Society [and] the European Tissue Repair Society. 9 (2), 66-76 (2001).
- Haughey, B. H., Panje, W. R. A porcine model for multiple musculocutaneous flaps. The Laryngoscope. 99 (2), 204-212 (1989).
- Ibrahim, Z., et al. A modified heterotopic swine hind limb transplant model for translational vascularized composite allotransplantation (VCA) research. Journal of Visualized Experiments. (80), e50475 (2013).
- Rosh, E. H., Vistnes, L. M., Ksander, G. A. The panniculus carnosus in the domestic pic. Plastic and Reconstructive Surgery. 59 (1), 94-97 (1977).
- Alessa, M. A., et al. Porcine as a training module for head and neck microvascular reconstruction. Journal of Visualized Experiments. (139), e58104 (2018).
- Minqiang, X., Jie, L., Dali, M., Lanhua, M. Transmidline abdominal skin flap model in pig: Refinements and advancements. Journal of Reconstructive Microsurgery. 28 (02), 111-118 (2012).
- Bodin, F., et al. Porcine model for free-flap breast reconstruction training. Journal of Plastic, Reconstructive & Aesthetic Surgery. 68 (10), 1402-1409 (2015).
- Kadono, K., Gruszynski, M., Azari, K., Kupiec-Weglinski, J. W. Vascularized composite allotransplantation versus solid organ transplantation: Innate-adaptive immune interphase. Current Opinion in Organ Transplantation. 24 (6), 714-720 (2019).
- Kruit, A. S., et al. Rectus Abdominis flap replantation after 18 h hypothermic extracorporeal perfusion-A Porcine Model. Journal of Clinical Medicine. 10 (17), 3858 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved