A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Quantitative Determination of De Novo Fatty Acid Synthesis in Brown Adipose Tissue Using Deuterium Oxide
* These authors contributed equally
In This Article
Summary
Here, we present an inexpensive quantitative method utilizing deuterium oxide and gas chromatography mass spectrometry (GCMS) for the analysis of total fatty acid de novo lipogenesis in brown adipose tissue in vivo.
Abstract
Fatty acid synthesis is a complex and highly energy demanding metabolic pathway with important functional roles in the control of whole-body metabolic homeostasis and other physiological and pathological processes. Contrary to other key metabolic pathways, such as glucose disposal, fatty acid synthesis is not routinely functionally assessed, leading to incomplete interpretations of metabolic status. In addition, there is a lack of publicly available detailed protocols suitable for newcomers in the field. Here, we describe an inexpensive quantitative method utilizing deuterium oxide and gas chromatography mass spectrometry (GCMS) for the analysis of total fatty acid de novo synthesis in brown adipose tissue in vivo. This method measures the synthesis of the products of fatty acid synthase independently of a carbon source, and it is potentially useful for virtually any tissue, in any mouse model, and under any external perturbation. Details on the sample preparation for GCMS and downstream calculations are provided. We focus on the analysis of brown fat due to its high levels of de novo fatty acid synthesis and critical roles in maintaining metabolic homeostasis.
Introduction
Obesity and associated metabolic diseases are a pandemic that endanger present and future generations1,2. Commonly simplified as the consequence of the imbalance between energy intake and expenditure, the metabolic dysregulation associated with obesity affects a large number of metabolic pathways controlled by environmental and endogenous factors3. However, only a few pathways are routinely tested in animal models of metabolic dysregulation.
As an example, glucose disposal is routinely measured by glucose and insulin tolerance tests, probably due to the simplicity of using portable glucose monitors4. Whole body glucose and lipid oxidation relative rates are also routinely estimated based on the respiratory exchange ratio from indirect calorimetry assays5,6. However, the majority of all other aspects of metabolism are not routinely functionally assessed. This leads to incomplete interpretations of the metabolic status and missed therapeutic options. One of the main such pathways is de novo lipogenesis.
De novo lipogenesis (DNL) is the process by which new fatty acids are generated from precursors. Glucose is considered to be the main precursor contributing to whole-body DNL7, however other precursors, such as acetate, fructose, lactate, and branched chain amino acids, have been shown to be relevant carbon sources in a spatial and condition dependent manner8,9,10,11,12. DNL is a key contributor to metabolic homeostasis and is essential for normal development13. Additionally, alterations in DNL have been associated with cancer14,15 and metabolic16,17,18 and cardiovascular diseases19,20.
The DNL pathway is composed of the core enzymatic components ATP citrate lyase (ACLY), acetyl-CoA carboxylase (ACC1/2), and fatty acid synthase (FAS) which primarily produce palmitate, a 16-carbon saturated fatty acid. However, odd chain and branched chain fatty acids can also be produced at lower rates9. Elongases and desaturases further modify these fatty acids, creating a diverse range of fatty acids species useful for a variety of functions (e.g., long-term energy storage and manipulation of membrane fluidity).
The expression of the DNL enzymatic machinery is controlled by a short number of transcription factors. The most well described to date include the sterol regulatory element binding protein (SREBP) family, carbohydrate response element binding protein (ChREBP), and liver X receptor (LXR)21,22,23,24,25,26. Despite an apparent overlap in their functions, individual regulations based on cell type dominancy and physiological or pathological conditions have been reported21,22,27,28.
Remarkably, a number of inhibitors for selected steps of the DNL pathway have been approved for clinical use or are in the preclinical or clinical stages of development for a number of diseases, including obesity, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH), and cardiovascular disease29. These efforts highlight the relevance of DNL in health and disease.
In recent years, the employment of methods to quantitatively assess de novo fatty acid synthesis has increased30. The most common method for assessing this is the use of heavy labelled water (D2O), where the heavy labelled hydrogen gets incorporated into acyl chains during synthesis both directly and indirectly, via deuterium exchange with the hydrogens of the DNL substrates NAPDH, acetyl-CoA, and malonyl-CoA. Although this approach is gaining in popularity, there is a lack of publicly available detailed protocols suitable for newcomers in the field. Here, we outline a method for quantitatively assessing the de novo synthesis of products of FAS using D2O and gas chromatography mass spectrometry (GCMS), with calculations previously developed by Lee et al.31. This method measures de novo fatty acid synthesis independently of a carbon source, and it is potentially useful for virtually any tissue, in any mouse model, and under any external perturbation. Here, we focus on the analysis of brown adipose tissue (BAT) due to its high levels of DNL and critical roles in maintaining metabolic homeostasis.
Protocol
All experiments were approved by the Institutional Animal Care and Use Committee at Cincinnati Children's Hospital Medical Center.
1. Preparation of D2O
NOTE: To avoid experimental variation, prepare sufficient solution/drinking water for all mice for the duration of the experiment.
- For intraperitoneal injection: generate 0.9% w/v saline D2O by dissolving 9 g of NaCl per liter of D2O. Filter through a non-pyrogenic 0.2 µm filter to sterilize.
- For drinking D2O-water: generate 8% v/v D2O-enriched water to be used as drinking water by mixing 80 mL of D2O per 920 mL of regular drinking water. Regular drinking water can be obtained from the mouse facility. Filter through a non-pyrogenic 0.2 µm filter to sterilize.
2. Modulation of BAT activity by temperature acclimation
- Separate the mice so there are two mice per cage 2 weeks prior to the start of temperature acclimation. Change the water supply to water bottles for the mice to adapt to and maintain all the cages at 22 °C.
- Prepare the mouse environmental chambers 1 week before starting the temperature acclimation by setting the appropriate temperatures: 30 °C for thermoneutrality, 22 °C for room temperature, and 18 °C for cold exposure.
- At the start of temperature acclimation, replace the cages with new ones with no environmental enrichment (to avoid nests). Move the cages to their respective temperatures.
NOTE: Cages assigned to thermoneutrality will stay at 30 °C for 4 weeks. Cages assigned to room temperature will stay at 22 °C for 4 weeks. Cages assigned to cold exposure will become progressively colder on a weekly schedule: 18 °C for the first week, 14 °C for the second week, 10 °C for the third week, and 6 °C for the fourth week. - Change soiled cages weekly for all conditions. Additionally, replace food and water bottles with new ones preadapted at the appropriate temperature for at least 24 h.
NOTE: Be mindful about the food amounts added every week, especially for mice in the cold, since they will consume a significantly greater amount of food compared to normal conditions. Provide food ad libitum.
3. Administration of D2O
- Inject each animal with 0.9% w/v saline-D2O at 35 µL/g of body weight, 12 h-3 days before tissue collection, via intraperitoneal injection using a 1 mL syringe and 26 G needle.
NOTE: Please see the discussion section for more information on selecting an appropriate labelling time. - Change the water bottles to bottles containing 8% v/v D2O-enriched drinking water.
4. Plasma and tissue collection, processing, and storage
- At the end of the experiment, sacrifice the mice using approved methodologies (e.g., carbon dioxide overdose followed by cervical dislocation).
- Use heat/cool pads or other methods to avoid sudden temperature changes before euthanasia that may affect the results. Euthanize the mice following approved methodologies. Proceed immediately to blood and tissue collection.
- Collect blood through cardiac puncture using a 26 G needle and store in an ethylenediaminetetraacetic acid blood collection tube. Keep the blood on ice until further processing.
- Cut open the skin along the middle line of the back of the mouse from the lower area of the thoracic cavity up to the upper area of the neck, while pulling the skin up to avoid affecting tissues below the skin. The interscapular BAT is located between the shoulder blades under a thin layer of white adipose tissue, and it is composed of two pyramidal shaped lobes.
- Clean the tissue by washing in ice cold phosphate buffered saline (PBS). Dab on a paper towel to eliminate excess liquid, weigh on an analytical scale, and collect in a microtube.
- Immediately flash freeze the tissue using liquid nitrogen. Other BAT depots can also be collected.
- Centrifuge the blood samples at 10,000 x g for 10 min at 4 °C. After centrifugation, carefully collect the plasma without disturbing the red blood cell pellet. Transfer it to a new ice-cold microtube and flash freeze in liquid nitrogen.
- Store brown fat and plasma samples at -80 °C until use.
5. Lipid extraction from adipose tissue
- Before beginning the extraction
- Prepare a 1 mM solution of hexadecenoic-d31 acid in methanol in a glass vial. This will serve as the internal fatty acid standard.
- Pre-cool the required amount of chloroform (CHCl3) and methanol (CH3OH) in a -80 °C freezer, or on dry ice.
NOTE: The antioxidant di-tert-butyl-4-methylphenol (BHT) may be added to the CHCl3 at a concentration of 0.01% w/v (2.5 mg/25 mL) to prevent oxidation of the double bonds in unsaturated fatty acids. - Pre-label microcentrifuge tubes for each tissue sample and an extra tube to be used for a blank extraction.
CAUTION: CH3OH and CHCl3 are highly volatile and toxic if inhaled. Use only in fume hoods.
NOTE: Different brands of microcentrifuge tubes have differing levels of background palmitate and capabilities in terms of preventing solvent leakage. We recommend that a variety of tubes be tested first, to ensure that solvent leakage is prevented and that the tubes have minimal levels of contaminating palmitate. Please see Yao et al.32 for further discussion.
- Take the samples out of the freezer and place on dry ice.
- Place the pre-labelled microcentrifuge tube on an analytical balance and tare the balance. Place tweezers and a scalpel/steel razor blade on dry ice for 10-20 s to cool.
- Use the tweezers to take the frozen tissue sample out of the tube and place on a plastic weigh boat. The weigh boat may be placed on a flat block of dry ice or another pre-cooled surface.
- Using the scalpel or steel razor blade, dissect a small portion of the tissue, equivalent to 5-15 mg in weight. Place in the microcentrifuge tube and record the exact weight. Repeat for each specimen. Samples can be stored in the freezer at this point or can be advanced to the steps below for lipid extraction.
NOTE: Ensure that the scalpel is properly cleaned with 70% ethanol between samples and a fresh weigh boat is used between each sample.
- Add 1 µL/mg of 10 mM hexadecenoic-d31(C16:0-d31) acid to each sample.
CAUTION: The following steps (5.4 to 5.8) should be carried out under a fume hood due to the inhalation risk of the solvents. - Add 250 µL of CH3OH, 250 µL of H2O, and 500 µL of CHCl3 to each sample with three 5 mm stainless steel beads. Place the tubes in a pre-cooled block of a grinding mill and mix the samples at a vibrational frequency of 25 Hz for 5 min, or use guidelines recommended by the manufacturer for tissue samples. Remove the beads using a magnet.
- Centrifuge the samples at 12,000 x g for 10 min at 4 °C.
NOTE: After centrifugation, a clear biphasic separation should be observed with the upper aqueous phase containing polar metabolites and the lower organic phase containing lipids and fatty acids. If no separation is seen, add 250 µL of H2O and repeat the vortex and centrifugation steps. - Using a micropipette, take a fixed volume from the bottom phase of each sample into correspondingly labelled microcentrifuge tubes.
- Add 500 µL of CHCl3 to the remaining sample and repeat steps 5.6-5.7.
- Place the samples under nitrogen gas or in a CHCl3-resistant refrigerated centrifugal vacuum at 4 °C until completely dry. Dried samples can be stored at -20 °C until ready for derivatization.
NOTE: The top layer of each sample can also be collected at this point and dried as in step 5.9 in order to analyze polar metabolites.
6. Preparation of fatty acid methyl esters (FAMEs) and GCMS analysis
- Acid-catalyzed esterification and transesterification to prepare FAMEs
CAUTION: The following steps should be carried out under a fume hood due to the inhalation risk of the solvents.- If the samples have been stored in freezer, dry under nitrogen for 5 min to ensure that no water is present.
- Using MS-grade solvents, pipette 98 mL of anhydrous CH3OH into a glass media bottle. Slowly add 2 mL of anhydrous sulfuric acid in the fume hood to make 2% H2SO4 in CH3OH. Mix by swirling the closed bottle.
- Add 500 µL of 2% H2SO4 in CH3OH solution to each sample and vortex briefly.
- Incubate the samples on a heat block at 50 °C for 2 h.
- Remove the samples from the heat block and add 100 µL of saturated NaCl solution and 500 µL of hexane to each sample.
- Vortex the samples vigorously at room temperature for 1 min. Leave the samples to sit for 1 min; two phases should be apparent after this.
- Collect the upper phase into a fresh microcentrifuge tube (see note in section 5.1.3 for appropriate selection of the microcentrifuge tube).
- To maximize the yield, repeat steps 6.1.5-6.1.7, collecting the second sample into the same labelled tubes.
- Dry the samples at room temperature under nitrogen gas.
- Resuspend the samples in 20 µL/mg of hexane, relative to the original tissue weight, and transfer immediately to glass GC vial with a glass insert.
NOTE: Work quickly while transferring samples to minimize evaporation.
- GCMS analysis
- To determine the abundance of FAME isotopologues, inject the samples on a single quadrupole gas chromatography mass spectrometer (GCMS).
NOTE: While many column types can be used to detect palmitate, the following temperature program was established for a GCMS column that has been developed for the separation of fatty acid cis/trans isomers, as detailed in the Table of Materials. This column has a length of 50 m with an 0.25 mm inner diameter. - Inject 1 µL of sample in a split/splitless inlet at an inlet temperature of 270 °C, using helium as the carrier gas, flowing at 1 mL/min. Use a splitless injection for low abundant fatty acids with a total flow of 19 mL/min, a septum purge of 3 mL/min, and a purge flow to split vent of 15 mL/min at 0.75 min. Use a split ratio of 10:1-40:1 for high abundant fatty acids such as palmitate and oleate.
- Use the following oven parameters: an initial temperature of 80 °C; increase by 20 °C/min increments to 170 °C; increase by 1 °C/min increments to 204 °C; increase by 20 °C/min increments to 250 °C; and then hold at 250 °C for 10 min.
- Use the following mass selective detector (MSD) parameters: an electron impact ionization mode of 70 eV and scan over the range of 50-400 m/z with a scan speed of 1,562 (u/s) and a frequency of 4.1 scans/s. Use a transfer line at 280 °C, an ion source at 230 °C, and a quadrupole at 150 °C.
NOTE: Other columns may be used, but the temperature program will vary. - Sample sequence: Randomize the sample order of injection and inject at least two or three hexane blanks at the beginning of the sequence, after every fifth sample within the sequence, and two or three at the end of the sequence.
- Use instrument specific software, or free open-access software such as Metabolite-Detector33, to integrate the ions in Table 1 for each fatty acid methyl ester.
NOTE: We have outlined ions that cover M1-M5 isotopologues in Table 1 that we have found covers the amount of deuterium incorporation with this labelling window. However, this may need to be expanded if de novo flux is significantly higher and/or a longer labelling time is used. - Use the abundance of each integrated ion to generate a mass isotopomer distribution, where the ion intensities can be converted to fractional abundance so that the sum of the mass isotopomer distribution equals one. Please see the example spreadsheet in the Supplementary File.
NOTE: In order to accurately determine deuterium incorporation, correction of the natural isotope abundance then has to be employed to allow for the presence of naturally occurring isotopes such as 13C, 15N, and 2H. This is performed by applying a correction matrix as outlined by Fernandez et al.34,35, and cannot be performed by subtracting the MID of an unlabelled measured metabolite from a labelled metabolite. In practice, we recommend the use of freely available software such as fluxfix36, polyMID37, or IsoCor38 to turn raw data into fractional MIDs, and we have provided the formula for isotope correction for the methyl ester products of FAS in Table 1. - To determine the quantity of palmitate present, utilize the following formula:
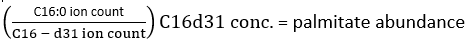
where ion count refers to the sum of all palmitate isotopologues integrated in Table 1 and C16:0-d31 refers to the internal standard hexadecenoic-d31. The internal standard forms a separate peak to that of the endogenous palmitate. The relative abundance of other fatty acids may also be determined, but fatty acid specific isotope internal standards (with mass shifts greater than that observed from D2O incorporation) or external standard curves may need to be employed for full quantitation. Use the blank extracted sample to determine the amount of background palmitate and subtract this from the final tissue value. - Calculate the molar enrichment (ME) of palmitate by the following equation:
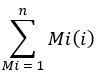
where Mi is the normalized, fractional abundance of a palmitate isotopologue, and n is the number of possible palmitate isotopologues. For example, the ME of a palmitate molecule with the following fractional distribution, M1 = 0.25, M2 = 0.08, M3 = 0.02, is: (0.025*1) + (0.08*2) + (0.02*3) = 0.245 (See Supplementary File).
- To determine the abundance of FAME isotopologues, inject the samples on a single quadrupole gas chromatography mass spectrometer (GCMS).
7. Deuterium acetone exchange of plasma samples to determine body water enrichment
- Reaction
- Prepare 10 standards of deuterium in water, ranging from 0-9% v/v.
- Prepare a 5% v/v acetone/acetonitrile solution allowing for 4 µL per sample, including the standards from step 5.1.1.
- In labelled, safe-lock microcentrifuge tubes, combine 10 µL of each plasma sample or standard, 4 µL of 10 M NaOH, and 4 µL of 5% acetone/acetonitrile. Perform this in triplicate for each sample.
- Mix the samples gently by pipetting. Allow the samples to incubate at room temperature overnight.
- Extraction
- After incubation, add 450-550 mg of Na2SO4 to each sample.
- In the fume hood, add 600 µL of CHCl3 to each tube and vigorously vortex for 15 s.
- Centrifuge the samples at 300 x g for 2 min.
- Under a fume hood, transfer triplicate, 80 µL aliquots of the supernatant from each sample into labelled, glass GCMS vials with glass inserts and cap tightly.
- GCMS analysis
- Separate samples on a column (30 m, 0.25 mm i.d, Agilent DB-35MS) and analyze on the attached mass spectrometer.
- Inject 1 µL of sample in a split/splitless inlet, with a split ratio of 40:1, a helium flow of 1 mL/min, and an inlet temperature of 270 °C.
- Use the following oven parameters: an initial temperature of 60 °C, increase by 20 °C/min increments to 100 °C, increase by 50 °C/min increments to 220 °C, and then hold at 220 °C for 1 min.
- Use the following mass selective detector (MSD) parameters: electron impact ionization mode at 70 eV with select ion monitoring of 58 and 59 m/z. Use a transfer line at 280 °C, an ion source at 230 °C, and a quadrupole at 150 °C.
NOTE: Other low-bleed, bonded, crosslinked, mid-polarity columns may be used, but temperature program will vary.
- Set the liquid sampler method to begin with a CHCl3 wash and then a randomized sequence of the samples, including additional CHCl3 wash steps every six samples.
NOTE: If acetone is used as a needle wash for the autosampler, replace with CHCl3 or hexane and inject multiple blank CHCl3 samples until any contaminating acetone peak is no longer evident in the chromatogram. - Using this method, an acetone peak elutes at around 1.25 min. Integrate the data and calculate the fractional abundance of acetone enrichment following steps 6.2.6-6.2.7, with an adjustment to analyze peaks containing an m/z ratio of 58-60 as indicated in Table 1.
- Use the standards to generate a standard curve to determine the percentage of body water enrichment (p) of the test samples, based on the fractional enrichment of acetone.
- Separate samples on a column (30 m, 0.25 mm i.d, Agilent DB-35MS) and analyze on the attached mass spectrometer.
8. In vivo de novo lipogenesis calculations
- Calculate the fraction of newly synthesized fatty acids (FNS) that are direct products of FAS (i.e., palmitate, odd chain fatty acids, and mmBCFAs) in each specimen with the following equation:
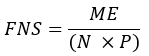
where ME is the average molar enrichment of a palmitate molecule (step 6.2.9), p is the deuterium enrichment in water from the corresponding plasma sample (step 7.3.4), and N is the number of exchangeable hydrogen atoms on palmitate where a deuterium can be incorporated. - Determine N using the equation below which was established by Lee et al.31:
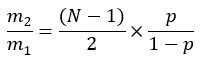
- Determine the molar amount of newly synthesized fatty acids (MNS) by:
MNS = FNS x total fatty acid amount (nmol/mg tissue).
NOTE: For example, if obtaining a palmitate molar enrichment (ME) of 0.245, a deuterium enrichment in body water (p) of 0.045, and a calculated N number of 22, the fractional synthesis of palmitate is 0.247. If the amount of palmitate present in the tissue is 2 mmol/mg, then the mmol of newly synthesized palmitate is 0.494 mmol/mg (see Supplementary File).
Results
Based on the D2O dosing described in step 1, we typically find that body water is enriched in the range of 2.5% to 6%, and that a baseline level of deuterium enrichment in body water is rapidly achieved in 1 h and maintained for the duration of the study via 8% enriched drinking water (Figure 1). Continuous steady state body water enrichment is an assumption of the calculations used in step 6, and so we recommend experimental validation of the kinetics of body water enric...
Discussion
Understanding the balance and interaction between complex metabolic pathways is an indispensable step toward understanding the biological basis of metabolic related diseases. Here, we show a noninvasive and inexpensive methodology to determine changes in de novo fatty acid synthesis. This method is adapted from previously published methods which developed calculations for estimating de novo synthesis flux from fatty acid deuterium enrichment31 and for using deuterium-acetone exch...
Disclosures
The authors have nothing to disclosure.
Acknowledgements
We thank the Sanchez-Gurmaches and Wallace lab members for valuable discussions. This work was supported by grants from the American Heart Association (18CDA34080527 to JSG and 19POST34380545 to RM), the NIH (R21OD031907 to JSG), a CCHMC Trustee Award, a CCHMC Center for Pediatric Genomics Award, and a CCHMC Center for Mendelian Genomics & Therapeutics Award. This work was supported in part by NIH P30 DK078392 of the Digestive Diseases Research Core Center in Cincinnati. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. RT and MW were supported by a UCD Ad Astra Fellowship.
Materials
| Name | Company | Catalog Number | Comments |
| 4 mL Glass Vials | Fisher Scientific | 14-955-334 | |
| 0.2 µm filter | Olympus Plastic | 25-244 | |
| 26G needeled syringes | BD | 309597 | |
| Acetone | Merck | 34850 | |
| Acetonitrile | Merck | 900667 | |
| Blue GC screw cap with septa | Agilent | 5190-1599 | |
| Centrifuge | Eppendorf | 5424R | |
| Chloroform | Sigma | 366927 | |
| Deuterium oxide | Sigma | 151882 | |
| Di-tert-butyl-4-methylphenol (BHT) Select FAME Column | Merck | B1378 | |
| Di-tert-butyl-4-methylphenol (BHT) Select FAME Column | Agilent | CP7419 | |
| EDTA tube | Sarstedt | 411395105 | |
| Ethanol | Merck | 51976 | |
| Hexadecenoic-d31 Acid | Larodan | 71-1631 | |
| Hexane | Merck | 34859 | |
| Methanol | Merck | 34860 | |
| Microcentrifuge tube | Olympus Plastic | 24-282 | |
| Mouse environmental chamber | Caron | Caron 7001-33 | |
| Potasium Chloride | Fisher Bioreagents | BP366-500 | |
| Potasium Phosphate | MP Biomedicals | 194727 | |
| SafeLock microcentrifuge tubes | Eppendorf | 30120086 | |
| Screw top amber GC vial | Agilent | 5182-0716 | |
| Sodium Chloride | Fisher Bioreagents | BP358-212 | |
| Sodium Hydroxide | Merck | S5881 | |
| Sodium Phosphate, dibasic | Fisher Bioreagents | BP332-500 | |
| Sodium Sulfate | Merck | 239313 | |
| Sulfuric Acid | Merck | 258105 | |
| Vial insert | Agilent | 5183-2088 |
References
- . The Lancet, Diabetes Endocrinology. Childhood obesity: a growing pandemic. The Lancet. Diabetes & Endocrinology. 10 (1), 1 (2022).
- Gonzalez-Muniesa, P., et al. Obesity. Nature Reviews Disease Primers. 3, 17034 (2017).
- Müller, T. D., Blüher, M., Tschöp, M. H., DiMarchi, R. D. Anti-obesity drug discovery: advances and challenges. Nature Reviews Drug Discovery. 21 (3), 201-223 (2021).
- Virtue, S., Vidal-Puig, A. GTTs and ITTs in mice: simple tests, complex answers. Nature Metabolism. 3 (7), 883-886 (2021).
- Müller, T. D., Klingenspor, M., Tschöp, M. H. Revisiting energy expenditure: how to correct mouse metabolic rate for body mass. Nature Metabolism. 3 (9), 1134-1136 (2021).
- Virtue, S., Lelliott, C. J., Vidal-Puig, A. What is the most appropriate covariate in ANCOVA when analysing metabolic rate. Nature Metabolism. 3 (12), 1585 (2021).
- Hellerstein, M. K. De novo lipogenesis in humans: metabolic and regulatory aspects. European Journal of Clinical Nutrition. 53 Suppl 1, S53-S65 (1999).
- Zhao, S., et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 579 (7800), 586-591 (2020).
- Wallace, M., et al. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nature Chemical Biology. 14 (11), 1021-1031 (2018).
- Zhao, S., et al. ATP-citrate lyase controls a glucose-to-acetate metabolic switch. Cell Reports. 17 (4), 1037-1052 (2016).
- Green, C. R., et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nature Chemical Biology. 12 (1), 15-21 (2016).
- Zhang, Z., et al. Serine catabolism generates liver NADPH and supports hepatic lipogenesis. Nature Metabolism. 3 (12), 1608-1620 (2021).
- Chirala, S. S., et al. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proceedings of the National Academy of Sciences. 100 (11), 6358-6363 (2003).
- Icard, P., et al. ATP citrate lyase: A central metabolic enzyme in cancer. Cancer Letters. 471, 125-134 (2020).
- Fhu, C. W., Ali, A. Fatty acid synthase: an emerging target in cancer. Molecules. 25 (17), 3935 (2020).
- Lawitz, E. J., et al. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clinical Gastroenterology and Hepatology. 16 (12), 1983e3-1991e3 (2018).
- Smith, G. I., et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. The Journal of Clinical Investigation. 130 (3), 1453-1460 (2020).
- Imamura, F., et al. Fatty acids in the de novo lipogenesis pathway and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLoS Medicine. 17 (6), e1003102 (2020).
- Lai, H. T. M., et al. Serial plasma phospholipid fatty acids in the de novo lipogenesis pathway and total mortality, cause-specific mortality, and cardiovascular diseases in the cardiovascular health study. Journal of the American Heart Association. 8 (22), e012881 (2019).
- Ference, B. A., et al. Mendelian randomization study of ACLY and cardiovascular disease. The New England Journal of Medicine. 380 (11), 1033-1042 (2019).
- Herman, M. A., et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 484 (7394), 333-338 (2012).
- Sanchez-Gurmaches, J., et al. Brown fat AKT2 Is a cold-induced kinase that stimulates ChREBP-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metabolism. 27 (1), 195e6-209e6 (2018).
- Wang, X., et al. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. II. Purification and characterization. The Journal of Biological Chemistry. 268 (19), 14497-14504 (1993).
- Briggs, M. R., Yokoyama, C., Wang, X., Brown, M. S., Goldstein, J. L. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. I. Identification of the protein and delineation of its target nucleotide sequence. The Journal of Biological Chemistry. 268 (19), 14490-14496 (1993).
- Yokoyama, C., et al. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 75 (1), 187-197 (1993).
- Chen, G., Liang, G., Ou, J., Goldstein, J. L., Brown, M. S. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proceedings of the National Academy of Sciences. 101 (31), 11245-11250 (2004).
- Denechaud, P. D., et al. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. The Journal of Clinical Investigation. 118 (3), 956-964 (2008).
- Crewe, C., et al. SREBP-regulated adipocyte lipogenesis is dependent on substrate availability and redox modulation of mTORC1. JCI Insight. 5 (15), e129397 (2019).
- Batchuluun, B., Pinkosky, S. L., Steinberg, G. R. Lipogenesis inhibitors: therapeutic opportunities and challenges. Nature Reviews Drug Discovery. 21 (4), 283-305 (2022).
- Wallace, M., Metallo, C. M. Tracing insights into de novo lipogenesis in liver and adipose tissues. Seminars in Cell & Developmental Biology. 108, 65-71 (2020).
- Lee, W. N., et al. In vivo measurement of fatty acids and cholesterol synthesis using D2O and mass isotopomer analysis. The American Journal of Physiology. 266 (5 Pt 1), E699-E708 (1994).
- Yao, C. H., Liu, G. Y., Yang, K., Gross, R. W., Patti, G. J. Inaccurate quantitation of palmitate in metabolomics and isotope tracer studies due to plastics. Metabolomics. 12, 143 (2016).
- Hiller, K., et al. MetaboliteDetector: comprehensive analysis tool for targeted and nontargeted GC/MS based metabolome analysis. Analytical Chemistry. 81 (9), 3429-3439 (2009).
- Fernandez, C. A., Des Rosiers, C., Previs, S. F., David, F., Brunengraber, H. Correction of 13C mass isotopomer distributions for natural stable isotope abundance. Journal of Mass Spectrometry. 31 (3), 255-262 (1996).
- Midani, F. S., Wynn, M. L., Schnell, S. The importance of accurately correcting for the natural abundance of stable isotopes. Analytical Biochemistry. 520, 27-43 (2017).
- Trefely, S., Ashwell, P., Snyder, N. W. FluxFix: automatic isotopologue normalization for metabolic tracer analysis. BMC Bioinformatics. 17 (1), 485 (2016).
- Jeong, H., et al. Correcting for naturally occurring mass isotopologue abundances in stable-isotope tracing experiments with PolyMID. Metabolites. 11 (5), 310 (2021).
- Millard, P., et al. IsoCor: isotope correction for high-resolution MS labeling experiments. Bioinformatics. 35 (21), 4484-4487 (2019).
- Brunengraber, D. Z., et al. Influence of diet on the modeling of adipose tissue triglycerides during growth. American Journal of Physiology. Endocrinology and Metabolsim. 285 (4), E917-E925 (2003).
- Svensson, R. U., et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nature Medicine. 22 (10), 1108-1119 (2016).
- Hellerstein, M. K., Neese, R. A. Mass isotopomer distribution analysis: a technique for measuring biosynthesis and turnover of polymers. The American Journal of Physiology. 263 (5 Pt 1), E988-E1001 (1992).
- Hellerstein, M. K., Neese, R. A. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. The American Journal of Physiology. 276 (6), E1146-E1170 (1999).
- Kelleher, J. K., Masterson, T. M. Model equations for condensation biosynthesis using stable isotopes and radioisotopes. The American Journal of Physiology. 262 (1 Pt 1), E118-E125 (1992).
- Kelleher, J. K., Nickol, G. B. Isotopomer spectral analysis: utilizing nonlinear models in isotopic flux studies. Methods in Enzymology. 561, 303-330 (2015).
- Argus, J. P., et al. Development and application of FASA, a model for quantifying fatty acid metabolism using stable isotope labeling. Cell Reports. 25 (10), 2919.e8-2934.e8 (2018).
- Guilherme, A., et al. Control of adipocyte thermogenesis and lipogenesis through β3-adrenergic and thyroid hormone signal integration. Cell Reports. 31 (5), 107598 (2020).
- Guilherme, A., et al. Neuronal modulation of brown adipose activity through perturbation of white adipocyte lipogenesis. Molecular Metabolism. 16, 116-125 (2018).
- Guilherme, A., et al. Adipocyte lipid synthesis coupled to neuronal control of thermogenic programming. Molecular Metabolism. 6 (8), 781-796 (2017).
- Lodhi, I. J., et al. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell Metabolism. 16 (2), 189-201 (2012).
- McCormack, J. G., Denton, R. M. Evidence that fatty acid synthesis in the interscapular brown adipose tissue of cold-adapted rats is increased in vivo by insulin by mechanisms involving parallel activation of pyruvate dehydrogenase and acetyl-coenzyme A carboxylase. The Biochemistry Journal. 166 (3), 627-630 (1977).
- Trayhurn, P. Fatty acid synthesis in vivo in brown adipose tissue, liver and white adipose tissue of the cold-acclimated rat. FEBS Letters. 104 (1), 13-16 (1979).
- Negron, S. G., Ercan-Sencicek, A. G., Freed, J., Walters, M., Lin, Z. Both proliferation and lipogenesis of brown adipocytes contribute to postnatal brown adipose tissue growth in mice. Science Reports. 10 (1), 20335 (2020).
- Schlein, C., et al. Endogenous fatty acid synthesis drives brown adipose tissue involution. Cell Reports. 34 (2), 108624 (2021).
- Mottillo, E. P., et al. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic beta3-adrenergic receptor activation. Journal of Lipid Research. 55 (11), 2276-2286 (2014).
- Adlanmerini, M., et al. Circadian lipid synthesis in brown fat maintains murine body temperature during chronic cold. Proceedings of the National Academy of Sciences. 116 (37), 18691-18699 (2019).
- Yu, X. X., Lewin, D. A., Forrest, W., Adams, S. H. Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: prediction from differential gene expression and confirmation in vivo. FASEB Journal. 16 (2), 155-168 (2002).
- Kushner, D. J., Baker, A., Dunstall, T. G. Pharmacological uses and perspectives of heavy water and deuterated compounds. Canadian Journal of Physiology and Pharmacology. 77 (2), 79-88 (1999).
- Diraison, F., Pachiaudi, C., Beylot, M. Measuring lipogenesis and cholesterol synthesis in humans with deuterated water: use of simple gas chromatographic/mass spectrometric techniques. Journal of Mass Spectrometry. 32 (1), 81-86 (1997).
- Yang, D., et al. Assay of low deuterium enrichment of water by isotopic exchange with [U-13C3]acetone and gas chromatography-mass spectrometry. Analytical Biochemistry. 258 (2), 315-321 (1998).
- Fu, X., et al. Measurement of lipogenic flux by deuterium resolved mass spectrometry. Nature Communications. 12 (1), 3756 (2021).
- Shah, V., Herath, K., Previs, S. F., Hubbard, B. K., Roddy, T. P. Headspace analyses of acetone: a rapid method for measuring the 2H-labeling of water. Analytical Biochemistry. 404 (2), 235-237 (2010).
- Argus, J. P., Yu, A. K., Wang, E. S., Williams, K. J., Bensinger, S. J. An optimized method for measuring fatty acids and cholesterol in stable isotope-labeled cells. Journal of Lipid Research. 58 (2), 460-468 (2017).
- Belew, G. D., Jones, J. G. De novo lipogenesis in non-alcoholic fatty liver disease: Quantification with stable isotope tracers. European Journal of Clinical Investigation. 52 (3), e13733 (2022).
- Zhang, Z., Chen, L., Liu, L., Su, X., Rabinowitz, J. D. Chemical basis for deuterium labeling of fat and NADPH. Journal of the American Chemical Society. 139 (41), 14368-14371 (2017).
- Belew, G. D., et al. Transfer of glucose hydrogens via acetyl-CoA, malonyl-CoA, and NADPH to fatty acids during de novo lipogenesis. Journal of Lipid Research. 60 (12), 2050-2056 (2019).
- Diraison, F., Pachiaudi, C., Beylot, M. In vivo measurement of plasma cholesterol and fatty acid synthesis with deuterated water: determination of the average number of deuterium atoms incorporated. Metabolism. 45 (7), 817-821 (1996).
- Ajie, H. O., et al. In vivo study of the biosynthesis of long-chain fatty acids using deuterated water. The American Journal of Physiology. 269 (2 Pt 1), E247-E252 (1995).
- Schloerb, P. R., Friis-Hansen, B. J., Edelman, I. S., Solomon, A. K., Moore, F. D. The measurement of total body water in the human subject by deuterium oxide dilution; with a consideration of the dynamics of deuterium distribution. The Journal of Clinical Investigation. 29 (10), 1296-1310 (1950).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved