A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
In Vivo Luminal Measurement of Distension-Evoked Urothelial ATP Release in Rodents
In This Article
Summary
This protocol describes the procedure for measuring ATP concentrations in the lumen of the bladder in an anesthetized rodent.
Abstract
ATP, released from the urothelium in response to bladder distension, is thought to play a significant sensory role in the control of micturition. Therefore, accurate measurement of urothelial ATP release in a physiological setting is an important first step in studying the mechanisms that control purinergic signaling in the urinary bladder. Existing techniques to study mechanically evoked urothelial ATP release utilize cultured cells plated on flexible supports or bladder tissue pinned into Ussing chambers; however, each of these techniques does not fully emulate conditions in the intact bladder. Therefore, an experimental setup was developed to directly measure ATP concentrations in the lumen of the rodent urinary bladder.
In this setup, the bladders of anesthetized rodents are perfused through catheters in both the dome of the bladder and via the external urethral orifice. Pressure in the bladder is increased by capping the urethral catheter while perfusing sterile fluid into the bladder through the dome. Measurement of intravesical pressure is achieved using a pressure transducer attached to the bladder dome catheter, akin to the setup used for cystometry. Once the desired pressure is reached, the urethral catheter's cap is removed, and fluid collected for ATP quantification by luciferin-luciferase assay. Through this experimental setup, the mechanisms controlling both mechanical and chemical stimulation of urothelial ATP release can be interrogated by including various agonists or antagonists into the perfusate or by comparing results between wildtype and genetically modified animals.
Introduction
Urinary ATP is thought to play a significant sensory role in the control of micturition1. For example, it is thought that ATP is released from the urothelium in response to distension where it can act on receptors on bladder afferent nerves to increase their excitability, leading to sensations of fullness2. Thus, it is also thought that urinary ATP could be an important player in the development of bladder pathologies. In support of this hypothesis, urinary ATP concentrations are significantly increased in patients suffering from overactive bladder (OAB)3, bladder pain syndrome/interstitial cystitis (BPS/IC)4, or a urinary tract infection (UTI)5,6, all conditions characterized by increased urgency, frequency and, sometimes, pain. Conversely, patients suffering from underactive bladder (UAB), which is characterized by an inability to empty one's bladder and can sometimes include a decreased ability to sense bladder fullness, have been shown to have decreased urinary ATP concentrations7. Experimentally, manipulation of urinary ATP concentrations can alter bladder reflexes in the rat; increasing ATP concentrations by blocking endogenous ATPases in the bladder lumen can increase voiding frequency, while decreasing ATP concentrations by instilling exogenous ATPases into the bladder reduces voiding frequency8. Thus, the importance of urinary ATP to bladder function is clear.
Given the apparent importance of urinary ATP to bladder pathology, accurate measurement of urothelial ATP release is an important step in understanding the mechanisms that control release. Many studies have been completed using different experimental models to measure urothelial ATP release. Foremost among these are cell cultures, either primary cultures or cell lines. However, the use of cultured urothelial cells is complicated by the fact that urothelial cells do not take on their physiological polarized morphology unless they are grown on special permeable membranes (such as Transwell technology [well inserts])9. Thus, it is difficult to relate any ATP release measured to physiology. Urothelial cells grown on well inserts can polarize and form a barrier akin to what is seen in vivo; however, the growth of a fully differentiated urothelium can take days or weeks. Additionally, while it is possible to mount well inserts into an Ussing chamber and apply pressure to the apical side to cause stretch, it is difficult to apply enough pressure to mimic conditions inside the bladder during pathology (i.e., pressures of 30 cm H2O or above). Whole bladder tissue can also be mounted in an Ussing chamber for stretch experiments, but this removes the bladder from the organism along with the trophic factors maintaining urothelial cell health and, hence, urothelial barrier function. Therefore, the most physiologically relevant way to study the release of ATP from the urothelium in response to stretch or pressure is in vivo. The surgical techniques needed to set up the experiment are identical to those commonly used in animal cystometry and, therefore, should be easily performed by anyone familiar with that technique.
In this protocol, we will describe the technique used to examine luminal ATP in female Sprague Dawley rats weighing approximately 200-250 g, as the transurethral catheterization described below is much easier in females; however, transurethral catheterization can also be performed in male rodents10. As transurethral catheterization has now been performed in mice of both sexes as well11, these experiments can easily be adapted for mice or rats of either sex or of varying sizes, depending on the needs of the research team.
Protocol
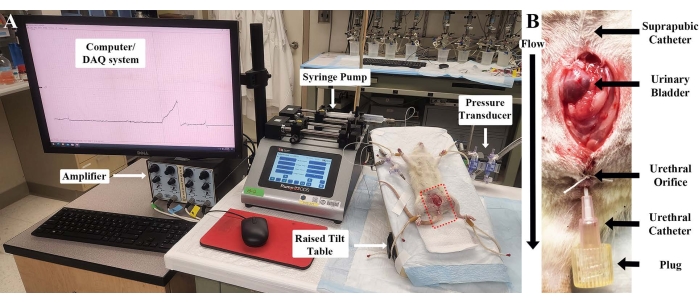
All procedures carried out in rodents must adhere to the applicable guidelines and be approved by the local institutional ethics review committee. The experiments performed for this manuscript were carried out in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh School of Medicine. See Figure 1 for a modified version of the standard rodent cystometry setup used in this protocol.
1. Laboratory animals
- Maintain the rats in social housing (multiple rodents in one cage) with a 12 h light/dark cycle and ad libitum access to water and food pellets.
2. Anesthesia and ganglionic block
- Induce initial anesthesia by placing the animal in a closed box gassed with 4%-5% isoflurane in O2 (1 L/min).
- Anesthetize the animal using urethane.
- Inject urethane subcutaneously bilaterally (1/2 dose on each side of the animal) at a dose of 1.2 g/kg. Place the animal in a cage to allow the urethane to take effect, which generally takes 2 h.
- Alternatively, administer urethane intraperitoneally (i.p.) by injecting the full dose in two separate doses ~10 min apart.
- After waiting for the appropriate time for the urethane to take effect (s.c.: 2 h, i.p.: 30 min), test for a proper plane of anesthesia by pinching the foot of the animal using forceps. If a reflex is observed, administer an additional dose of urethane (0.05-0.1 mL i.p.), wait for 15 min, and test again. Continue to monitor the animal for the proper plane of anesthesia throughout the procedure.
- To prevent a contraction of the bladder during distension, inject the animal with a ganglionic blocking agent, such as hexamethonium (20 mg/kg, i.p.).
- Apply ophthalmic ointment to the animal's eyes to prevent drying during the experiment.
3. Surgical procedure-suprapubic bladder catheterization
- Shave the abdomen of the animal and perform a midline laparotomy to expose the urinary bladder.
- Prepare a catheter by flaring one end of a short (~10-15 cm) length of PE50 intramedic tubing using a flame. Place a 22 G needle into the other end of the tubing and fill with Krebs solution (see Table of Materials for the composition).
- Place a small loop of 3-0 silk suture over the dome of the bladder and perform a small cystostomy (using fine scissors or an 18 G needle) large enough to insert the flared end of the catheter made above. With one hand holding the catheter in place, use the other hand to tighten the loop of the suture to secure the catheter in place. Finish securing the catheter by tying two knots in the suture and pulling the catheter back until the flared head is in contact with the bladder wall.
- Alternatively, secure the catheter using a classic purse-string suture technique, as previously described for cystometry12.
NOTE: It is imperative not to introduce air bubbles into the bladder during this procedure.
- Alternatively, secure the catheter using a classic purse-string suture technique, as previously described for cystometry12.
- Test the setup for leaks by infusing a small amount of Krebs solution through the catheter. If the fluid leaks out of the cystostomy, resecure the catheter with additional suture around the cystostomy.
4. Transurethral catheterization
- Dip the end of a 20 G x 1" I.V. catheter (with the needle removed) in surgical lubricant.
- Hold the external urethral meatus gently with a pair of forceps and insert the tip of the catheter into the urethral orifice in the direction of the tail until the tip causes the wall of the adjacent vaginal opening to deform. Rotate the catheter 90° (bringing the Luer-Lock end of the catheter toward the tail) and gently advance. Fully insert the catheter until the Luer-Lock hub is approximately 5 mm distal from the external urethral opening.
NOTE: Do not insert the catheter too far, which may cause the tip to poke the inside wall of the bladder. If resistance is felt while advancing the catheter, stop and begin again or risk puncturing the urethra. See the discussion section on tips to increase successful catheterization. - Secure the catheter and prevent leakage around the catheter by looping a short length of 3-0 silk suture around the external urethral meatus and tie it off tightly. Secure the catheter to the tail with tape to prevent it from accidentally being pulled out.
- Once catheterized, gently infuse Krebs solution into the bladder through the suprapubic bladder catheter and confirm that the fluid flows out of the urethral catheter and not around it. If necessary, retie the suture around the external urethral orifice.
- Close the abdominal incision over the bladder using a 3-0 silk suture.
5. Experimental setup
- Secure the animal on a board capable of being inclined to aid in the draining of intravesical fluid through the urethral catheter. Place a heating pad and absorbent underpad between the animal and the board to maintain body heat and absorb fluid draining from the urethral catheter.
- Connect the suprapubic catheter to a three-way stopcock, which connects the catheter to a syringe pump and a pressure transducer. Connect the pressure transducer to a computer by way of an amplifier and a data acquisition system.
NOTE: Care should be taken to prevent air bubbles from forming in the tubing connecting the syringe pump, transducer, and bladder catheter. - Calibrate the bladder pressure recording using the procedure suggested by the manufacturer of the pressure transducer and/or data acquisition software.
- Infuse Krebs solution through the suprapubic catheter at a rate of 0.1 mL/min and allow the fluid to drain from the urethral catheter for 1 h to wash out any residual ATP released during the catheter implantations.
- After this washout period, cap the urethral catheter using a Luer-Lock plug and measure the pressure in the bladder. Look for a slow rise in the intravesical pressure to a pressure of 30 cm H2O without a sharp increase in pressure, which would indicate a bladder contraction (see Figure 2). Remove the plug from the urethral catheter when the pressure reaches 30 cm H2O to prevent damage to the bladder.
NOTE: If bladder contractions continue to occur after 1 h, give an additional dose of hexamethonium (5 mg/kg dose i.p.).
6. Collection of samples
- Infuse the bladder at 0.1 mL/min and collect the eluate from the urethral catheter. Test 100 µL aliquots of the eluate immediately for ATP (see below) or freeze for later batch quantification.
- To test the effect of bladder distension on luminal ATP concentrations, cap the urethral catheter with the plug and monitor bladder pressure until it reaches the desired level. Then, uncap the urethral catheter and collect the eluate for ATP measurement or freezing, as described above.
- After each distension, allow the bladder to rest and wash out for 10-15 min before taking additional samples. Take a total of 3-5 predistension samples and 3-5 samples at each desired distention pressure to demonstrate repeatability.
- To test the effect of drugs on the release of ATP, switch the Krebs solution infusing the bladder to Krebs containing the drug of choice. Perfuse at 0.1 mL/min for 10-15 min for the drug to have an effect, and then collect the samples from non-distended and distended bladders as described in steps 6.1 and 6.2.
7. Quantifying ATP from collected samples
- Quantify ATP in the collected 100 µL samples using a commercially available luciferin/luciferase assay kit following the manufacturer's instructions and a luminometer.
- To quantify ATP, combine 100 µL samples of perfusate with 50 µL of the assay mix and place them in the luminometer for reading. To convert the Relative Light Units (RLUs) reported by the luminometer to a concentration of ATP, make serial dilutions of ATP in Krebs solution ranging from 1 µM to 10 pM in 10-fold dilutions to create a standard curve and read them in the luminometer. Plot the resulting readings on a graph and perform a non-linear (quadratic) regression to extrapolate concentrations from the samples taken from the animal.
NOTE: It is important to make ATP standards for any drug solution tested in the experiment, as many drugs interfere with the luciferin/luciferase reaction, which must be corrected for.
- To quantify ATP, combine 100 µL samples of perfusate with 50 µL of the assay mix and place them in the luminometer for reading. To convert the Relative Light Units (RLUs) reported by the luminometer to a concentration of ATP, make serial dilutions of ATP in Krebs solution ranging from 1 µM to 10 pM in 10-fold dilutions to create a standard curve and read them in the luminometer. Plot the resulting readings on a graph and perform a non-linear (quadratic) regression to extrapolate concentrations from the samples taken from the animal.
8. Euthanasia of animals
- When the experiment is completed and all the samples are collected, humanely euthanize the animal according to USDA guidelines and the National Research Council's Guide for the Care and Use of Laboratory Animals.
- Remove the anesthetized animal from the experimental setup, place it in a closed box, and gas it with 100% CO2. Ensure that the fill rate is equal to 30%-70% of the chamber volume per minute (e.g., 3-7 L/min for a box with a 10 L volume). Continue the CO2 flow for at least 1 min after respiration ceases.
- Use a secondary form of euthanasia to ensure death.
- Perform a thoracotomy as a secondary form of euthanasia by grasping the small flap of skin at the caudal end of the sternum and cutting a small hole in the skin and musculature at the diaphragm with a sharp pair of scissors. Complete the thoracotomy by inserting the scissors into the opening and cutting rostrally through the rib cage and exposing the thoracic cavity.
NOTE: The euthanized animal should be disposed of according to institutional guidelines.
- Perform a thoracotomy as a secondary form of euthanasia by grasping the small flap of skin at the caudal end of the sternum and cutting a small hole in the skin and musculature at the diaphragm with a sharp pair of scissors. Complete the thoracotomy by inserting the scissors into the opening and cutting rostrally through the rib cage and exposing the thoracic cavity.
Results
The described protocol allows for the accurate measurement of urothelial ATP release in vivo from the lumen of the bladder, using a modified version of the standard rodent cystometry setup (see Figure 1). This allows the researcher to examine the effects of drugs on stretch-mediated ATP release in a physiological setting.
Discussion
The majority of research into urothelial ATP release is conducted in cultured cells, using either immortalized cell lines or primary cultures of rodent urothelial cells. While these models have the benefit of being relatively high throughput (i.e., one culture/passage can make many plates/dishes of cells), their physiological relevance is diminished due to: 1) the inability of urothelial cells to grow polarized unless they are grown on special supports and 2) the difficulty in exposing cultured cells to physiological lev...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to JMB (DK117884).
Materials
| Name | Company | Catalog Number | Comments |
| amplifier | World Precision Instruments (WPI) | SYS-TBM4M | |
| ATP assay kit | Sigma-Aldrich, Inc. | FLAA-1KT | |
| data acquisition system/ software | DataQ Instruments | DI-1100 | Software included, requires Windows-based computer |
| Hexamethonium bromide | Sigma-Aldrich, Inc. | H0879 | 20 mg/kg dose |
| Isoflurane | Covetrus North America | 29404 | |
| lidocaine | Covetrus North America | 2468 | |
| Luer Lock plugs | Fisher Scientific | NC0455253 | |
| luminometer (GloMax 20/20) | Promega | E5311 | |
| Polyethylene (PE50) tubing | Fisher Scientific | 14-170-12B | |
| Pump 33 DDS syringe pump | Harvard Apparatus | 703333 | |
| pressure transducers | World Precision Instruments (WPI) | BLPR2 | |
| surgical instruments (scissors, hemostats, forceps, etc.) | Fine Science Tools | multiple numbers | |
| surgical lubricant | Fisher Scientific | 10-000-694 | |
| Sur-Vet I.V. catheter | Covetrus North America | 50603 | 20 G x 1 inch |
| tiltable surgical table (Plas Labs) | Fisher Scientific | 01-288-30A | |
| Tubing connectors | Fisher Scientific | 14-826-19E | allows Luer-Lock connectors to attach to tubing |
| Urethane | Sigma-Aldrich, Inc. | U2500 | 0.5 g/mL conc., 1.2 g/kg dose |
References
- Burnstock, G. Purinergic signalling in the urinary tract in health and disease. Purinergic Signalling. 10 (1), 103-155 (2014).
- Birder, L. A. Urothelial signaling. Autonomic Neuroscience: Basic & Clinical. 153 (1-2), 33-40 (2010).
- Silva-Ramos, M., et al. Urinary ATP may be a dynamic biomarker of detrusor overactivity in women with overactive bladder syndrome. PLoS One. 8 (5), 64696 (2013).
- Sun, Y., Chai, T. C. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. American Journal of Physiology - Cell Physiology. 290 (1), 27-34 (2006).
- Gill, K., et al. Urinary ATP as an indicator of infection and inflammation of the urinary tract in patients with lower urinary tract symptoms. BMC Urology. 15 (1), 7 (2015).
- Säve, S., Persson, K. Extracellular ATP and P2Y receptor activation induce a proinflammatory host response in the human urinary tract. Infection and Immunity. 78 (8), 3609-3615 (2010).
- Munoz, A., Smith, C. P., Boone, T. B., Somogyi, G. T. Overactive and underactive bladder dysfunction is reflected by alterations in urothelial ATP and NO release. Neurochemistry International. 58 (3), 295-300 (2011).
- Beckel, J. M., et al. Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. The Journal of Physiology. 593 (8), 1857-1871 (2015).
- Zhang, Y. Y., Ludwikowski, B., Hurst, R., Frey, P. Expansion and long-term culture of differentiated normal rat urothelial cells in vitro. In Vitro Cellular & Developmental Biology. Animal. 37 (7), 419-429 (2001).
- Lee, S., Carrasco, A., Meacham, R. B., Malykhina, A. P. Transurethral instillation procedure in adult male mouse. Journal of Visualized Experiments. (129), e56663 (2017).
- Zychlinsky Scharff, A., Albert, M. L., Ingersoll, M. A. Urinary tract infection in a small animal model: Transurethral catheterization of male and female mice. Journal of Visualized Experiments. (130), e54432 (2017).
- Uvin, P., et al. The use of cystometry in small rodents: a study of bladder chemosensation. Journal of Visualized Experiments. (66), e3869 (2012).
- Leitao, J. M., Esteves da Silva, J. C. Firefly luciferase inhibition. Journal of Photochemistry and Photobiology B. 101 (1), 1-8 (2010).
- Auld, D. S., Thorne, N., Nguyen, D. T., Inglese, J. A specific mechanism for nonspecific activation in reporter-gene assays. ACS Chemical Biology. 3 (8), 463-470 (2008).
- Harvey, E. N. Studies on the oxidation of luciferin without luciferase and the mechanism of bioluminescence. Journal of Biological Chemistry. 78 (2), 369-375 (1928).
- Wang, J., Jackson, D. G., Dahl, G. The food dye FD&C Blue No. 1 is a selective inhibitor of the ATP release channel Panx1. The Journal of General Physiology. 141 (5), 649-656 (2013).
- Avazpour, M., Shiri, S., Delpisheh, A., Abbasi, A. M. Simultaneous determination of brilliant blue FCF and carmoisine in food samples by aqueous two-phase system and spectrophometric detection. Journal of Basic Research in Medical Sciences. 1 (1), 56-65 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved